Abstract
Objective: To explore the effectiveness of combining an artificial intelligence (AI) film reading system with a cervical liquid-based ThinPrep cytology test (TCT) in cervical cancer screening. Methods: A total of 1200 adult women who underwent cervical cancer screening in the Gynecology Department of The Fifth People’s Hospital of Jinan from July 2022 to June 2023 were included in the study. All participants underwent TCT followed by both manual and AI examination. The AI examination was performed using an AI film reading system that employed advanced machine learning algorithms and image processing techniques to analyze digital TCT slides. Pathological tissue biopsy was performed on all cases with abnormalities, and the results were used as the gold standard to analyze the effectiveness of the different screening methods. Results: TCT screening results revealed that the average time for manual film reading was shorter than that for the AI film reading system (P<0.001). The AI film reading system significantly detected more lesions than the manual film reading method (P<0.001). The overall compliance rate between AI imaging and manual imaging interpretation was 79.75%, with a corresponding Kappa value of 0.588, indicating moderate agreement between the two methods. The accuracy of the AI screening system for low-grade lesions and inflammation was 87.47%, compared to 79.41% for manual screening (P=0.018). For high-grade cancer lesions, the accuracy rates were 82.54% for AI and 75.90% for manual screening (P=0.241). The AI screening system had a sensitivity of 67.53% (104/154) for detecting high-grade lesions and cancers, higher than the 40.91% (63/154) sensitivity of manual screening. However, the specificity of the AI screening system was 94.07% (349/371), while manual screening had a specificity of 94.61% (351/371). The Youden index for AI screening system was 0.616, significantly higher than the 0.355 for manual screening. Conclusion: In TCT screening, the AI screening system outperforms manual screening. The combination of the AI film reading system and TCT may hold significant value in cervical cancer screening, as well as in the early diagnosis and treatment of the disease.
Keywords: Computer intelligent film reading system, liquid based cytology, cervical cancer, screening, efficiency
Introduction
Cervical cancer is a prevalent malignancy among adult women, characterized by high incidence and mortality rates. According to World Health Organization (WHO) statistics, there are approximately 459,000 new cases of cervical cancer worldwide each year, with about 131,500 cases occurring in China, representing 28.7% of the global total [1]. The incidence of cervical cancer is increasing among younger women, necessitating greater attention to and emphasis on its prevention and treatment [2].
Presently, cytological smear testing is a crucial method for the screening and early diagnosis of cervical cancer. However, traditional smear methods have certain limitations in clinical practice. Studies have reported a high false positive rate of 20% to 50%, thereby restricting their clinical utility and failing to meet the demands of modern clinical gynecology [3]. With the innovative development of information technology, computer-aided detection (CAD) systems, an artificial intelligence (AI) auxiliary system, have gradually been applied in clinical settings as automated systems for initial screening based on cytology. Currently, these systems are widely used in clinical settings abroad but have not yet been applied domestically [4-6]. Research reports suggest that CAD systems can improve screening efficiency, increase detection rates of lesions, and offer certain advantages compared to traditional screening methods [7]. However, there are reports indicating that CAD systems are not highly sensitive in cervical cancer screening, so it is necessary to further explore their application value in ThinPrep cytology test (TCT) screening for cervical cancer [8].
This study enhanced the AI reading system and employed it in TCT, integrating visual convolutional neural network (CNN) algorithms and microscopic imaging technology for automated localization, identification, and classification of cervical lesion cells. The aim of this study is to assess the effectiveness of the AI reading system in TCT screening by analyzing data from 1200 adult women undergoing cervical cancer screening.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee of The Fifth People’s Hospital of Jinan. Written informed consent was obtained from all patients involved.
Participants
A total of 1200 adult females who underwent cervical cancer screening in the Gynecology Department of The Fifth People’s Hospital of Jinan from July 2022 to June 2023 were included.
Inclusion criteria: (1) Age 20-65 years with a history of sexual activity. (2) No acute reproductive system inflammation. (3) No history of cervical surgery. (4) Non-pregnant and non-menstruating. (5) Specimen cell count >5000. (6) Willing to undergo TCT.
Exclusion criteria: (1) Within 6 weeks of a cervical cytology examination. (2) Within 3 months of cervical surgery or treatment. (3) Acute or subacute infection in the vagina or cervix. (4) Specimen cell count does not meet the requirements for TCT.
The mean age of participants was 39.65±4.47 years (range 21-64 years); mean BMI was 20.24±3.12 kg/m2; parity ranged from 1 to 4 (mean 2.04±0.25); number of deliveries ranged from 1 to 3 (mean 1.85±0.30); age at first delivery ranged from 21 to 29 years (mean 23.61±1.04 years); 924 were married and 276 were unmarried.
We calculated the required sample size with an expected sensitivity of 0.93 [9], an allowable error of 0.05, and a confidence level of 95% (Z-value of 1.96) using the sample size calculation formula: N=Z2(1-P)P/E2. Where: N is the sample size. Z is the Z-value for the normal distribution, which is 1.96 for a 95% confidence level. P is the expected sensitivity, which is 0.93. E is the allowable error, which is 0.05. Calculations give: N≈99.92. Therefore, when the expected sensitivity is 0.93, the allowable error is 0.05, and the confidence level is 95%, the required sample size is approximately 100. Hence, a sample size of 1200 is sufficient for this study.
Methods
Establishment of AI system database
The AI-assisted system is based on a visual CNN combined with cell microscopic imaging analysis technology. All the TCT specimens collected during the preliminary stage were used. After staining, the slides were scanned with a 20× objective lens, and the images were stitched and analyzed to form full-field digital slides. Experienced clinical cytopathologists then selected and labeled images with abnormal and normal cells. Using a Faster Region-based CNN (R-CNN) combined with the feature pyramid network (FPN) target determination method, the system automatically completed the localization and classification of diseased cells. Images containing cells were screened and input into a feature extraction network for the local image blocks. The extracted block features, along with learnable classification label features, were fed into a transformer encoder labeled with annotations. The output classification label features were then input into a multilayer perceptron to obtain the classification results of the slides according to The Bethesda System (TBS). This included qualitative results (normal, abnormal, and unsatisfactory), and an abnormal probability severity score (labeling abnormal cells and providing confidence intervals).
TCT
Participants were required to abstain from drug use, douching, and sexual activity for 24 hours prior to formal sampling. A speculum was used to ensure complete exposure of the cervix, and subsequently, a sampling brush was deployed to gather shed cells from the junction of the squamous epithelium in the examinee. The brush was gently rotated clockwise to 3-5 times under light pressure, after which the collected cell sample was washed and placed into a preservation solution. A TCT slide staining system was employed to perform cleaning, transferring, fixing, staining, and sealing, resulting in the production of a single-layer TCT slide. Two highly experienced pathologists conducted blind microscopic examinations, and any discrepancies were resolved through discussion. A domestically manufactured slide scanning and analysis system was utilized for intelligent computerized digital section reading. The AIassisted slide review utilizes digital slide intelligent reading, scanning each slide for approximately 3 minutes to generate a 4096×2816 pixel digital scan image, which was then saved. The image can be zoomed in or out at will. The results were strictly determined according to the TBS.
Pathological biopsy
Multiple-point pathological tissue biopsies were conducted for individuals with suspected malignant lesions. Cervical biopsy forceps were used during colposcopy to collect tissue samples from suspected lesion sites. Biopsies were typically taken at the 3, 6, 9, and 12 o’clock positions for individuals without clear lesions. If colposcopy yielded unsatisfactory results, endocervical curettage was performed concurrently, and the collected biopsy tissues are preserved in formalin. The slides were reviewed and diagnosed by the same pathologist.
Diagnosis criteria
(1) TCT judgment: Categories: Normal or no malignant lesions (NILM), atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells that cannot exclude a high-grade lesion (ASC-H), high-grade squamous intraepithelial lesion (HSIL), squamous cell carcinoma (SCC), atypical glandular cells of undetermined significance (AGUS), and adenocarcinoma (AC). Classification: ASC-US, ASC-H, LSIL, HSIL, SCC, and atypical glandular cells (AGC) are classified as positive, while NILM is classified as negative [10].
(2) Pathological examination judgment: According to the pathological results of female reproductive system diseases formulated by the WHO, the results were divided into: normal or inflammation, low-grade lesions (cervical intraepithelial neoplasia grade I (CINI)), high-grade lesions (cervical intraepithelial neoplasia grade II (CINII), cervical intraepithelial neoplasia grade III (CINIII), and carcinoma in situ), early invasive cancer, and invasive cancer [11].
Primary and secondary outcomes
Primary outcomes
Sensitivity and specificity of detecting high-grade lesions and cancers using the AI film reading system compared to manual examination.
Secondary outcomes
Efficiency: Measuring the time required for the AI film reading system to process and analyze TCT slides compared to manual reading. Consistency: Assessing the agreement between AI and manual readings using the Kappa statistic. Accuracy in different lesion types: Comparing the accuracy of AI and manual readings in identifying low-grade lesions, inflammation, and high-grade lesions.
Statistical methods
Data analysis was performed using SPSS 22.0 statistical software. Measurement data were presented as mean ± standard deviation (SD), and count data were expressed as number of cases and percentages. Agreement with pathological results was assessed using the Kappa consistency test. The chi-square test was applied for count data (%), expressed as χ2. For the comparison of the sensitivity and specificity of manual examination and AI, a paired three-dimensional chi-square test was performed. A P-value of <0.05 indicated statistical significance.
Results
Efficiency comparison of different film readings in TCT
In TCT screening, the average time for manual film reading was shorter than that for the AI film reading system (P<0.01, Table 1).
Table 1.
Efficiency comparison of different film readings in TCT
| Film reading method | Sample size | Average time spent (min/piece) |
|---|---|---|
| AI film reading system | 1200 | 2.78±0.34 |
| Manual film reading | 1200 | 4.82±1.01 |
| t | 3.298 | |
| P | <0.001 |
AI: artificial intelligence; TCT: ThinPrep cytology test.
Imaging results of different groups in TCT
The screening results of 1200 adult females are shown in Table 2 and Figure 1. The AI film reading system significantly detected more lesions than the manual film reading method (χ2=28.0662, P<0.001).
Table 2.
Imaging results of different groups in TCT [n (%)]
| Film reading method | Sample size | NILM | ASC-US | ASC-H | LSIL | HSIL and SCC | χ2 | p |
|---|---|---|---|---|---|---|---|---|
| AI film reading system | 1200 | 648 (54.0) | 127 (10.58) | 54 (4.5) | 276 (23.0) | 95 (7.92) | 28.066 | <0.001 |
| Manual film reading | 1200 | 622 (51.83) | 215 (17.92) | 46 (3.83) | 237 (19.75) | 80 (6.67) |
AI: artificial intelligence; TCT: ThinPrep cytology test; NILM: negative for intraepithelial lesion or malignancy; ASC-US: atypical squamous cells of undetermined significance; ASC-H: atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion; SCC: squamous cell carcinoma.
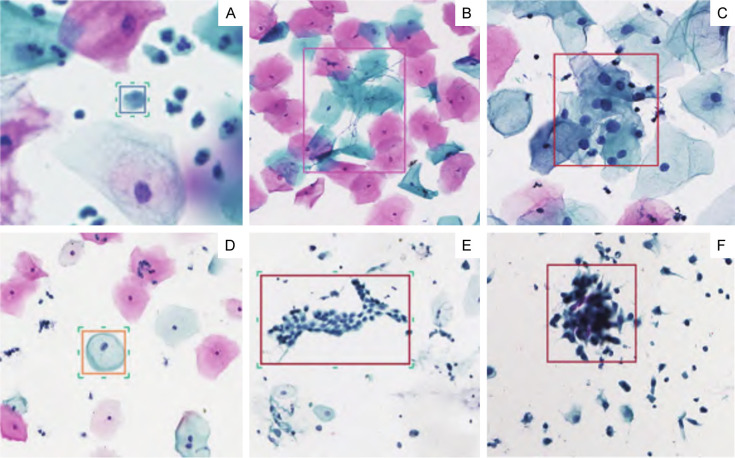
Figure 1.
The conditions of cervical cancer were detected using the AI (artificial intelligence) imaging system. A. Bacterial infections detected by the AI system. B. Another instance of bacterial infections detected by the AI system. C. ASC-US (atypical squamous cells of undetermined significance) classified by the AI system. D. LSIL (low-grade squamous intraepithelial lesion) identified by the AI system. E. HSIL (high-grade squamous intraepithelial lesion) detected by the AI system. F. Another instance of HSIL detected by the AI system.
Consistency of different imaging results in TCT
Through analysis, 957 patients were classified into the same grade by both imaging techniques. Among the cases with different ratings, 24 cases identified as NILM by the AI imaging system were determined as ASC-US by manual imaging interpretation, and 18 cases identified as ASC-US by the AI system were determined as NILM by manual interpretation. The overall compliance rate between AI imaging and manual imaging interpretation was 79.75%, with a corresponding Kappa value of 0.588, indicating moderate agreement between the two groups (Table 3).
Table 3.
TCT different imaging grades and consistency
| AI imaging system | Total | Manual film reading | Consistency | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| NILM | ASC-US | LSIL | ASC-H | HSIL and cancer | Compliance rate | Kappa value | ||
| NILM | 648 | 604 | 24 | 0 | 0 | 0 | - | - |
| ASC-US | 127 | 4 | 101 | 37 | 2 | 1 | - | - |
| LSIL | 276 | 10 | 71 | 161 | 6 | 12 | - | - |
| ASC-H | 54 | 3 | 12 | 13 | 30 | 6 | - | - |
| HSIL and cancer | 95 | 1 | 7 | 22 | 8 | 61 | - | - |
| Total | 1200 | 622 | 215 | 237 | 46 | 80 | 79.750 | 0.588 |
AI: artificial intelligence; TCT: ThinPrep cytology test; NILM: negative for intraepithelial lesion or malignancy; ASC-US: atypical squamous cells of undetermined significance; ASC-H: atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion; SCC: squamous cell carcinoma.
Comparison of different TCT readings with cervical cancer pathology examination
A total of 525 patients underwent cervical biopsy in this group, with 286 cases (54.48%) showing normal or inflammatory results, 85 cases (16.19%) showing CIN I, 71 cases (13.52%) showing CIN II, and 83 cases (15.81%) showing CIN III or cancer. One case of CIN I was judged as normal by the AI screening system, and one case of CIN III was judged as normal by manual screening (Table 4).
Table 4.
Comparison of TCT readings with pathology biopsy results
| Pathological results | Total | AI imaging system | Manual film reading | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| NILM | ASC-US | LSIL | ASC-H | HSIL and cancer | NILM | ASC-US | LSIL | ASC-H | HSIL and cancer | ||
| Inflammation | 286 | 19 | 115 | 137 | 9 | 6 | 22 | 134 | 116 | 8 | 5 |
| CIN I | 85 | 2 | 29 | 47 | 6 | 1 | 1 | 15 | 63 | 5 | 1 |
| CIN II | 71 | 1 | 7 | 23 | 14 | 26 | 0 | 27 | 31 | 10 | 3 |
| CIN III and cancer | 83 | 0 | 16 | 3 | 15 | 49 | 1 | 14 | 18 | 13 | 37 |
| Total | 525 | 22 | 167 | 210 | 44 | 82 | 24 | 190 | 228 | 36 | 47 |
AI: artificial intelligence; TCT: ThinPrep cytology test; NILM: negative for intraepithelial lesion or malignancy; ASC-US: atypical squamous cells of undetermined significance; ASC-H: atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion; SCC: squamous cell carcinoma; CIN: cervical intraepithelial neoplasia.
Through analysis, the accuracy of the AI screening system for low-grade lesions and inflammation was 87.47% (349/399), while manual screening was 79.41% (351/442) (P=0.018). However, the accuracy rates for high-grade lesions and cancer were 82.54% (104/126) and 75.90% (63/83) for the AI and manual systems, respectively (P=0.241, Table 5).
Table 5.
Accuracy of TCT screening for cervical cancer (%)
| Film reading method | Pathological results | Accuracy (%) | ||
|---|---|---|---|---|
|
| ||||
| Low grade lesions and inflammation | High grade lesions and cancer | |||
| AI imaging system | Low grade lesions and inflammation | 349 | 50 | 87.47 |
| High grade lesions and cancer | 22 | 104 | 82.54 | |
| Manual film reading | Low grade lesions and inflammation | 351 | 91 | 79.41 |
| High grade lesions and cancer | 20 | 63 | 75.90 | |
AI: artificial intelligence; TCT: ThinPrep cytology test.
Analysis of efficacy of TCT screening for cervical cancer
First, the overall sensitivity and specificity of the AI screening system and manual screening were statistically tested (Table 6, P<0.05), followed by separate tests for sensitivity and specificity (Tables 7, 8, P<0.05). The AI screening system has a sensitivity of 67.53% (104/154) for detecting high-grade lesions and cancers, which was higher than the 40.91% (63/154) sensitivity of manual screening. However, the specificity of the AI screening system was 94.07% (349/371), compared to 94.61% (351/371) for manual screening.
Table 6.
Comparison of overall sensitivity and specificity
| AI imaging system | χ | p | |||||
|---|---|---|---|---|---|---|---|
| + | + | - | - | ||||
| Manual film reading | Pathological biopsy | + | - | + | - | 24.360 | <0.001 |
| + | + | 49 | 0 | 14 | 0 | ||
| + | - | 0 | 11 | 0 | 9 | ||
| - | + | 55 | 0 | 36 | 0 | ||
| - | - | 0 | 11 | 0 | 340 | ||
AI: artificial intelligence; “+” means positive; “-” indicates negative; Pathological biopsy indicates gold standard.
Table 7.
Sensitivity comparison (patients)
| Manual film reading | Total | χ | p | |||
|---|---|---|---|---|---|---|
| + | - | |||||
| AI imaging system | + | 49 | 14 | 63 | 4.34 | 0.037 |
| - | 55 | 36 | 91 | |||
| Total | 104 | 50 | 154 | |||
AI: artificial intelligence; “+” means positive; “-” indicates negative.
Table 8.
Comparison of specificity (non-patient)
| Manual film reading | Total | χ | p | |||
|---|---|---|---|---|---|---|
| + | - | |||||
| AI imaging system | + | 11 | 9 | 20 | 82.19 | <0.001 |
| - | 11 | 340 | 351 | |||
| Total | 22 | 349 | 371 | |||
AI: artificial intelligence; “+” means positive; “-” indicates negative.
At this stage, it was essential to use an index commonly employed in receiver operating characteristic (ROC) analysis to comprehensively evaluate sensitivity and specificity - the Youden index (sensitivity + specificity - 1). The Youden index for the AI screening system was 0.616, significantly higher than the 0.355 for manual screening.
Discussion
Cervical cancer involves a chronic pathological and physiological process that transitions from quantitative to qualitative changes. The pre-cancerous process is prolonged, with a low survival rate in the advanced stages. Timely detection and treatment of cervical cancer are crucial for enhancing patient prognosis. Currently, molecular biology and serology are essential for the early differential diagnosis of cervical tumors, laying the foundation for cervical cancer diagnosis and treatment. Nevertheless, TCT remains the preferred method for cervical cancer screening. The subjective factors and visual interpretation by physicians can impact the effectiveness of screening [12].
This study explored the efficacy of an AI-assisted cytology diagnostic system in improving the accuracy and efficiency of cervical cancer screening. Our results demonstrate that the AI imaging system significantly enhances the efficiency and accuracy of cervical cancer screening compared with traditional manual methods. The AI system, which utilizes advanced machine learning algorithms and image processing techniques, offers substantial improvements in identifying and classifying cervical lesions.
Our findings align with several studies indicating that AI-assisted cytology can enhance screening performance [13-15]. Bao et al. reported that AI-assisted systems improved the sensitivity and specificity of cervical cancer screening, achieving an overall consistency rate of 94.7% with a kappa value of 0.921 [15]. Our study found similar results, with a kappa value of 0.588 and an overall agreement rate of 79.75% between AI and manual readings, suggesting the high reliability of the AI system.
Cytological examination has many advantages; however, cytology-based screening strategies place high demands on the quality of the healthcare system. Liquid-based cytology technology helps better prepare samples and reduce the rate of unsatisfactory specimens [16,17]. Nevertheless, compared with traditional cytology, its effectiveness in detecting precancerous lesions or cancer is not significantly improved. Additionally, there is considerable heterogeneity in cytological classification among different cytologists [18]. AI-assisted cytology systems offer opportunities to address these challenges [19].
The AI system’s ability to accurately identify and classify cervical lesions was evident in the detection of low-grade lesions and inflammation, where the AI system achieved an accuracy of 87.47%, surpassing the 79.41% accuracy of manual readings, which is consistent with related research reports [20-22]. This improved performance can be attributed to the AI system’s capability to consistently analyze high-resolution digital images, reducing human error and variability. The CNN algorithms employed by the AI system enable precise localization and classification of cervical lesions, which may be challenging for manual reviewers due to the subjective nature of visual interpretation.
Both methods show no significant difference in accuracy for screening high-grade lesions and cancer, with rates of 82.54% and 75.90%, respectively. As a result, clinical doctors should promptly diagnose and intervene following the identification of high-grade lesions and cancer during TCT screening to avoid missing the optimal intervention opportunity [15,23,24]. Additionally, the AI system demonstrated higher sensitivity in detecting high-grade lesions and cancer (67.53% vs. 40.91% for manual readings) while maintaining similar specificity (94.07% vs. 94.61%). This heightened sensitivity is crucial for early detection and intervention, potentially improving patient outcomes.
Moreover, the Youden index of the AI screening system was 0.616, higher than the 0.355 of manual screening, indicating that the AI screening system is superior to manual screening.
Several studies have highlighted the practical benefits of AI in clinical practice [25-27]. Tan et al. found that an automatic model for cervical cancer screening based on CNN algorithms performed better than manual methods in detecting cervical lesions [23]. Our study corroborates these findings, particularly regarding the accuracy of detecting low-grade lesions and inflammation, indicating the AI system’s effectiveness in early cervical lesion screening.
Our findings showed that the AI system significantly reduced the average time for slide analysis compared with manual methods, enhancing overall screening efficiency. Moreover, the integration of AI in clinical workflows can mitigate the impact of human factors on diagnostic accuracy. AI systems could help standardize the diagnostic process, reducing inter- and intra-observer variability in cytological assessments. Our study supports this, demonstrating that the AI system provides consistent and reliable results, as reflected by the high agreement rate with manual readings and substantial kappa value.
Despite these promising results, some limitations should be noted. The sample size, which was adequate for initial validation, may not fully represent the broader population. Future studies should include larger and more diverse cohorts to confirm our findings. Moreover, the AI system’s reliance on high-quality digital images means that any issues with image acquisition or staining quality could impact its performance. Addressing these technical challenges is essential for broader clinical implementation. Furthermore, while the AI system demonstrated higher accuracy and efficiency, it did not entirely eliminate the need for human oversight. Certain cases, such as those involving atypical cells or inadequate staining, still require expert review to ensure diagnostic accuracy. This highlights the importance of integrating AI as a complementary tool rather than as a complete replacement for manual methods.
Further studies with larger sample sizes and diverse populations are necessary to fully validate these results and address the system’s limitations. Integrating AI with traditional methods offers a promising approach to advancing cervical cancer screening and improving patient outcomes.
In conclusion, our study confirms that the AI imaging system significantly improves the accuracy and efficiency of cervical cancer screening, reinforcing the potential of AI to enhance early detection and diagnosis of cervical lesions.
Disclosure of conflict of interest
None.
References
- 1.Hu SH, Zhang CH, Jin WW, Feng XH, Zhou P. The relationship between the results of cervical liquid-based cytology and high-risk HPV infection in cervical precancerous lesions. Med Forum. 2023;27:63–65. [Google Scholar]
- 2.Zhao F, Ma DY, Wang TT, Zhang Y, Dong Y, Zhao J. Stratified study of cytological P16, HR-HPV, liquid-based cytology prediction in high-grade cervical precancerous lesions. Chin J Fam Plann Gynecotokol. 2023;15:61–65. 70. [Google Scholar]
- 3.Chen J, Yan SN, Kang WJ. Efficacy of thinprep cytologic test combined with pap smear in the diagnosis of cervical cancer. Acta Medicinae Sinica. 2023;36:144–148. [Google Scholar]
- 4.Lee J, Kang BJ, Kim SH, Park GE. Evaluation of Computer-Aided Detection (CAD) in screening automated breast ultrasound based on characteristics of CAD marks and false-positive marks. Diagnostics (Basel) 2022;12:583. doi: 10.3390/diagnostics12030583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suman S, Tiwari AK, Singh K. Computer-aided diagnostic system for hypertensive retinopathy: a review. Comput Methods Programs Biomed. 2023;240:107627. doi: 10.1016/j.cmpb.2023.107627. [DOI] [PubMed] [Google Scholar]
- 6.Maas MHJ, Neumann H, Shirin H, Katz LH, Benson AA, Kahloon A, Soons E, Hazzan R, Landsman MJ, Lebwohl B, Lewis SK, Sivanathan V, Ngamruengphong S, Jacob H, Siersema PD. A computer-aided polyp detection system in screening and surveillance colonoscopy: an international, multicentre, randomised, tandem trial. Lancet Digit Health. 2024;6:e157–e165. doi: 10.1016/S2589-7500(23)00242-X. [DOI] [PubMed] [Google Scholar]
- 7.Pan YY, Li SH, Song J, Bu DL, Su SZ. The value of liquid-based cytology, HPV testing and DNA quantitative analysis in cervical cancer screening. Chin J Clin Obstet Gynecol. 2023;24:69–70. [Google Scholar]
- 8.Zhou Q, Wu XH, Liu J, Li L, Zhu XQ, Bai P, Sheng XG. Guidelines to the diagnosis and treatment of cervical cancer (4th edition) Chin J Pract Gynecol Obstet. 2018;34:613–622. [Google Scholar]
- 9.Jia AD, Li BZ, Zhang CC. Detection of cervical cancer cells based on strong feature CNN-SVM network. Neurocomputing. 2020;411:112–127. [Google Scholar]
- 10.Zhu Y, Wang Y. New advances in screening techniques for cervical cancer. Chin J Clin Obstet Gynecol. 2023;24:105–107. [Google Scholar]
- 11.Guo X, Liu Y, Wang R, Kang YL, Du Y. Application of an artificial intelligence-assisted system in cytological diagnosis of cer vical lesions. Carcinog Teratog Mutag. 2022;34:361–365. [Google Scholar]
- 12.Che S, Liu D, Liu S, Luo PF. Applicational progress and challenges of the artificial intelligence-aided cervical cancer cytological screening. Chin J Clin Lab Manage. 2019;7:193–198. [Google Scholar]
- 13.Bao H, Bi H, Zhang X, Zhao Y, Dong Y, Luo X, Zhou D, You Z, Wu Y, Liu Z, Zhang Y, Liu J, Fang L, Wang L. Artificial intelligence-assisted cytology for detection of cervical intraepithelial neoplasia or invasive cancer: a multicenter, clinical-based, observational study. Gynecol Oncol. 2020;159:171–178. doi: 10.1016/j.ygyno.2020.07.099. [DOI] [PubMed] [Google Scholar]
- 14.Wang CW, Liou YA, Lin YJ, Chang CC, Chu PH, Lee YC, Wang CH, Chao TK. Artificial intelligence-assisted fast screening cervical high grade squamous intraepithelial lesion and squamous cell carcinoma diagnosis and treatment planning. Sci Rep. 2021;11:16244. doi: 10.1038/s41598-021-95545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao H, Sun X, Zhang Y, Pang B, Li H, Zhou L, Wu F, Cao D, Wang J, Turic B, Wang L. The artificial intelligence-assisted cytology diagnostic system in large-scale cervical cancer screening: a population-based cohort study of 0.7 million women. Cancer Med. 2020;9:6896–6906. doi: 10.1002/cam4.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronco G, Cuzick J, Pierotti P, Cariaggi MP, Dalla Palma P, Naldoni C, Ghiringhello B, Giorgi-Rossi P, Minucci D, Parisio F, Pojer A, Schiboni ML, Sintoni C, Zorzi M, Segnan N, Confortini M. Accuracy of liquid based versus conventional cytology: overall results of new technologies for cervical cancer screening: randomised controlled trial. BMJ. 2007;335:28. doi: 10.1136/bmj.39196.740995.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, Bulten J. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008;111:167–177. doi: 10.1097/01.AOG.0000296488.85807.b3. [DOI] [PubMed] [Google Scholar]
- 18.Renshaw AA, Holladay EB, Geils KB. Results of multiple-slide, blinded review of Papanicolaou slides in the context of litigation. Determining what can be detected regularly and reliably. Cancer. 2005;105:263–269. doi: 10.1002/cncr.21319. [DOI] [PubMed] [Google Scholar]
- 19.William W, Ware A, Basaza-Ejiri AH, Obungoloch J. A review of image analysis and machine learning techniques for automated cervical cancer screening from pap-smear images. Comput Methods Programs Biomed. 2018;164:15–22. doi: 10.1016/j.cmpb.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Bogani G, Ditto A, Martinelli F, Signorelli M, Chiappa V, Leone Roberti Maggiore U, Taverna F, Lombardo C, Borghi C, Scaffa C, Lorusso D, Raspagliesi F. Artificial intelligence estimates the impact of human papillomavirus types in influencing the risk of cervical dysplasia recurrence: progress toward a more personalized approach. Eur J Cancer Prev. 2019;28:81–86. doi: 10.1097/CEJ.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 21.Tian R, Cui Z, He D, Tian X, Gao Q, Ma X, Yang JR, Wu J, Das BC, Severinov K, Hitzeroth II, Debata PR, Xu W, Zhong H, Fan W, Chen Y, Jin Z, Cao C, Yu M, Xie W, Huang Z, Bao Y, Xie H, Yao S, Hu Z. Risk stratification of cervical lesions using capture sequencing and machine learning method based on HPV and human integrated genomic profiles. Carcinogenesis. 2019;40:1220–1228. doi: 10.1093/carcin/bgz094. [DOI] [PubMed] [Google Scholar]
- 22.Rezende MT, Bianchi AGC, Carneiro CM. Cervical cancer: automation of Pap test screening. Diagn Cytopathol. 2021;49:559–574. doi: 10.1002/dc.24708. [DOI] [PubMed] [Google Scholar]
- 23.Tan X, Li K, Zhang J, Wang W, Wu B, Wu J, Li X, Huang X. Automatic model for cervical cancer screening based on convolutional neural network: a retrospective, multicohort, multicenter study. Cancer Cell Int. 2021;21:35. doi: 10.1186/s12935-020-01742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang DH, Hou SQ, Chen HL. Value of liquid-based thin-layer cytology combined with high-risk human papillomavirus nucleic acid in screening cervical cancer and precancerous lesions. Med Innovation Chin. 2020;17:147–150. [Google Scholar]
- 25.Lin H, Yu L. Medical artificial intelligent research: translating artificial intelligence into clinical practice. Ann Transl Med. 2020;8:695. doi: 10.21037/atm-2020-mair-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo J, Pan M, Mo K, Mao Y, Zou D. Emerging role of artificial intelligence in diagnosis, classification and clinical management of glioma. Semin Cancer Biol. 2023;91:110–123. doi: 10.1016/j.semcancer.2023.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Yin J, Ngiam KY, Teo HH. Role of artificial intelligence applications in real-life clinical practice: systematic review. J Med Internet Res. 2021;23:e25759. doi: 10.2196/25759. [DOI] [PMC free article] [PubMed] [Google Scholar]