Abstract
Objective: To explore the clinical efficacy of individualized health education (IHE) and care mode based on magnetic resonance imaging (MRI) combined with Mini-Mental State Examination (MMSE) for lung cancer patients with brain metastases undergoing radiotherapy. Methods: This retrospective study involved 50 lung cancer patients with brain metastases. Patients were divided into a control group (n=25, conventional care) and an intervention group (n=25, individualized health education (IHE) care) according to their nursing model. Both groups underwent enhanced brain MRI scans. The patients were assessed using the Mini Mental State Scale (MMSE) before and at 1 month after radiotherapy. At the same time, Montreal Cognitive Assessment (MoCA) was used to assess the degree of cognitive impairment in both groups before and after the intervention. Finally, the European Organization for Research and Treatment of Cancer (EORTC QLQ-C30) questionnaire was used to evaluate the overall health status and quality of life (QOL) (including physical function, emotional function, and social function) of the two groups of patients after radiotherapy. The patients’ self-care ability in daily life was assessed using Alzheimer’s Disease Collaborative Study Activities of Daily Living (ADCS-ADL). Results: Following intervention, there was no significant difference in MMSE total scores between the control and intervention groups (P > 0.05), or in physical function scores (P > 0.05). However, the intervention group had significantly higher overall QOL scores compared to the control group (P < 0.05), particularly in emotional and social function (P < 0.05). There was no significant difference in total MoCA scores between the two groups (P > 0.05), but the intervention group showed superior scores in visual-spatial, executive function, naming, and attention compared to the control group (all P < 0.05). Following intervention, the intervention group demonstrated better ADCS-ADL scores than the control group (P < 0.05). Conclusion: The IHE mode effectively improved emotional and social functions and enhanced QOL in lung cancer patients with brain metastases undergoing radiotherapy.
Keywords: Magnetic resonance imaging, individualized health education and nursing model, lung cancer patients with brain metastases
Introduction
Globally, the incidence and mortality rates of lung cancer remain high, constituting a significant public health concern. According to the latest statistics, approximately 1.5 million new cases of lung cancer are diagnosed each year, associated with 1.3 million deaths [1]. Notably, the brain is a frequent site of distant metastasis for lung cancer, with an incidence ranging from 20% to 60% [2,3]. These metastases cause a variety of symptoms including headache, dizziness, nausea, seizures, visual impairment, gait disturbances, dysphasia, severely affecting patients’ quality of life and treatment outcome [4,5]. Currently, radiotherapy is one of the primary methods for treating lung cancer brain metastases. However, during radiotherapy, patients may experience exacerbation of existing symptoms or even develop new health problems. In such circumstances, providing effective health education and nursing support is crucial [6]. Personalized health education models, using a patient-centered nursing strategy that considers individual patient differences, have demonstrated positive outcomes in clinical settings. By combining predictive nursing approaches with health education tools, it is possible to enhance patients’ treatment experiences and outcomes [7,8].
In terms of diagnosis, timely and accurate detection of lung cancer brain metastases is crucial for formulating treatment plans and assessing prognosis. In recent years, with advancements in medical technology, magnetic resonance imaging (MRI) has emerged as a superior diagnostic tool compared to traditional computed tomography (CT) scans due to its high sensitivity and resolution for detecting brain metastases [9-11]. MRI not only provides detailed information about metastatic lesions such as their location, size, and presence of associated edema but also assesses the presence of tumor hemorrhage and compression of surrounding brain tissue [12-14]. Consequently, MRI plays a critical role in guiding treatment. Although local surgery combined with radiotherapy can significantly improve the prognosis for most brain metastases patients, radiotherapy may also cause damage to brain tissue, affecting patients’ neurocognitive function [15-17]. Research indicates that lung cancer brain metastases primarily occur in the cerebral hemispheres, with severe cases spreading to the brainstem and cerebellum. This spread is closely linked to specific cognitive impairments, such as memory decline, mood fluctuations, and decreased information processing ability [18-20].
The core components of the individualized health education (IHE) model include comprehensive assessment of patients to understand their health status, lifestyle habits, cognitive abilities, and their understanding and attitudes towards the disease. Based on the assessment results, personalized health education plans are formulated, covering disease knowledge, self-management techniques during treatment, and lifestyle adjustment recommendations. Multiple educational methods and tools are employed to enhance patient learning. Continuous follow-up and assessment of patients’ learning progress and changes in health status are conducted, with educational plans adjusted as needed. The IHE model, by enhancing patients’ self-management ability and increasing their active participation in treatment, can effectively improve patients’ treatment adherence, thereby enhancing treatment outcomes and quality of life. However, despite the occurrence of varying degrees of cognitive impairment in patients after radiotherapy, research on interventions targeting the neurocognitive function of radiotherapy patients through IHE remains relatively limited.
This study selected 50 lung cancer patients with brain metastases who underwent scheduled radiotherapy as the research subjects. MRI was utilized as the diagnostic tool, while the Mini-Mental State Examination (MMSE) and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ C30) were employed to evaluate the neurocognitive function and quality of life (QOL) of the patients. This study aimed to analyze the impact of the IHE model on the neurocognitive function and QOL of patients with lung cancer brain metastases. Through the comprehensive assessment conducted in this study, we provide further evidence-based support and practical guidance for the comprehensive treatment and care of patients with lung cancer brain metastases.
Materials and methods
Research participants
In this retrospective study, 50 patients with lung cancer brain metastases (BMS) (35 males and 15 females, aged 48-77 years, average age (52.14±6.75) years) who were hospitalized in Sir Run Run Shaw Hospital of Zhejiang University from April 2019 to March 2022 and scheduled for radiotherapy were selected as the study subjects. The enrolled patients were sub-grouped into a control group (n=25) and an intervention group (n=25) according to their nursing protocols. No difference was observed in gender, age, or other baseline data between the two groups (all P > 0.05).
Inclusion criteria: 1) Lung cancer patients with brain metastases [21]; 2) Patients who underwent the cognitive function test on time; 3) Patients with an age of 18 or above; 4) Patients with no history of brain surgery or brain radiotherapy; 5) Patients with an expected survival time of more than 90 days.
Exclusion criteria: 1) Patients with a previous history of stroke, dementia, Parkinson’s disease, Alzheimer’s disease, or brain dysfunction, combined history of mental illness, personality disorder, intellectual disability, brain injury, or brain disease; 2) Patients with impaired hearing/vision or unable to complete the study for other reasons; 3) Patients with other brain dysfunction; and 4) Patients who received other treatments that affected neurocognitive function.
This study adhered to the principles outlined in the Helsinki Declaration and obtained approval from the Ethics Committee of the Sir Run Run Shaw Hospital, Zhejiang University.
Types of testing
The participants in control group only received routine nursing, which mainly included psychological counseling, real-time monitoring of disease process, and guidance on daily living activities. In the intervention group, MMSE was adopted to evaluate cognitive dysfunction, and IHE mode of nursing was given as follows:
I. Assessment protocol: senior nurses (with over 5 years of experience in oncology radiation therapy) conducted evaluations before radiotherapy, at the end of treatment, and at the first- and third-months post-treatment. MMSE is a tool for assessing cognitive function, including orientation, memory, attention and numeracy, language, and visuospatial skills [21,22]. It is scored on a scale of 0 to 30, with higher scores indicating better cognitive function, with 24 to 27 indicating possible cognitive impairment and 27 to 30 indicating normal cognitive function. QLQ-C30 is a questionnaire designed specifically for cancer patients to assess quality of life. It covers four areas of overall health status, physical function, emotional state, and social function, and provides a complete picture of the quality of life of cancer patients after treatment by comprehensively evaluating these aspects, helping the medical team to better understand the needs of patients and provide appropriate support.
II. MRI examination: enhanced MRI scan, axial and sagittal images were obtained.
III. Education on association between cognitive function and brain area function: targeted health education was conducted based on MMSE scores and MRI results, focusing on guiding patients and their primary caregivers to pay attention to social activities, life skills, and emotional changes. The education content covers the influence of different brain regions on cognitive function, such as the cerebral cortex’s influence on language ability, the frontal lobe’s impact on personality and thinking ability, and the hippocampus on memory function. This approach aimed to deepen patients’ understanding of the condition and improvement in self-management abilities.
IV. Personalized nursing and educational activities: during hospitalization, emphasis was placed on the diagnosis and treatment of high-risk populations and home care, adopting early detection, intervention, and treatment measures, and emphasizing the importance of cognitive exercises. By assessing the needs of patients and caregivers, appropriate educational tools and methods were selected to provide personalized education. Various educational materials and information frameworks were utilized, with regular updates to meet the social, psychological, and medical needs of patients. Guidance was provided on proper medication management and home environment modifications, such as adding handrails and bed rails, to reduce accidental injury [24,25].
V. Diet and nutrition: a balanced diet was advocated, including an increased intake of high-calorie, high-protein foods, along with the consumption of fresh vegetables and fruits.
VI. Palliative care: efforts were made to reduce uncertainty in treatment decisions, provide psychological support, and manage pain.
VII. Social and economic support: supports were offered to economically challenged patients, connecting them with community resources to aid in addressing their needs.
VIII. Continued follow-up: maintaining consistent systematic follow-up to address patient concerns and proactively collaborate with patients to resolve issues.
Observational measurements
Mini Mental State Scale (MMSE) was utilized to assess cognitive impairment. Follow-up MRI 3 months after nursing was conducted to observe tumor changes in patients with brain metastases. The Chinese version of the Simplified European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30) was used to evaluate the overall health status and quality of life of both of the groups of patients after radiotherapy. In addition, both groups of patients underwent Montreal Cognitive Assessment (MoCA) and Alzheimer’s Disease Collaborative Study Activities of Daily Living (ADCS-ADL) assessments three months after radiotherapy completion. These assessments were conducted by experienced doctors who also provided necessary guidance and explanation to ensure understanding by patients and their families. Explicit consent was obtained from patients before the evaluations, and patients or their families were directly involved in completing the questionnaires to enhance the accuracy and reliability of the data.
MMSE is a simple tool for evaluating cognitive function, which covers several cognitive fields. The MMSE score ranges from 0 to 30, and the higher the score, the better the cognitive function. MMSE includes the following parts: A. Orientation: to assess the patient’s awareness of time and place. For example, patients are asked about the current date, season, place, and city. B. Memory: to evaluate short-term and long-term memory. For example, patients are asked to repeat a few words and then recall them again after a few minutes. C. Attention and calculation ability: to assess the patient’s attention and calculation ability by doing simple calculations or memorizing numbers. D. Language ability: including language function tests such as naming objects, repeating sentences, and writing ability. E. Visual spatial skills: to assess the cognitive ability of visual spatial relations by drawing figures or connecting numbers. MMSE scores between 24 and 27 indicate cognitive dysfunction, and between 27 and 30 suggest normal cognitive function.
According to the criteria for evaluating therapeutic efficacy of solid tumors and interpreted jointly by two radiologists based on recent MRI results. (1) Lesion Control: The lesion shows shrinkage or disappearance, with stable or increased volume and maximum diameter. Contrast-enhanced MRI reveals smooth ring-like, thin-walled ring enhancement or cystic changes overlapping with the original irradiation site, without significant enhancement and no clinical deterioration. (2) Suspicious Progression: The lesion exhibits homogeneous or heterogeneous, thick-walled enhancement, with increased peritumoral edema. Symptoms and signs can be alleviated with hormones and dehydrating agents. Follow-up shows reduced lesion enhancement, decreased edema, and the lesion gradually stabilizes. Wrinkling of the cyst wall forms a ring-like pattern, indicating actual lesion control. (3) Recurrence: The lesion shows homogeneous or heterogeneous, nodular enhancement, accompanied by significant enlargement of peritumoral edema. Follow-up shows increased lesion enhancement and enlarged edema area, with an increase in lesion volume.
QLQ-C30 is a questionnaire specifically designed to assess the quality of life of cancer patients. It covers multiple domains to evaluate overall health and functional status. A. Overall health scores: this section primarily assesses the patient’s general health status and quality of life. It typically includes evaluations of the patient’s overall health perception and satisfaction with their quality of life, reflecting the patient’s overall feelings about their health status. B. Body functions: body functions assessment covers various aspects such as the severity of physical pain, daily activities (like walking and self-care abilities), sleep quality, and fatigue. These indicators help understand the patient’s physical functional status and health impairments. C. Emotional function: emotional function assessment focuses on the patient’s emotional state, including anxiety, depression, and emotional fluctuations. This evaluation provides insights into the impact of cancer and its treatment on the patient’s emotional health, as well as their ability to cope with emotions. D. Social function: the social function section assesses the patient’s performance in social interactions and daily social activities. It includes the frequency and quality of interactions with family and friends, and how cancer affects their social life. Through comprehensive assessment across these four domains, the QLQ-C30 questionnaire provides a holistic view of the quality of life of cancer patients post-treatment. It helps healthcare teams better understand patient needs and provide appropriate support.
MoCA is a tool used to assess mild cognitive impairment and is widely used for early diagnosis of various brain function disorders. It evaluates the following domains: A. Visual spatial score: assess the patient’s performance in visual spatial relations and constructional abilities. B. Executive function score: measure the patient’s abilities in planning, organizing, problem-solving, and flexible thinking. C. Naming and attention score: evaluate the patient’s abilities in naming objects and sustaining attention. D. Speech language score: measure the patient’s fluency of speech and ability to comprehend complex language commands. E. Abstraction score: assess the patient’s abilities in abstract thinking and reasoning. F. Delayed memory score: measure the patient’s ability to recall information after a period of time. G. Directional ability score: evaluate the patient’s ability in orientation to time and place.
ADCS-ADL is a standardized tool for evaluating patients’ self-care ability in daily life, paying special attention to the life skills of patients with mild to moderate cognitive impairment. ADCS-ADL includes the following aspects: A. Diet: whether the patient can eat independently or needs help? B. Dressing: whether the patient can dress independently or needs help? C. Personal hygiene: the patient’s self-care ability in personal hygiene such as bathing and brushing teeth. D. Telephone use: whether patients can make and receive calls independently? E. Independence of other daily activities, such as shopping, cooking and cleaning. Each task has a corresponding scoring standard, and the higher the score, the better the daily living ability. Through these detailed assessments, we can fully understand the daily living and independence of patients.
Statistical analysis
SPSS 23.0 was used for statistical analysis. Measured data conforming to a normal distribution were expressed as (x±sd). Inter-group comparison was performed using independent sample t test, and a paired t-test was applied for the intra-group comparison before and one month after intervention. Statistical data were used by percentage (%) and χ2 tests. For pairwise comparison with α=0.05 test level, P < 0.05 was considered significant.
Results
Comparison of clinical data between two groups
Table 1 shows the comparison of clinical data between the two groups. In the control group, there were 17 males and 8 females with a mean age of (51.37±11.43) years; there were 15 cases with 3 or more intracranial metastases and 10 cases with fewer than 3 lesions. In the intervention group, there were 18 males and 7 females with a mean age of (53.26±12.35) years. There were 12 cases with 3 or more intracranial metastatic lesions and 13 cases with fewer than 3 lesions. There were no significant differences (all P > 0.05) observed between the control and intervention groups in terms of gender, age, maximum lesion diameter, number of intracranial metastases, cases of hypertension, or cases of diabetes.
Table 1.
Comparison of clinical data between the two groups
| Item | Control group (n=25) | Intervention group (n=25) | P |
|---|---|---|---|
| Gender | 0.193 | ||
| Men | 17 | 18 | |
| Women | 8 | 7 | |
| Average age (years) | 51.37±11.43 | 53.26±12.35 | 0.216 |
| Number of intracranial metastases | 0.146 | ||
| 3 or higher | 15 | 12 | |
| < 3 | 10 | 13 | |
| Hypertension | 2 | 1 | 0.552 |
| Diabetes | 1 | 3 | 0.297 |
MRI imaging findings
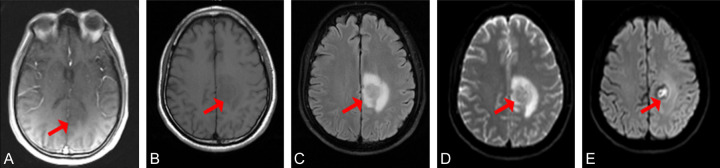
Two experienced diagnostic radiologists evaluated the optimal delayed enhanced scanstage for the lesion. The results showed that no obvious lesions were observed in T1 phase. As the delay time increased, the contour of the lesion gradually became clearer, the degree of enhancement gradually increased, and the edges gradually became more obvious. The visible lesion with a size of 22x20 mm forms an increasingly enhanced contrast with the surrounding brain tissue (Figure 1).
Figure 1.
MRI images of a 58-year-old male patient with adenocarcinoma of the left lung. A-E. Images from phases T1 through T5, respectively. The red arrows indicate the location of the lesion.
Comparison of cognitive function between the two groups
Analysis of the MMSE scores revealed no significant changes before and after patients received interventions (P > 0.05), and this was consistent across both control and intervention groups (P > 0.05). Details are shown in Figure 2.
Figure 2.
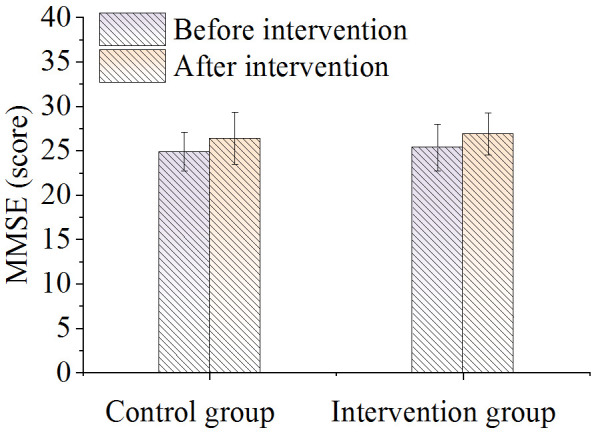
Comparison of MMSE scores.
Comparison of QLQ-C30 between the two groups
Compared to before nursing, the post-intervention QLQ-C30 scores of the two groups increased greatly (P < 0.05). Initially, there were no significant differences in QLQ-C30 score between the groups before intervention. However, the QLQ-C30 scores in the intervention group were significantly higher compared to the control group after nursing intervention (P < 0.05), as shown in Figure 3.
Figure 3.
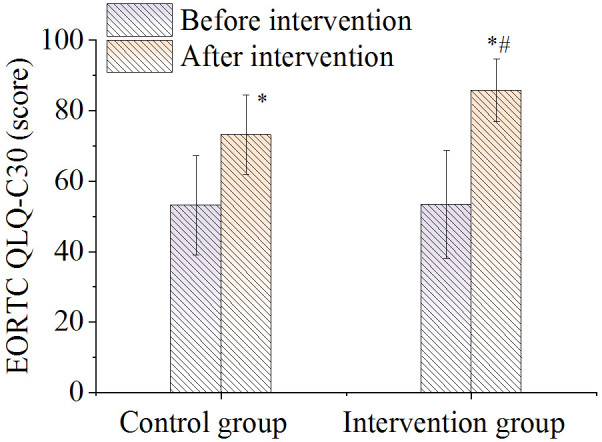
Comparison of total QLQ-C30 scores. Note: *P < 0.05, compared with before intervention value; #P < 0.05, compared with control group.
Comparison of QOL between the two groups
There was no difference in the score of overall health status between the two groups before nursing. After nursing it was significantly higher in the intervention group compared to the control group (P < 0.05), as shown in Figure 4. As shown in Figure 5, there were no significant differences in the body function scores between the two groups before and after nursing care (P > 0.05). As shown in Figures 6 and 7, there were no differences in emotional function scores and social function scores between the two groups before nursing care (P > 0.05). After nursing care, the scores in the intervention group improved (P < 0.05) and were superior to those of the control group (P < 0.05).
Figure 4.
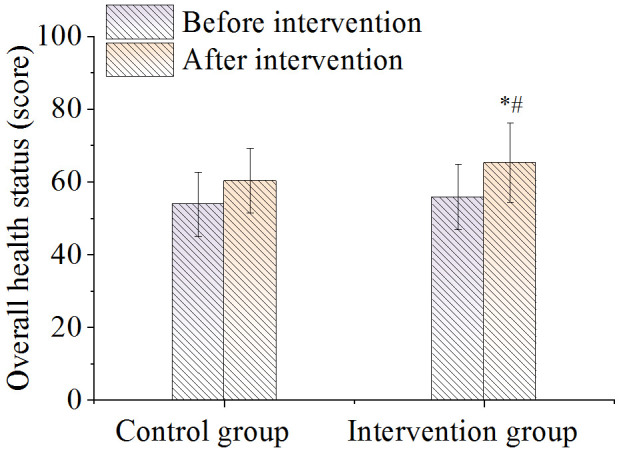
Comparison of overall health scores. Note: *P < 0.05, compared with before intervention value; #P < 0.05, compared with control group.
Figure 5.
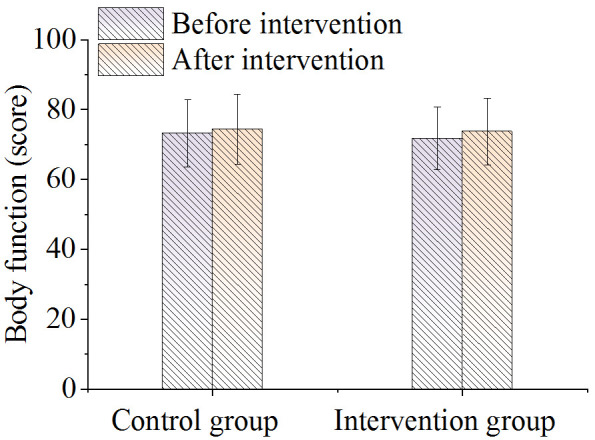
Comparison of body functions.
Figure 6.
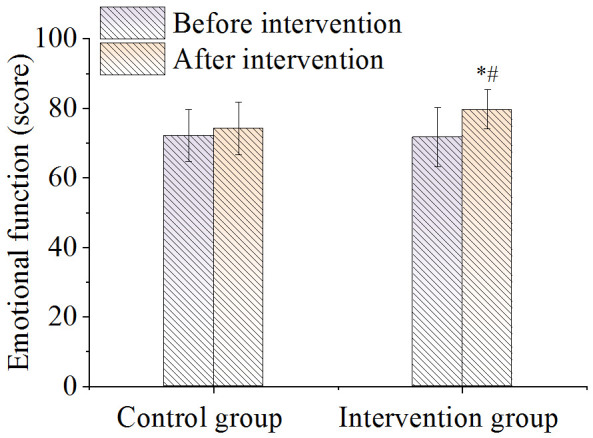
Comparison of emotional function. Note: *P < 0.05, compared with before intervention value; #P < 0.05, compared with control group.
Figure 7.
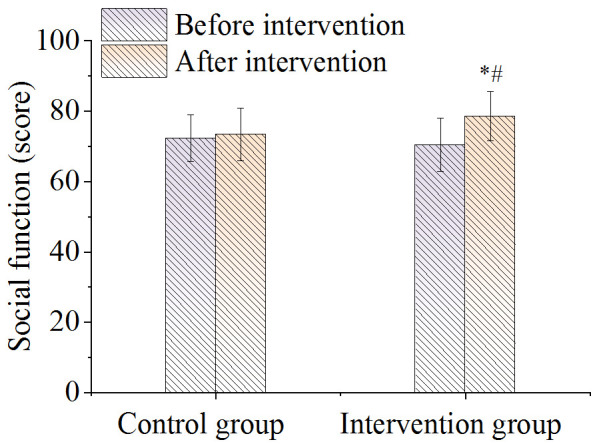
Comparison of social function. Note: *P < 0.05, compared with before intervention value; #P < 0.05, compared with control group.
Comparison of MoCA scores between the two groups
As shown in Figure 8, after intervention, there was no significant difference in the total score of MoCA between the two groups of patients (P > 0.05). The intervention group showed better visual space score, executive function score and naming and attention score than the control group (all P < 0.05); however, there was no significant difference in speech language score, abstraction score, delayed memory score, or directional ability score between the two groups (all P > 0.05).
Figure 8.
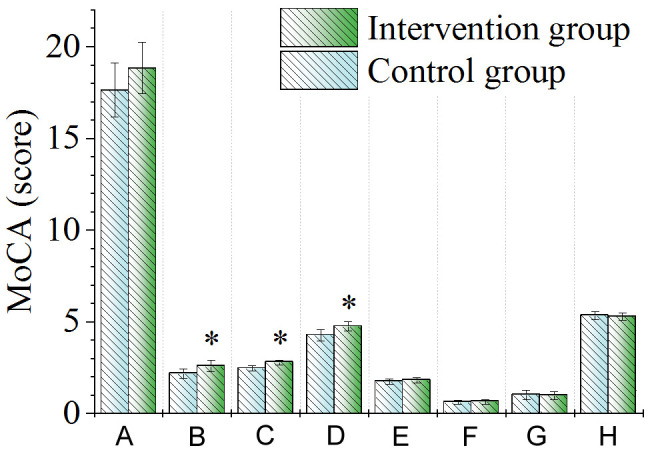
Comparison of MoCA scale scores. A. Total score; B. Visual space score; C. Executive function score; D. Naming and attention score; E. Speech language score; F. Abstraction score; G. Delayed memory score; H. Directional ability score. Note: *P < 0.05, compared with control group. MoCA, Montreal Cognitive Assessment.
Comparison of ADCS-ADL scale scores between the two groups
As shown in Figure 9, there was no significant difference in ADCS-ADL scores between the two groups before the intervention (P > 0.05). After intervention, the score of the intervention group was higher than that of the control group (P < 0.05).
Figure 9.
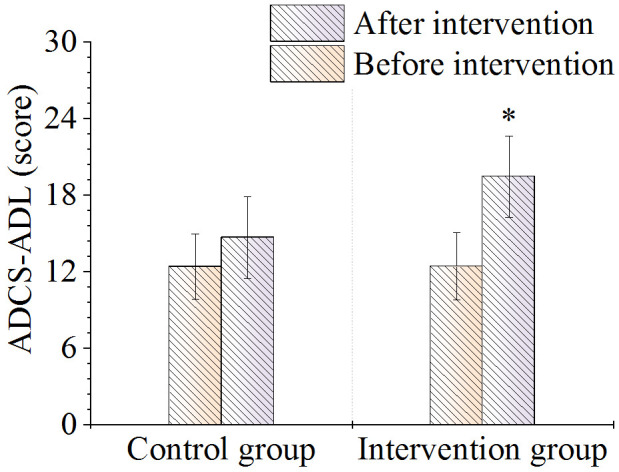
Comparison of ADCS-ADL scale scores. Note: *P < 0.05, compared with control group. ADCS-ADL, Alzheimer’s Disease Collaborative Study Activities of Daily Living.
Discussion
With the development of MRI and computed tomography, the detection rate of lung cancer brain metastases (BMS) has also improved. Metastatic brain cancer typically originates from malignant tumors spreading to the brain parenchyma or meninges. The primary malignant tumors are mostly lung cancer, and the brain metastatic lesions are usually located at the junction of gray and white matters, often manifesting as multiple metastases and a few single lesions [26]. Effectively controlling the progression of BMS and alleviating its clinical symptoms are of great significance for improving the quality of life of patients. The application of MRI enhancement technology can improve the accuracy of detecting lung cancer brain metastases [27-29]. In our study, MRI enhancement had a high diagnostic detection rate for lung cancer brain metastases, which can accurately reflect the location, shape, size, number, and edema range of patients’ brain metastases, whether there was bleeding and the degree of bleeding, and the compression of surrounding brain tissue to provide a powerful basis for treatment choice. Also, post-radiotherapy MRI imaging of lung cancer brain metastases provided information regarding lesion status, allowing observation of dynamic changes (progression/disease control/suspicious progression) in the lesions.
In evaluating the clinical efficacy of radiotherapy for brain metastases, it is critical to consider not only the patients’ progression-free survival but also their cognitive function, which can be evaluated by the MMSE score. The effects of radiotherapy on neurocognitive function in patients with BMS and its biological mechanism are still controversial. Research [30] concluded that controlling the local lesions of BMS after whole brain radiotherapy could eliminate micro-metastases and residual cancer cells, and significantly prolong survival. However, while killing tumor cells, radiotherapy can also kill healthy cells surrounding the lesion, causing damage to the nervous system and leading to cognitive dysfunction. The challenge often lies in balancing tumor control with the risk of radiation-induced damage to the nervous system. So far, the specific characteristics of cognitive dysfunction and self-efficacy associated with radiotherapy for brain tumors are not thoroughly defined [31-33]. Jia et al. [34] evaluated cognitive dysfunction in elderly cancer patients undergoing initial chemotherapy and identified predictive factors for cognitive decline by using MMSE as the primary tool, similar to our study. The results indicated that MMSE was effective in detecting mild to moderate cognitive impairment in an elderly population. The findings of Jia et al. demonstrated that abnormal MMSE score at baseline was one of the independent predictors of cognitive decline during chemotherapy, highlighting the importance of MMSE in early detection and prediction of cognitive changes. This study compared cognitive function between the intervention and control groups using MMSE, underscoring its utility in monitoring nursing effects. Overall, MMSE plays a crucial role in detecting and managing cognitive dysfunction in elderly patients, aiding in the early identification of issues and implementation of appropriate intervention.
The Individualized Health Education (IHE) model is a new nursing method that provides tailored nursing based on the individual differences of patients [35,36]. The use of video to disseminate health knowledge allows patients to revisit the material as needed, enhancing their understanding of their treatment progression, boosting their engagement with the treatment process, and speeding up recovery. The internet model combined with the action research method can provide better continuous care for patients [37]. In terms of clinical outcomes measured in this study, no significant differences were observed in MMSE scores between the control group and the intervention group immediately following the nursing interventions, or at 1 and 3 months post-radiotherapy. However, QOL score and overall health status score showed significant improvement, especially in emotional and social functions. This indicates that personalized health education and nursing can significantly improve QOL of patients.
The results of this study indicate that there were no significant differences in total MoCA score between the two groups, suggesting that IHE mode did not lead to immediate and significant improvement in overall cognitive function. However, in terms of visual space score, executive function score, and naming and attention score, the intervention group outperformed the control group significantly, implying the effectiveness of the IHE mode for specific cognitive domains, particularly those closely associated with everyday life skills. Personalized health education can selectively enhance patients’ partial cognitive abilities, possibly because the IHE mode stimulates these cognitive domains more directly through specific, targeted educational activities and exercises. Additionally, based on the results of the ADCS-ADL scale, there were no significant differences in the self-care ability for daily activities between the two groups before the intervention. However, three months after the intervention, the intervention group’s ability for self-care in daily activities was significantly superior to that of the control group. This finding underscores the value of the IHE mode in enhancing the daily life self-care abilities of patients with lung cancer brain metastases, which directly contributes to their overall quality of life and sense of self-efficacy. For patients with lung cancer brain metastases, maintaining or even improving independence in daily activities is crucial for their quality of life and psychological well-being. The IHE mode is a beneficial intervention for these patients, facilitating improvement in certain key cognitive functions and daily life skills through personalized education and support. He et al. (2022) [36] investigated the effects of a 16-week dance program on symptom alleviation and improvement of quality of life in breast cancer patients undergoing adjuvant chemotherapy. In terms of research objectives, this study aligns with our study in aiming to enhance symptoms and quality of life in cancer patients through specific interventions (dance program or personalized care). Both studies evaluated the impact of the intervention on patients’ quality of life, with significant improvement observed in the intervention group, emphasizing the importance of personalized care interventions in enhancing the quality of life of cancer patients. This demonstrates that personalized care interventions tailored to cancer patients’ specific needs and cultural background can effectively manage symptoms and enhance quality of life.
Conclusion
The IHE model can effectively improve the emotional function and social function of lung cancer patients with brain metastases and improve the QOL. A limitation of this study is its relatively small sample size, which may introduce certain biases in the results. Therefore, it is necessary to continue collecting cases in subsequent stages to expand the sample size and confirm the research findings, providing new avenues for the care model of lung cancer brain metastases (BMS). We plan to conduct prospective studies and incorporate additional clinical observation indicators for analysis. This will help to comprehensively evaluate the effectiveness of nursing interventions and to further validate and optimize the care model we described.
Disclosure of conflict of interest
None.
References
- 1.Zhang Q, Abdo R, Iosef C, Kaneko T, Cecchini M, Han VK, Li SS. The spatial transcriptomic landscape of non-small cell lung cancer brain metastasis. Nat Commun. 2022;13:5983. doi: 10.1038/s41467-022-33365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y, Cui Y, Zheng X, Zhao Y, Sun G. Small-cell lung cancer brain metastasis: from molecular mechanisms to diagnosis and treatment. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166557. doi: 10.1016/j.bbadis.2022.166557. [DOI] [PubMed] [Google Scholar]
- 3.Liu W, Powell CA, Wang Q. Tumor microenvironment in lung cancer-derived brain metastasis. Chin Med J (Engl) 2022;135:1781–1791. doi: 10.1097/CM9.0000000000002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Chen R, Wa Y, Ding S, Yang Y, Liao J, Tong L, Xiao G. Tumor immune microenvironment and immunotherapy in brain metastasis from non-small cell lung cancer. Front Immunol. 2022;13:829451. doi: 10.3389/fimmu.2022.829451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rios-Hoyo A, Arriola E. Immunotherapy and brain metastasis in lung cancer: connecting bench side science to the clinic. Front Immunol. 2023;14:1221097. doi: 10.3389/fimmu.2023.1221097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua YC, Gao DZ, Wang KY, Ding XS, Xu WR, Li YB, Shi WW, Sun SB, Li XY. Bevacizumab reduces peritumoral brain edema in lung cancer brain metastases after radiotherapy. Thorac Cancer. 2023;14:3133–3139. doi: 10.1111/1759-7714.15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soeda S. An individualized mental health education programme for Japanese managers. Occup Med (Lond) 2020;70:176–182. doi: 10.1093/occmed/kqaa025. [DOI] [PubMed] [Google Scholar]
- 8.Kattari L. Civic education’s role in advancing health equity for LGBTQIA2S+ youth. Health Promot Pract. 2023;24:59–61. doi: 10.1177/15248399221129887. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchiya K. Editorial comment: new insights into the MRI diagnosis of brain metastasis from lung cancer. AJR Am J Roentgenol. 2021;217:1193–1194. doi: 10.2214/AJR.21.26288. [DOI] [PubMed] [Google Scholar]
- 10.Deng F, Liu Z, Fang W, Niu L, Chu X, Cheng Q, Zhang Z, Zhou R, Yang G. MRI radiomics for brain metastasis sub-pathology classification from non-small cell lung cancer: a machine learning, multicenter study. Phys Eng Sci Med. 2023;46:1309–1320. doi: 10.1007/s13246-023-01300-0. [DOI] [PubMed] [Google Scholar]
- 11.Mentzelopoulos A, Gkiatis K, Karanasiou I, Karavasilis E, Papathanasiou M, Efstathopoulos E, Kelekis N, Kouloulias V, Matsopoulos GK. Chemotherapy-induced brain effects in small-cell lung cancer patients: a multimodal MRI study. Brain Topogr. 2021;34:167–181. doi: 10.1007/s10548-020-00811-3. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Huang WJ, Han N, Jiang YL, Ma LY, Zhang J. MRI features and whole-lesion apparent diffusion coefficient histogram analysis of brain metastasis from non-small cell lung cancer for differentiating epidermal growth factor receptor mutation status. Clin Radiol. 2023;78:e243–e250. doi: 10.1016/j.crad.2022.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Yu R, Chang H, Yan W, Wang D, Li F, Cui Y, Wang Y, Wang X, Yan Q, Liu X, Jia W, Zeng Q. Identifying pathological subtypes of brain metastasis from lung cancer using MRI-based deep learning approach: a multicenter study. J Imaging Inform Med. 2024;37:976–987. doi: 10.1007/s10278-024-00988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam JG, Hong H, Choi SH, Park CM, Goo JM, Kim YT, Kim H. No prognostic impact of staging brain MRI in patients with stage IA non-small cell lung cancer. Radiology. 2022;303:632–643. doi: 10.1148/radiol.212101. [DOI] [PubMed] [Google Scholar]
- 15.Zhao R, Kong W, Shang J, Zhe H, Wang YY. Hippocampal-sparing whole-brain radiotherapy for lung cancer. Clin Lung Cancer. 2017;18:127–131. doi: 10.1016/j.cllc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Wu D, Shen B, Chen M, Zhou X, Zhang P, Qiu G, Ji Y, Du X, Yang Y. Serum lactate dehydrogenase predicts brain metastasis and survival in limited-stage small cell lung cancer patients treated with thoracic radiotherapy and prophylactic cranial irradiation. Strahlenther Onkol. 2022;198:1094–1104. doi: 10.1007/s00066-022-01977-4. [DOI] [PubMed] [Google Scholar]
- 17.Kandaz M, Guler OC, Yazar U, Canyilmaz E, Yoney A. Whole brain radiotherapy plus conventional boost in non-small cell lung cancer patients with brain metastasis: a retrospective analysis of overall survival. Turk Neurosurg. 2019;29:464–469. doi: 10.5137/1019-5149.JTN.22896-18.3. [DOI] [PubMed] [Google Scholar]
- 18.Dhanawat A, Noronha V, Ramaswamy A, Gattani S, Castelino R, Dhekale R, Mahajan S, Patil V, Menon N, Daptardar A, Gota V, Banavali S, Badwe R, Prabhash K. The prevalence of cognitive impairment in older Indian persons with cancer and brain metastases. Ecancermedicalscience. 2022;16:1372. doi: 10.3332/ecancer.2022.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haldbo-Classen L, Amidi A, Lukacova S, Wu LM, Oettingen GV, Lassen-Ramshad Y, Zachariae R, Kallehauge JF, Høyer M. Cognitive impairment following radiation to hippocampus and other brain structures in adults with primary brain tumours. Radiother Oncol. 2020;148:1–7. doi: 10.1016/j.radonc.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Ng DQ, Chan D, Agrawal P, Zhao W, Xu X, Acharya M, Chan A. Evidence of brain-derived neurotrophic factor in ameliorating cancer-related cognitive impairment: a systematic review of human studies. Crit Rev Oncol Hematol. 2022;176:103748. doi: 10.1016/j.critrevonc.2022.103748. [DOI] [PubMed] [Google Scholar]
- 21.Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19:e43–e55. doi: 10.1016/S1470-2045(17)30689-7. [DOI] [PubMed] [Google Scholar]
- 22.Jia X, Wang Z, Huang F, Su C, Du W, Jiang H, Wang H, Wang J, Wang F, Su W, Xiao H, Wang Y, Zhang B. A comparison of the Mini-Mental State Examination (MMSE) with the Montreal Cognitive Assessment (MoCA) for mild cognitive impairment screening in Chinese middle-aged and older population: a cross-sectional study. BMC Psychiatry. 2021;21:485. doi: 10.1186/s12888-021-03495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cocks K, Wells JR, Johnson C, Schmidt H, Koller M, Oerlemans S, Velikova G, Pinto M, Tomaszewski KA, Aaronson NK, Exall E, Finbow C, Fitzsimmons D, Grant L, Groenvold M, Tolley C, Wheelwright S, Bottomley A European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Group. Content validity of the EORTC quality of life questionnaire QLQ-C30 for use in cancer. Eur J Cancer. 2023;178:128–138. doi: 10.1016/j.ejca.2022.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Qian Y, Yang L, Cheng L. Self-efficacy level investigation on patients with hematologic malignancies and study on related factors. Nursing and Rehabilitation. 2015;14:16–20. [Google Scholar]
- 25.Qiao SN, Liu MJ, Tian SM. Evaluation of application effect of action method in telephone follow-up of cancer pain patients at home. China Chinese Journal of Pain Medicine. 2018;24:4. [Google Scholar]
- 26.Parida PK, Marquez-Palencia M, Nair V, Kaushik AK, Kim K, Sudderth J, Quesada-Diaz E, Cajigas A, Vemireddy V, Gonzalez-Ericsson PI, Sanders ME, Mobley BC, Huffman K, Sahoo S, Alluri P, Lewis C, Peng Y, Bachoo RM, Arteaga CL, Hanker AB, DeBerardinis RJ, Malladi S. Metabolic diversity within breast cancer brain-tropic cells determines metastatic fitness. Cell Metab. 2022;34:90–105. e7. doi: 10.1016/j.cmet.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eggen AC, Wind TT, Bosma I, Kramer MCA, van Laar PJ, van der Weide HL, Hospers GAP, Jalving M. Value of screening and follow-up brain MRI scans in patients with metastatic melanoma. Cancer Med. 2021;10:8395–8404. doi: 10.1002/cam4.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Ding V, Luo S, Choi E, Hellyer J, Myall N, Henry S, Wood D, Stehr H, Ji H, Nagpal S, Hayden Gephart M, Wakelee H, Neal J, Han SS. Predictive model to guide brain magnetic resonance imaging surveillance in patients with metastatic lung cancer: impact on real-world outcomes. JCO Precis Oncol. 2022;6:e2200220. doi: 10.1200/PO.22.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai M, Kashiwagi N, Nakanishi K, Maeda N, Nakaya Y, Tanaka J, Watanabe S, Hongyo H, Tanaka Y, Yamada S, Kawata A, Toda S, Takano K, Arita H, Tomiyama N. Nonbrain metastases seen on magnetic resonance imaging during metastatic brain tumor screening. Jpn J Radiol. 2023;41:367–381. doi: 10.1007/s11604-022-01362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li PJ, Luo J, Liu GE, Liu DH, Shen SS, Li XJ, Ma H. Radiation therapy for patients with brain metastases from non-small cell lung cancer without driven gene mutation. Chin Med J (Engl) 2020;133:2359–2361. doi: 10.1097/CM9.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakazaki K, Yomo S, Kondoh T, Serizawa T, Kenai H, Kawagishi J, Sato S, Nagano O, Aiyama H, Kawai H, Hasegawa T, Iwai Y, Nagatomo Y, Kida Y, Nishigaki M. Salvage gamma knife radiosurgery for active brain metastases from small-cell lung cancer after whole-brain radiation therapy: a retrospective multi-institutional study (JLGK1701) J Neuroonco. 2020;147:67–76. doi: 10.1007/s11060-020-03397-9. [DOI] [PubMed] [Google Scholar]
- 32.Du TQ, Li X, Zhong WS, Tian JD, Zhao YX, Liu D. Brain metastases of lung cancer: comparison of survival outcomes among whole brain radiotherapy, whole brain radiotherapy with consecutive boost, and simultaneous integrated boost. J Cancer Res Clin Oncol. 2021;147:569–577. doi: 10.1007/s00432-020-03359-8. [DOI] [PubMed] [Google Scholar]
- 33.Petty WJ, Urbanic JJ, Ahmed T, Hughes R, Levine B, Rusthoven K, Papagikos M, Ruiz JR, Lally BE, Chan M, Clark H, D’Agostino RB Jr, Blackstock AW. Long-term outcomes of a phase 2 trial of chemotherapy with consolidative radiation therapy for oligometastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2018;102:527–535. doi: 10.1016/j.ijrobp.2018.06.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia F, Cheng X, Zeng H, Miao J, Hou M. Clinical research on stereotactic radiosurgery combined with epithermal growth factor tyrosine kinase inhibitors in the treatment of brain metastasis of non-small cell lung cancer. J BUON. 2019;24:578–584. [PubMed] [Google Scholar]
- 35.Shibata K, Iwasa K, Takanaka T, Yachi T, Okazaki A, Shiba Y, Kasahara K. Curative thoraco-systemic therapy plus local treatment to the brain for extensive disease-small-cell lung cancer with metastasis only to the brain. Jpn J Clin Oncol. 2019;49:687–690. doi: 10.1093/jjco/hyz079. [DOI] [PubMed] [Google Scholar]
- 36.He X, Ng MSN, Choi KC, So WKW. Effects of a 16-week dance intervention on the symptom cluster of fatigue-sleep disturbance-depression and quality of life among patients with breast cancer undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Nurs Stud. 2022;133:104317. doi: 10.1016/j.ijnurstu.2022.104317. [DOI] [PubMed] [Google Scholar]
- 37.Peng Y, Wan H, Hu X, Xiong F, Cao Y. Internet+Continuous nursing mode in home nursing of patients with T-Tube after hepatolithiasis surgery. Comput Math Methods Med. 2022;2022:9490483. doi: 10.1155/2022/9490483. [DOI] [PMC free article] [PubMed] [Google Scholar]