Abstract
Objectives: To investigate the role of Morinda officinalis polysaccharide (MOP) in the protein expression of the Wnt/β-catenin signaling cascade during the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs), and to elucidate the mechanisms by which MOP enhances osteogenic differentiation at the cellular level. Methods: BMSCs were isolated and cultured using the whole bone marrow adherence method, followed by flow cytometry for the detection of BMSC marker antigens. Two groups were prepared: a low-dose MOP (L-MOP, 10 µg/mL) group and a high-dose MOP (H-MOP, 40 µg/mL) group. MTT assays and cell clone formation assays were performed to evaluate the effects of different MOP doses on BMSC proliferation. Alizarin red staining (ARS) and alkaline phosphatase (ALP) staining were conducted to assess the impact of varying MOP doses on nodule calcification and ALP activity in BMSCs. Additionally, western blot assays were carried out to determine the effects of different MOP concentrations on the expression levels of osteogenesis-related factors and Wnt/β-catenin pathway proteins in BMSCs. Results: Highly purified BMSCs were successfully extracted. Subsequent assays demonstrated that BMSCs exhibited enhanced proliferation at all MOP doses, particularly at the H-MOP dose, compared to the control group. Both L-MOP and H-MOP increased calcium content and ALP activity in BMSCs, as well as elevated the expression of osteogenic factors and Wnt/β-catenin pathway proteins compared to the blank control group. However, the addition of Dickkopf-1 (DKK1) significantly reduced BMSC proliferation and osteogenic differentiation compared to the H-MOP group. Conclusions: MOP can enhance BMSC proliferation and osteogenic differentiation by activating the Wnt/β-catenin signaling pathway.
Keywords: Morinda officinalis polysaccharide, bone marrow mesenchymal stem cells, Wnt/β-catenin pathway, osteogenic differentiation
Introduction
Morinda officinalis polysaccharide (MOP) is a natural Chinese medicinal compound found predominantly in Morinda officinalis. It comprises various chemical constituents, such as flavonoids, polysaccharides, amino acids, and trace elements, and exhibits a range of efficacies including immune regulation, antioxidation, anti-fatigue, and osteoporosis prevention [1-7]. With the growing application of modern Chinese medicine, more studies have reported on the treatment of osteoporosis using these approaches [8-13]. The advantages of MOP have become prominent in treating metabolic bone disorders, especially osteoporosis [14-16].
Bone marrow mesenchymal stem cells (BMSCs), serving as seed cells in bone tissue engineering, are widely used in treating bone defects and osteoporosis [17,18]. The diminished osteogenic differentiation ability of BMSCs is a primary pathogenic mechanism in osteoporosis [19,20]. Therefore, enhancing the osteogenic differentiation of BMSCs to maintain bone metabolic balance is a promising strategy for preventing and treating osteoporosis.
The Wnt/β-catenin signaling pathway plays a significant role in bone metabolism, particularly in regulating the self-renewal and directed differentiation of BMSCs, as well as promoting preosteoblast proliferation and osteoblast formation [21-25]. Activation of the Wnt signaling pathway can expedite the aggregation of β-catenin, which in turn drives the osteogenic differentiation and bone formation of BMSCs [26]. A decline in the osteogenic differentiation ability of BMSCs can lead to a deficiency of osteoblasts within bone tissue, reduced total bone mass, and bone density, ultimately resulting in osteoporosis [27,28].
Published studies have demonstrated that MOP accelerates the proliferation of osteoblasts in vitro, enhances ALP activity, and reduces the level of Dickkopf-1 (DKK1) protein [7]. The alleviation of bone degradation is linked to the activation of the Wnt/β-catenin signaling pathway [29]. Elevating the content of proteins related to this pathway facilitates BMSCs in differentiating into osteoblasts and fortifies mineralized nodules. The Wnt/β-catenin signaling route is established as a crucial pathway for osteogenic differentiation in BMSCs [29,30].
In this study, BMSCs were treated with MOP to investigate its effects on their proliferation and differentiation into osteoblasts, and to explore the relevance of this mechanism with the Wnt/β-catenin signaling pathway. The findings provide a theoretical foundation for the application of MOP in diagnosing and treating bone diseases.
Materials and methods
Extraction and culture of BMSC cells
BMSCs were collected from the femurs of 4-week-old female Sprague-Dawley rats purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. The rats were anesthetized with a 10% Avertin intraperitoneal injection and then sacrificed by cervical dislocation. The following steps were performed: 1) The rats were immersed in 75% ethanol for 10 minutes. 2) The femurs were isolated and rinsed until the bone marrow was flushed out. 3) The bone marrow cells were collected and centrifuged. 4) The supernatant was discarded, and the remaining cells were suspended in alpha-modified Eagle’s medium (α-MEM, SH30265.01, Hyclone), containing 10% fetal bovine serum (SH30070.02, Hyclone, South Logan, UT, USA) and 100 U/mL penicillin and streptomycin. 5) The cells were inoculated into culture flasks. 6) The flasks were placed in a constant incubator (37°C, 5% CO2, saturated humidity) for 48 hours. The cells’ adhesion to the flask wall was observed, and the medium was renewed every three days until cell confluency exceeded 80%. 7) The cells were treated with trypsin for digestion and then continuously cultured. Subsequent assays were conducted after the cells were cultured to the third generation.
Processing and grouping of cells
Third-generation BMSCs in the logarithmic phase were implanted in a 12-well plate, then administered with DMEM (RASMX-90021) and cultured under conditions of 37°C and 5% CO2 for osteogenic differentiation. The cell groups included: Blank control group: BMSCs cultivated in DMEM only. L-MOP group: BMSCs cultivated in DMEM and treated with low-dose MOP (10 μg/mL, HPLC>90%, wkq-08910, Weikeqi-biotech, Chengdu, Sichuan, China) for 48 hours. H-MOP group: BMSCs cultivated in DMEM and treated with high-dose MOP (40 μg/mL) for 48 hours. H-MOP+DKK1 group: BMSCs cultivated in DMEM and treated with high-dose MOP (40 μg/mL) and DKK1 (100 ng/mL) for 48 hours. The following assays were performed on the BMSCs prepared and grouped in this procedure.
Identification of BMSC surface antigens
After digestion with trypsin, the BMSCs were centrifuged at 1000 rpm. The supernatant was removed, and PBS was added to re-suspend the BMSCs to a concentration of 1×104 cells/µL. Then, 200 µL of the cell suspension was transferred into an Eppendorf tube. The following antibodies were added respectively and mixed uniformly: CD34 (ab81289, 1:50), CD90 (ab307736, 1:500), CD73 (ab202122, 1:200), CD105 (ab231774, 1:500), and CD45 (ab40763, 1:20), along with identical types of control antibodies. After 30 minutes of incubation, the supernatant was discarded. A flow cytometer (BD Biosciences, San Jose, CA, USA) was used to examine the antigens on the surface of BMSCs. The antibodies were provided by Abcam, Waltham, MA, USA.
MTT-based cell activity detection
This assay aimed to observe the effect of different doses of MOP on BMSC activity. Each group had three replicates. Log-phase BMSCs were transferred into a 96-well plate and incubated until the cells adhered to the well walls. Following treatment with MOP, an MTT solution prepared from the MTT reagent (ST316) was added, and the cells were incubated further. After incubation, the medium was removed, and dimethyl sulfoxide (ST1276) was added to each well. A microplate reader (wavelength: 490 nm) was used to measure the absorbance of the cells, allowing the assessment of BMSC proliferative viability. The MTT reagent and dimethyl sulfoxide were provided by Beyotime Biotechnology, Shanghai, China.
Cell clone formation assay
Third-generation BMSCs in the logarithmic phase were implanted in 60 mm culture dishes. After adhering to the walls, the cells were treated with different doses of MOP (L-MOP and H-MOP) and cultured for two weeks. Subsequently, the colonies were fixed with 100% methanol for 10 minutes and then stained at room temperature for 30 minutes using 0.5% crystal violet (C0121; Beyotime Biotechnology, Shanghai, China). The state of cell clone formation was photographed, and the clone formation rate was calculated using the following formula, considering a cell mass with more than 50 cells as one clone:
Alizarin red staining
First, third-generation BMSCs from different groups were seeded at a concentration of 1×104 cells/µL into a 12-well culture plate and incubated (5% CO2, 37°C) for 24 hours. Then, an osteogenic induction medium was added to each well. The osteogenic induction medium consisted of DMEM supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 1% glutamine, 0.2% ascorbic acid, 1% sodium β-glycerophosphate, and 0.01% dexamethasone. The medium was replaced every three days for 28 days.
After the induction period, the cells were washed with PBS and fixed with 95% ethanol for 30 minutes. The ethanol was then removed, and the cells were rinsed again with PBS before being stained with 1 mL of alizarin red solution (ARS, G1452; Solarbio, Beijing, China) for 30 minutes. After staining, the ARS was discarded, and the cells were washed with PBS. The calcified nodules in the BMSCs were observed under a microscope. A Calcium Assay Kit (S1063S; Beyotime, Shanghai, China) was used for a quantitative analysis of the calcium content.
ALP staining
Osteogenic induction in this assay followed the method described in Section 2.7. Subsequently, the effect of different doses of MOP on the ALP activity of BMSCs was examined using an ALP staining kit (P0321S; Beyotime Biotechnology). The ALP staining status was observed under a microscope.
Western blot
Third-generation BMSCs were randomly divided into two groups: an osteogenic induction group, where BMSCs received treatment with different doses of MOP followed by osteogenic induction, and a non-osteogenic induction group, where BMSCs were treated only with different doses of MOP. These groups were prepared and processed as follows:
RIPA lysis buffer was used to extract total proteins from each group of cells and tissues.
Protein concentrations were quantified using BCA protein assay kits (R21250).
Proteins were denatured, separated by SDS-PAGE electrophoresis, and then transferred to a PVDF membrane.
The membrane was blocked at room temperature for 2 hours in a TBST solution prepared with 5% non-fat milk.
For the osteogenic induction group, the following primary antibodies were used: rabbit anti-human OCN (osteocalcin, ab133612; 1:1000), RUNX2 (Runt-related transcription factor 2, ab92336; 1:5000), Col I (Collagen I, ab34710; 1:1000), SP7 (Osterix, ab209484; 1:1000), and GAPDH (ab9485; 1:10000). These were incubated at 4°C overnight.
For the non-osteogenic induction group, the following primary antibodies were used: rabbit anti-human β-catenin (ab32572; 1:5000), p-GSK3β (ab75814; 1:10000), GSK3β (ab32391; 1:5000), and GAPDH (ab9485; 1:10000). These were also incubated overnight at 4°C.
Both groups were washed with TBST solution three times and then incubated with goat anti-rabbit IgG secondary antibody (1:5000) for 2 hours at room temperature. Finally, DAB chromogen (DA1016) was added for protein visualization, and a gel imaging system (5200) was used to record protein grayscale and capture images. Relative protein expressions were quantitatively analyzed, using GAPDH as the control.
In this assay, BCA protein assay kits were procured from Yuanye, Shanghai, China; all primary and secondary antibodies were bought from Abcam, Waltham, MA, USA; the DAB chromogen was produced by Solarbio, Beijing, China; and the gel imaging system was manufactured by Tanon, Shanghai, China.
Statistical analysis
Data were analyzed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA), and graphical representations were created with GraphPad Prism 9.0 (GraphPad Inc., La Jolla, CA, USA). Quantitative data were expressed as mean ± standard error ( ± SE). Differences among groups were compared using the t-test or ANOVA, followed by Tukey tests. A P-value below 0.05 was considered statistically significant.
Results
Identification of BMSCs
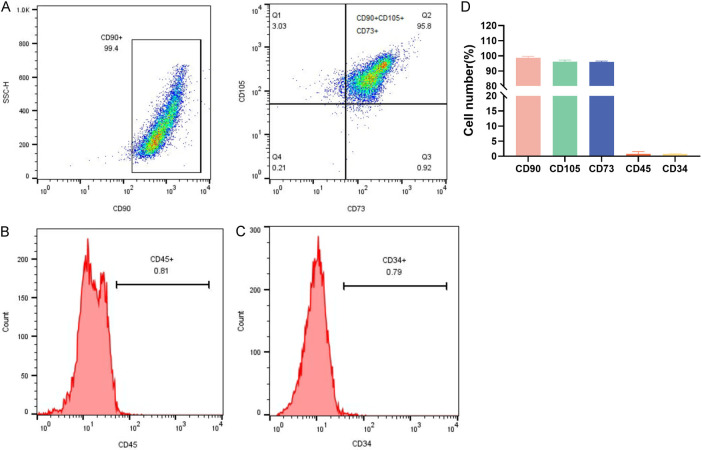
Flow cytometry analysis showed that the characteristic antigens CD90, CD105, and CD73 were positively expressed on the surface of BMSCs, while CD45 and CD34, characteristic of hematopoietic stem cells, were negatively expressed (Figure 1A-C). Quantitative analysis indicated that the expression levels of these BMSC-specific antigens were above 90%, whereas the expression levels of hematopoietic stem cell markers were below 5% (Figure 1D). This confirms that the isolated third-generation BMSCs were of high purity, suitable for experimental research.
Figure 1.
The separation, cultivation, and identification of Bone Marrow Mesenchymal Stem Cells (BMSCs). A: Positive antigens CD90, CD105 and CD73. B, C: Negative antigens CD34 and CD45. D: The proportions of BMSCs expressing different antigens.
MOP’s effect on BMSCs’ proliferation
BMSCs treated with varying doses of MOP (0, 5, 10, 20, 40, and 80 µg/mL) exhibited a dose-dependent increase in proliferative activity. Compared to the control group (0 µg/mL MOP), BMSCs showed significantly enhanced proliferation at 10 µg/mL, peaking at 40 µg/mL, and then declining at 80 µg/mL (Figure 2A). Based on these findings, 10 µg/mL and 40 µg/mL were selected as the doses for the L-MOP and H-MOP groups, respectively. Both L-MOP and H-MOP groups demonstrated significant increases in BMSC clone formation rates compared to the control group, with H-MOP being more conducive to cell clone formation (Figure 2B). These results indicate that within a certain range, the dose of MOP is directly proportional to BMSC proliferation.
Figure 2.
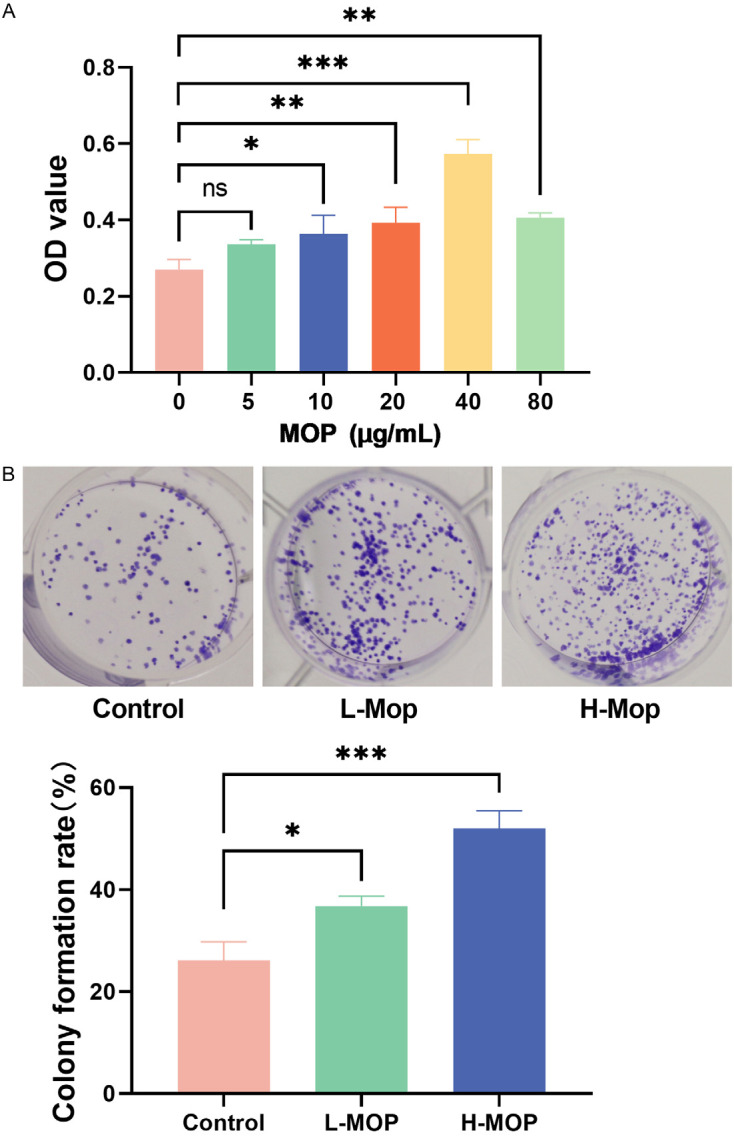
Bone marrow mesenchymal stem cells (BMSCs)’ proliferation with the interference of different doses of Morinda officinalis polysaccharide (MOP) (*P<0.05; **P<0.01; ***P<0.001). A: Screening of MOP doses. B: BMSC clone formation assay.
MOP’s effect on BMSCs’ osteogenic differentiation
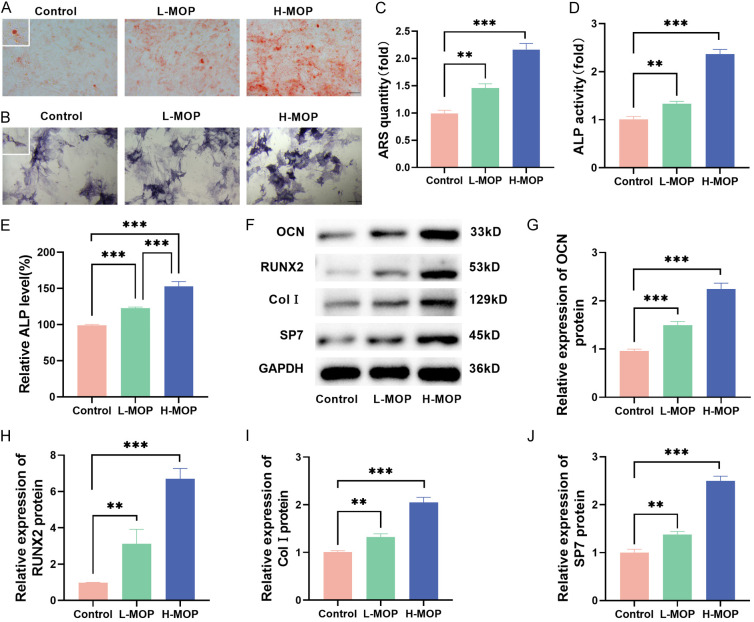
ARS staining and subsequent quantitative analysis revealed a significant increase in calcium deposition in BMSCs with increasing MOP doses (Figure 3A and 3C). ALP activity shows the same experimental results, higher ALP level in the H-MOP group compared to the L-MOP group (Figure 3B, 3D and 3E). The expression of osteogenic differentiation-related genes (OCN, RUNX2, Col I, and SP7) was also up-regulated following MOP treatment (Figure 3F-J). Overall, these findings demonstrate that MOP facilitates the osteogenic differentiation of BMSCs.
Figure 3.
Bone marrow mesenchymal stem cells (BMSCs)’ osteogenic differentiation with the interference of different doses of Morinda officinalis polysaccharide (MOP) (**P<0.01; ***P<0.001). A and C: ARS staining results. B and D: ALP staining results. E: Detected ALP activity. F-J: The expressed levels of osteogenic differentiation-related genes (including OCN, RUNX2, Col I, and SP7) detected with the Western blot method.
MOP’s effect on the Wnt/β-catenin signaling pathway in BMSCs
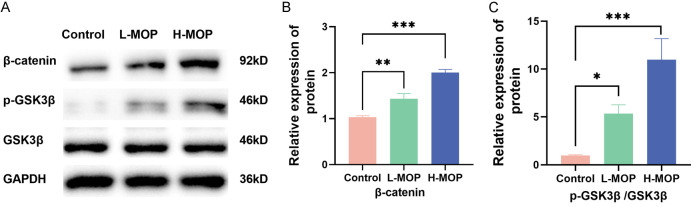
The Wnt/β-catenin signaling pathway, crucial for osteogenic differentiation, showed a significant increase in β-catenin and phosphorylated GSK3β (p-GSK3β/GSK3β) protein levels following MOP treatment in BMSCs (Figure 4A-C). This suggests that MOP activates the Wnt/β-catenin signaling pathway in BMSCs.
Figure 4.
Morinda officinalis polysaccharide (MOP)’s influences on the Wnt/β-catenin signaling pathway (*P<0.05; **P<0.01; ***P<0.001). A: Electrophoretic strips of the wnt/β-catenin signaling pathway-related proteins. B, C: Quantitative analysis of the β-catenin protein and p-GSK3β/GSK3β.
MOP’s action in boosting BMSCs’ proliferation by triggering the Wnt/β-catenin signaling pathway
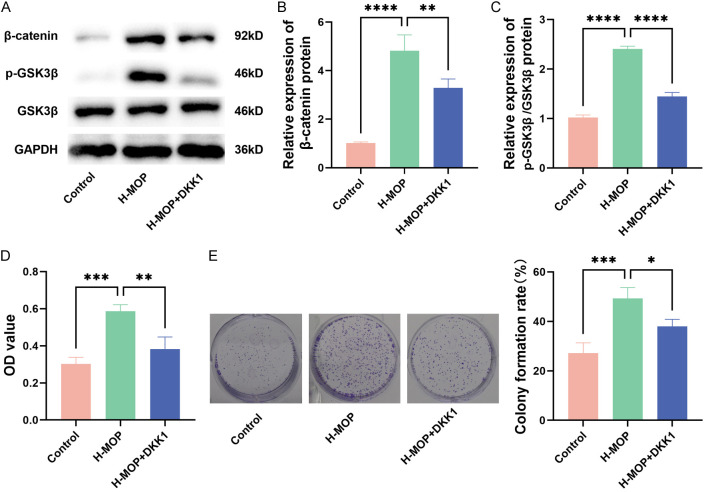
The H-MOP group was selected to elucidate MOP’s mechanism in enhancing the proliferation of BMSCs. As shown in Figure 5A-C, the protein levels of β-catenin and p-GSK3β/GSK3β in the H-MOP group were significantly higher compared to the control group, indicating activation of the Wnt/β-catenin pathway. However, after the H-MOP group received DKK1 (a Wnt/β-catenin pathway inhibitor), the levels of these proteins decreased (Figure 5A-C). MTT assay results demonstrated that the addition of DKK1 mitigated H-MOP’s enhancement of BMSC proliferation (Figure 5D) and reduced BMSC clone formation (Figure 5E), thereby suppressing BMSC proliferation. Overall, H-MOP’s positive effect on BMSC proliferation was inhibited when the Wnt/β-catenin pathway was blocked. This suggests that MOP promotes BMSC proliferation by activating the Wnt/β-catenin signaling pathway.
Figure 5.
Morinda officinalis polysaccharide (MOP)’s action in boosting bone marrow mesenchymal stem cells (BMSCs)’ proliferation by triggering the Wnt/β-catenin signaling pathway (*P<0.05; **P<0.01; ***P<0.001; ****P<0.0001). A-C: Protein strips in the Wnt/β-catenin signaling pathway and related quantitative analysis results. D: The proliferation of BMSCs assessed with the MTT method. E: BMSC clone formation assay.
MOP’s action in strengthening BMSCs’ osteogenic differentiation by activating the Wnt/β-catenin signaling pathway
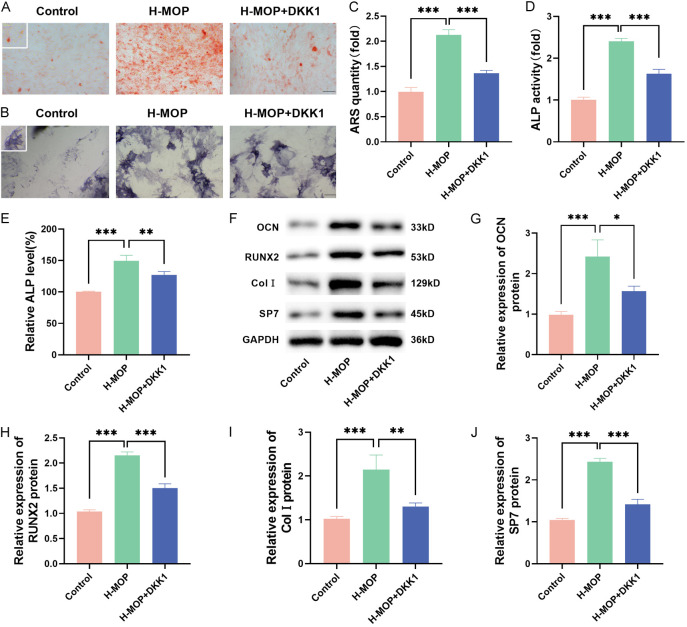
To further explore the regulatory mechanism of MOP on the osteogenic differentiation of BMSCs, the H-MOP group and H-MOP+DKK1 group were selected for further study. ARS and ALP staining results indicated that the addition of DKK1 significantly reduced calcium deposition and ALP activity in BMSCs (Figure 6A-D), showing lower ALP levels (Figure 6E). The protein levels of osteogenesis-related markers (OCN, RUNX2, Col I, and SP7) also decreased following the addition of DKK1 (Figure 6F-J). This indicates that inhibition of the Wnt/β-catenin pathway diminished the enhancing effect of MOP on the osteogenic differentiation of BMSCs, confirming that MOP facilitates osteogenic differentiation by activating the Wnt/β-catenin signaling pathway.
Figure 6.
Morinda officinalis polysaccharide (MOP)’s action in strengthening bone marrow mesenchymal stem cells (BMSCs)’ osteogenic differentiation by activating the Wnt/β-catenin signaling pathway (*P<0.05; **P<0.01; ***P<0.001). A and C: ARS staining results. B and D: ALP staining results. E: Detected ALP activity. F-J: The expressed levels of osteogenic differentiation-related genes (including OCN, RUNX2, Col I, and SP7) detected with the western blot method. ARS, Alizarin red staining; ALP, alkaline phosphatase.
Discussion
The specific functions of a cell are closely related to its surface markers, reflecting some fundamental characteristics of the cell [31]. Mesenchymal stem cells belong to a heterogeneous cell population; their surface antigens are not specific but can express markers of endothelial cells, mesenchymal cells, and epithelial cells [32]. In this work, CD90, CD105, and CD73 were selected as positive markers and CD45 and CD34 as negative markers. The results showed that more than 90% of CD90, CD105, and CD73 were positively identified, while less than 5% of CD45 and CD34 were negatively identified, meeting the criteria for human mesenchymal stem cells [33,34]. This indicates that the BMSCs were successfully isolated and are suitable for subsequent experimental research. As the time of passaging culture increases, the cells show a certain degree of aging, so the generation of BMSCs we chose to study was the third-generation.
In the human body, bone resorption and bone formation are controlled by osteoblasts. Osteoblasts undergo stages including proliferation, differentiation, and mineralization during bone formation [35]. The optical density (OD) values and colony formation rates are directly proportional to the cells’ ability to proliferate and form bones. MTT assays revealed that MOP significantly enhanced the proliferative activity of BMSCs in a dose-dependent manner within the range of 0-40 µg/mL. However, at concentrations above 40 µg/mL, OD values declined, identifying 40 µg/mL as the optimal dose for BMSC treatment.
In alignment with in vivo osteogenic differentiation, in vitro determination primarily involves assessing osteoblastic biological and genetic markers. Short-term differentiation is evaluated using ALP activity, while long-term differentiation is confirmed by ARS calcium nodules. After osteogenic differentiation, cells exhibit prominent osteoblastic characteristic genes such as OCN, Col I, RUNX2, and SP7 [36]. Col I, an extracellular matrix protein, stimulates osteoblast adhesion and differentiation, serving as a prerequisite for bone tissue formation and an early marker of osteoblastic differentiation [37]. OCN, a non-collagen protein synthesized and secreted by mature osteoblasts, appears in the final stages of osteoblast differentiation and intervenes in calcium ion homeostasis and bone mineralization through its ability to bind with Ca2+ [38,39]; RUNX2 is a central regulatory gene for the osteoblastic phenotype, enhancing the secretion of the osteoblastic extracellular matrix by binding to osteoblast-specific cis-acting elements in target gene promoters [40]. SP7, a zinc finger transcription factor unique to osteoblasts and downstream of RUNX2, is vital for osteoblast proliferation, differentiation, and bone development, regulating several osteoblast-specific differentiation markers including RUNX2 and Osteonectin (ON) [41,42]. Studies have shown that MOP treatment significantly enhances calcium deposition and ALP activity in BMSCs, with a marked increase in osteoblastic characteristic genes (OCN, RUNX2, Col I, and SP7), confirming MOP’s efficacy in facilitating osteogenic differentiation.
The Wnt/β-catenin signaling cascade is involved in the development of multiple organs and tissues [43,44]. β-catenin is essential in the early stages of osteoblast development, aiding in their maturation [45]. GSK3β, a negative regulator in the classic Wnt pathway, forms a multi-protein complex with its substrates, phosphorylating β-catenin when the Wnt pathway is inactive [46]. Upon Wnt pathway activation, Dishevelled (Dvl) phosphorylates a cysteine in GSK3β, coinciding with β-catenin dephosphorylation [47]. By influencing the Wnt signaling cascade, GSK3β and β-catenin are implicated in bone development and remodeling [48]. MOP administration increased both β-catenin and p-GSK3β/GSK3β levels in BMSCs, suggesting Wnt/β-catenin pathway activation as a consequence of MOP intervention. This may explain how MOP enhances the osteogenic differentiation of BMSCs.
Belonging to the DKK family, DKK1 functions as a secretory glycoprotein. When it binds to its respective receptor, Wnt signal transduction is suppressed. This binding regulates cell growth, differentiation, and movement, and potentially hinder bone development, leading to osteoporosis [49,50]. To elucidate MOP’s mechanism of action in BMSCs, the Wnt/β-catenin pathway was inhibited using DKK1. The addition of DKK1 resulted in diminished BMSC proliferation, as demonstrated by MTT and cell clone formation assays. Additionally, DKK1 significantly reduced calcium deposition, ALP activity, and the levels of genes associated with osteogenic differentiation in BMSCs. These experimental findings substantiate that MOP enhances BMSC proliferation and osteogenic differentiation by activating the Wnt/β-catenin signaling pathway.
This study lacks in vivo experiments, and the specific molecular mechanisms remain to be further validated. Therefore, future research should explore the osteogenic effect of MOP in animal models. Additionally, MOP should be applied in combination with other osteogenic drugs and biological scaffolds to investigate its potential synergistic effects on osteogenesis, aiming to develop new drugs or therapeutic strategies for treating osteoporosis.
In conclusion, by investigating the impact of varying MOP concentrations on BMSC proliferation and osteogenic differentiation, this study revealed that the Wnt/β-catenin signaling pathway is instrumental in MOP’s promotion of BMSC proliferation and osteogenic differentiation. MOP is an effective herbal formulation for stimulating osteogenic differentiation of BMSCs, which may aid in the therapeutic treatment of osteoporosis. This study may give data support for the use of Chinese herbs to treat osteoporosis, as well as a reference for future clinical trial design.
Acknowledgements
This work was supported by the Research Project of Guangdong Provincial Bureau of Traditional Chinese Medicine (20211279), Guangdong Medical Science and Technology Research Fund Project (B2021066), Guangdong Medical Science and Technology Research Fund Project (A2020612) and Campus Level Project of Guangdong Food and Drug Vocational College (2018ZR010).
Disclosure of conflict of interest
None.
References
- 1.Jiang Y, Chen L, Zeng J, Wang Y, Chen Y, Chen S, Xu J, He X. Anti-inflammatory monoterpenes from morinda (Morinda officinalis How.) Phytochemistry. 2024;220:114034. doi: 10.1016/j.phytochem.2024.114034. [DOI] [PubMed] [Google Scholar]
- 2.Zhang D, Zhang S, Jiang K, Li T, Yan C. Bioassay-guided isolation and evaluation of anti-osteoporotic polysaccharides from Morinda officinalis. J Ethnopharmacol. 2020;261:113113. doi: 10.1016/j.jep.2020.113113. [DOI] [PubMed] [Google Scholar]
- 3.Cha X, Han S, Yu J, Zhang S, Yu S, Fu D, Yao M, Zhang L, Feng G. Inulin with a low degree of polymerization protects human umbilical vein endothelial cells from hypoxia/reoxygenation-induced injury. Carbohydr Polym. 2019;216:97–106. doi: 10.1016/j.carbpol.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Wu P, Jiao F, Huang H, Liu D, Tang W, Liang J, Chen W. Morinda officinalis polysaccharide enable suppression of osteoclastic differentiation by exosomes derived from rat mesenchymal stem cells. Pharm Biol. 2022;60:1303–1316. doi: 10.1080/13880209.2022.2093385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu PY, Chen W, Huang H, Tang W, Liang J. Morinda officinalis polysaccharide regulates rat bone mesenchymal stem cell osteogenic-adipogenic differentiation in osteoporosis by upregulating miR-21 and activating the PI3K/AKT pathway. Kaohsiung J Med Sci. 2022;38:675–685. doi: 10.1002/kjm2.12544. [DOI] [PubMed] [Google Scholar]
- 6.Huang SC, Cao QQ, Cao YB, Yang YR, Xu TT, Yue K, Liu F, Tong ZX, Wang XB. Morinda officinalis polysaccharides improve meat quality by reducing oxidative damage in chickens suffering from tibial dyschondroplasia. Food Chem. 2021;344:128688. doi: 10.1016/j.foodchem.2020.128688. [DOI] [PubMed] [Google Scholar]
- 7.Liu M, Wang C, Zhang H, Guo H, Kang L, Li H, Li K. A systematic review on polysaccharides from Morinda officinalis How: advances in the preparation, structural characterization and pharmacological activities. J Ethnopharmacol. 2024;328:118090. doi: 10.1016/j.jep.2024.118090. [DOI] [PubMed] [Google Scholar]
- 8.Lei SS, Su J, Zhang Y, Huang XW, Wang XP, Huang MC, Li B, Shou D. Benefits and mechanisms of polysaccharides from Chinese medicinal herbs for anti-osteoporosis therapy: a review. Int J Biol Macromol. 2021;193:1996–2005. doi: 10.1016/j.ijbiomac.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Li D, Chen R, Gao S, Xu Z, Li N. Cell death regulation: a new way for natural products to treat osteoporosis. Pharmacol Res. 2023;187:106635. doi: 10.1016/j.phrs.2022.106635. [DOI] [PubMed] [Google Scholar]
- 10.Jia Y, Sun J, Zhao Y, Tang K, Zhu R, Zhao W, Wang R, Zhang Y, Lin N, Chen W. Chinese patent medicine for osteoporosis: a systematic review and meta-analysis. Bioengineered. 2022;13:5581–5597. doi: 10.1080/21655979.2022.2038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Fu SF, Yang Y, An R, Liu HY, Mao HP. Clinical practice of traditional Chinese medicine for the treatment of postmenopausal osteoporosis: a literature review. Climacteric. 2022;25:562–569. doi: 10.1080/13697137.2022.2102894. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Zeng L, Wu M, Huang H, Liang G, Yang W, Pan J, Liu J. Efficacy of Chinese patent medicine for primary osteoporosis: a network meta-analysis. Complement Ther Clin Pract. 2021;44:101419. doi: 10.1016/j.ctcp.2021.101419. [DOI] [PubMed] [Google Scholar]
- 13.Zhuo Y, Li M, Jiang Q, Ke H, Liang Q, Zeng LF, Fang J. Evolving roles of natural terpenoids from traditional Chinese medicine in the treatment of osteoporosis. Front Endocrinol (Lausanne) 2022;13:901545. doi: 10.3389/fendo.2022.901545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Z, Nian M, Lv H, Yue J, Qiao H, Yang X, Zheng X. Advances in anti-osteoporosis polysaccharides derived from medicinal herbs and other edible substances. Am J Chin Med. 2022;50:441–470. doi: 10.1142/S0192415X22500173. [DOI] [PubMed] [Google Scholar]
- 15.Yan C, Huang D, Shen X, Qin N, Jiang K, Zhang D, Zhang Q. Identification and characterization of a polysaccharide from the roots of Morinda officinalis, as an inducer of bone formation by up-regulation of target gene expression. Int J Biol Macromol. 2019;133:446–456. doi: 10.1016/j.ijbiomac.2019.04.084. [DOI] [PubMed] [Google Scholar]
- 16.Rong K, Chen P, Lang Y, Zhang Y, Wang Z, Wen F, Lu L. Morinda officinalis polysaccharide attenuates osteoporosis in rats underwent bilateral ovariectomy by suppressing the PGC-1α/PPARγ pathway. J Orthop Surg (Hong Kong) 2022;30:10225536221130824. doi: 10.1177/10225536221130824. [DOI] [PubMed] [Google Scholar]
- 17.Gholami Farashah MS, Mohammadi A, Javadi M, Soleimani Rad J, Shakouri SK, Meshgi S, Roshangar L. Bone marrow mesenchymal stem cells’ osteogenic potential: superiority or non-superiority to other sources of mesenchymal stem cells? Cell Tissue Bank. 2023;24:663–681. doi: 10.1007/s10561-022-10066-w. [DOI] [PubMed] [Google Scholar]
- 18.Ning K, Liu S, Yang B, Wang R, Man G, Wang DE, Xu H. Update on the effects of energy metabolism in bone marrow mesenchymal stem cells differentiation. Mol Metab. 2022;58:101450. doi: 10.1016/j.molmet.2022.101450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torrecillas-Baena B, Pulido-Escribano V, Dorado G, Gálvez-Moreno MÁ, Camacho-Cardenosa M, Casado-Díaz A. Clinical potential of mesenchymal stem cell-derived exosomes in bone regeneration. J Clin Med. 2023;12:4385. doi: 10.3390/jcm12134385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao X, Cui P, Hu G, Wang C, Jiang L, Zhao J, Xu J, Zhang X. PIP5k1β controls bone homeostasis through modulating both osteoclast and osteoblast differentiation. J Mol Cell Biol. 2020;12:55–70. doi: 10.1093/jmcb/mjz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Zhao Y, Xie Z, Li M, Liu Y, Tu X. Activating Wnt/β-catenin signaling in osteocytes promotes osteogenic differentiation of BMSCs through BMP-7. Int J Mol Sci. 2022;23:16045. doi: 10.3390/ijms232416045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen J, Sun Y, Liu X, Zhu Y, Bao B, Gao T, Chai Y, Xu J, Zheng X. EGFL6 regulates angiogenesis and osteogenesis in distraction osteogenesis via Wnt/β-catenin signaling. Stem Cell Res Ther. 2021;12:415. doi: 10.1186/s13287-021-02487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen G, Ren H, Shang Q, Zhao W, Zhang Z, Yu X, Tang K, Tang J, Yang Z, Liang D, Jiang X. Foxf1 knockdown promotes BMSC osteogenesis in part by activating the Wnt/β-catenin signalling pathway and prevents ovariectomy-induced bone loss. EBioMedicine. 2020;52:102626. doi: 10.1016/j.ebiom.2020.102626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Liang N, He B, Wu S, Wen D, Tang X, Shen X. SDF-1 induces directional chemotaxis of BMSCs at the intervertebral fusion site and promotes osteogenic differentiation by regulating Wnt/β-catenin in the bone marrow chimera spinal intervertebral fusion mouse model. Turk J Biol. 2022;47:14–28. doi: 10.55730/1300-0152.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu C, Shi H, Jiang X, Fan Y, Huang D, Qi X, Cheng Q. ZEB1 mediates bone marrow mesenchymal stem cell osteogenic differentiation partly via Wnt/β-catenin signaling. Front Mol Biosci. 2021;8:682728. doi: 10.3389/fmolb.2021.682728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teufel S, Hartmann C. Wnt-signaling in skeletal development. Curr Top Dev Biol. 2019;133:235–279. doi: 10.1016/bs.ctdb.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Johnston CB, Dagar M. Osteoporosis in older adults. Med Clin North Am. 2020;104:873–884. doi: 10.1016/j.mcna.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S. RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. Biomed Res Int. 2020;2020:6910312. doi: 10.1155/2020/6910312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JH, Xin HL, Xu YM, Shen Y, He YQ, Hsien-Yeh, Lin B, Song HT, Juan-Liu, Yang HY, Qin LP, Zhang QY, Du J. Morinda officinalis How. - A comprehensive review of traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2018;213:230–255. doi: 10.1016/j.jep.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Tu P, Zeng K, Jiang Y. Total glycosides and polysaccharides of Cistanche deserticola prevent osteoporosis by activating Wnt/β-catenin signaling pathway in SAMP6 mice. J Ethnopharmacol. 2021;271:113899. doi: 10.1016/j.jep.2021.113899. [DOI] [PubMed] [Google Scholar]
- 31.Tang CY, Wu M, Zhao D, Edwards D, McVicar A, Luo Y, Zhu G, Wang Y, Zhou HD, Chen W, Li YP. Runx1 is a central regulator of osteogenesis for bone homeostasis by orchestrating BMP and WNT signaling pathways. PLoS Genet. 2021;17:e1009233. doi: 10.1371/journal.pgen.1009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright A, Arthaud-Day ML, Weiss ML. Therapeutic use of mesenchymal stromal cells: the need for inclusive characterization guidelines to accommodate all tissue sources and species. Front Cell Dev Biol. 2021;9:632717. doi: 10.3389/fcell.2021.632717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, Nguyen GH, Le PTT, Hoang VT, Forsyth NR, Heke M, Nguyen LT. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7:272. doi: 10.1038/s41392-022-01134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salhotra A, Shah HN, Levi B, Longaker MT. Mechanisms of bone development and repair. Nat Rev Mol Cell Biol. 2020;21:696–711. doi: 10.1038/s41580-020-00279-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.An J, Yang H, Zhang Q, Liu C, Zhao J, Zhang L, Chen B. Natural products for treatment of osteoporosis: the effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016;147:46–58. doi: 10.1016/j.lfs.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 37.Botor M, Fus-Kujawa A, Uroczynska M, Stepien KL, Galicka A, Gawron K, Sieron AL. Osteogenesis imperfecta: current and prospective therapies. Biomolecules. 2021;11:1493. doi: 10.3390/biom11101493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schatz M, Saravanan S, d’Adesky ND, Bramlett H, Perez-Pinzon MA, Raval AP. Osteocalcin, ovarian senescence, and brain health. Front Neuroendocrinol. 2020;59:100861. doi: 10.1016/j.yfrne.2020.100861. [DOI] [PubMed] [Google Scholar]
- 39.Karsenty G. Osteocalcin: a multifaceted bone-derived hormone. Annu Rev Nutr. 2023;43:55–71. doi: 10.1146/annurev-nutr-061121-091348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komori T. Whole aspect of Runx2 functions in skeletal development. Int J Mol Sci. 2022;23:5776. doi: 10.3390/ijms23105776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hojo H, Ohba S. Sp7 action in the skeleton: its mode of action, functions, and relevance to skeletal diseases. Int J Mol Sci. 2022;23:5647. doi: 10.3390/ijms23105647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JS, Tokavanich N, Wein MN. SP7: from bone development to skeletal disease. Curr Osteoporos Rep. 2023;21:241–252. doi: 10.1007/s11914-023-00778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda K, Kobayashi Y, Koide M, Uehara S, Okamoto M, Ishihara A, Kayama T, Saito M, Marumo K. The regulation of bone metabolism and disorders by Wnt signaling. Int J Mol Sci. 2019;20:5525. doi: 10.3390/ijms20225525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rim EY, Clevers H, Nusse R. The Wnt pathway: from signaling mechanisms to synthetic modulators. Annu Rev Biochem. 2022;91:571–598. doi: 10.1146/annurev-biochem-040320-103615. [DOI] [PubMed] [Google Scholar]
- 45.Vlashi R, Zhang X, Wu M, Chen G. Wnt signaling: essential roles in osteoblast differentiation, bone metabolism and therapeutic implications for bone and skeletal disorders. Genes Dis. 2022;10:1291–1317. doi: 10.1016/j.gendis.2022.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang HL, Hart J, Fan L, Mustafi R, Bissonnette M. Upregulation of glycogen synthase kinase 3β in human colorectal adenocarcinomas correlates with accumulation of CTNNB1. Clin Colorectal Cancer. 2011;10:30–36. doi: 10.3816/CCC.2011.n.004. [DOI] [PubMed] [Google Scholar]
- 47.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Law SM, Zheng JJ. Premise and peril of Wnt signaling activation through GSK-3β inhibition. iScience. 2022;25:104159. doi: 10.1016/j.isci.2022.104159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baetta R, Banfi C. Dkk (Dickkopf) proteins. Arterioscler Thromb Vasc Biol. 2019;39:1330–1342. doi: 10.1161/ATVBAHA.119.312612. [DOI] [PubMed] [Google Scholar]
- 50.Jiang H, Zhang Z, Yu Y, Chu HY, Yu S, Yao S, Zhang G, Zhang BT. Drug discovery of DKK1 inhibitors. Front Pharmacol. 2022;13:847387. doi: 10.3389/fphar.2022.847387. [DOI] [PMC free article] [PubMed] [Google Scholar]