Abstract
Background: The low diagnosis and treatment rates of familial hypercholesterolemia (FH) have become a global issue. This study aims to explore the correlation between early-onset coronary artery disease (CAD) and FH in the Hakka population in Meizhou, Guangdong. Methods: Clinical data of Hakka patients with early-onset CAD, admitted to the Meizhou People’s Hospital from January 2023 to January 2024 were retrospectively analyzed. The patients were categorized into an FH group and a non-FH group. Biochemical indicators, lipid levels, echocardiographic parameters, clinical phenotypes, and genetic typing of early-onset CAD patients in the Hakka population were analyzed for their correlation with FH. Results: A total of 167 Hakka patients with early-onset CAD were included, among whom 22 patients had FH. The FH group showed lower triglyceride (TG) level [1.785 (1.40, 2.10) vs. 2.090 (1.80, 2.30), P = 0.002] and higher levels of total cholesterol (TC) [6.635 (5.60, 7.10) vs. 4.830 (4.00, 5.40), P<0.001], low-density lipoprotein cholesterol (LDL-C) [4.440 (3.90, 5.20) vs. 2.820 (2.40, 3.30), P<0.001], and apolipoprotein B (Apo B) [1.600 (1.30, 1.80) vs. 0.910 (0.70, 1.10), P<0.001]. FH was correlated with TG, TC, LDL-C and Apo B levels (r1 = -0.235; r2 = 0.441; r3 = 0.483; r4 = 0.538). TG is a risk factor while TC, LDL-C and Apo B are protective factors for FH. Conclusion: The incidence of FH is relatively high among early-onset CAD patients in the Hakka population in Meizhou. TG, TC, LDL-C, and Apo B levels are valuable in aiding clinical differential diagnosis of CAD patients with FH.
Keywords: Early-onset coronary artery disease, familial hypercholesterolemia, atherosclerosis, low-density lipoprotein cholesterol, apolipoprotein B, correlation
Introduction
Familial hypercholesterolemia (FH) is caused by abnormal lipoprotein metabolism, resulting in a lifelong elevation of low-density lipoprotein cholesterol (LDL-C) levels and the deposition of cholesterol in the plasma [1]. This leads to characteristic clinical features such as yellowish skin or tendon xanthomas and is often associated with valvular regurgitation, which is an autosomal dominant inherited disease [2]. A previous study suggested that homozygous FH (HoFH) is rare, with an incidence rate of 1 in 160,000 to 1 million, while the more common heterozygous type (HeFH) has an incidence rate of 1 in 250 to 500 [3]. Its significant familial clustering characteristics markedly increase the risk of atherosclerotic cardiovascular disease (ASCVD), posing a serious threat to public health [4].
Clinically, the accumulation of LDL-C is considered the initiating factor of atherosclerosis (AS), and lipid metabolism disorder forms the pathological basis of AS, which is closely associated with the occurrence and development of early-onset coronary artery disease (CAD) [5]. Studies have found that male FH patients have a 50% higher risk of developing CAD before the age of 50 compared to the general population, while female patients have a 60% higher risk before the age of 30 [6,7]. Furthermore, HoFH patients show a trend towards a younger onset of the disease, with the potential to develop CAD before the age of 20, and a significantly lower survival rate after the age of 30 [8]. In a study involving 1093 myocardial infarction patients under 35 years old, using the domestic application of Dutch Lipid Clinic Network (DLCN) scoring for FH detection revealed a high detection rate of 6.5% [9]. The younger population is more likely to carry FH mutation genes, and their mortality rate is significantly higher compared to the elderly [10]. Therefore, conducting genetic testing for FH in early-onset CAD patients is of great significance for early identification, accurate diagnosis, prevention, and treatment in clinical practice.
Currently, FH is still a major medical challenge due to low diagnosis and treatment rates worldwide [11]. There is limited research on the detection rate and clinical characteristics of FH based on Chinese data [12]. Moreover, there is a lack of studies on the detection and treatment status of FH in the Hakka population, which has a relatively unique genetic background [13]. Therefore, this retrospective study aims to explore the incidence, clinical phenotypes, and genetic typing of FH in early-onset CAD patients in the Hakka population in Meizhou, Guangdong to reveal the relationship between early-onset coronary artery disease and familial hypercholesterolemia in this population.
Materials and methods
General information
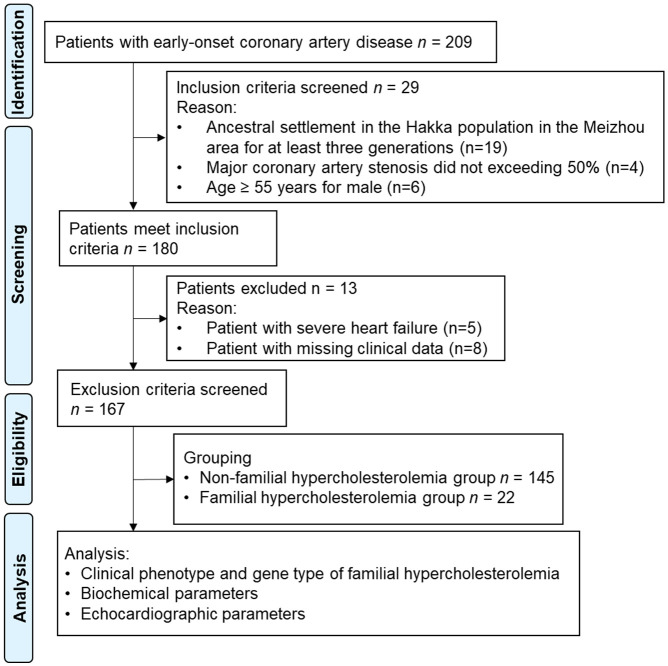
The clinical data of 167 Hakka patients with early-onset CAD admitted to the Meizhou People’s Hospital from January 2023 to January 2024 were retrospectively analyzed in this study. General patient information, including gender, age, BMI, blood pressure, smoking history, past medical history, and family medical history, were collected. This study was approved by the Medical Ethics Committee of Meizhou People’s Hospital. The study flow chart is shown in Figure 1.
Figure 1.
Flow chart of study.
Inclusion criteria: (1) Patients diagnosed with early-onset CAD according to the relevant guidelines [14]; (2) Self-confirmed history of myocardial infarction or previous coronary artery bypass grafting, or percutaneous coronary intervention; (3) Confirmation of at least one major coronary artery stenosis exceeding 50% by coronary CT or coronary angiography; (4) Coronary artery stenosis less than 50%, clinically diagnosed with coronary atherosclerosis, but with clinical symptoms, changes in electrocardiogram, and elevated blood levels of Cardiac Troponin; (5) Ancestral settlement in the Hakka population in the Meizhou area for at least three generations; (6) Onset of CAD; (7) Male ≤55 years, female ≤65 years; (8) Plasma LDL-C ≥3.4 mmol/L, or LDL-C <3.4 mmol/L after taking statins or other medical drugs.
Exclusion criteria: (1) Severe heart failure or arrhythmia; (2) Coexisting immune system diseases or other infectious diseases; (3) Coexisting coagulation abnormalities or severe hematologic diseases; (4) Coexisting significant organ dysfunction of the liver, kidneys, or multiple organ failure; (5) Coexisting thyroid dysfunction; (6) Women during pregnancy or lactation; (7) Missing clinical data or blood sample.
FH diagnostic criteria
Clinical diagnosis of FH was based on the “Expert Consensus on the Screening, Diagnosis, and Treatment of Familial Hypercholesterolemia in China”: 1) Serum LDL-C level ≥4.7 mmol/L in patients not receiving lipid-lowering drug therapy; 2) Presence of xanthoma or tendon xanthoma, or the presence of corneal arcus in individuals <45 years old; 3) Family history of FH or early-onset ASCVD, especially early-onset CAD.
Laboratory tests
The study subjects fasted for 8-12 hours overnight before blood collection. The next morning, 5 mL of peripheral venous blood was collected using biochemical collection tubes. Samples were centrifuged at 3000 r/min for 10 minutes using a Beckman Coulter automatic biochemical analyzer, and the serum was separated and stored at -80°C for testing. Biochemical parameters including fasting plasma glucose (FPG), uric acid (UA), creatinine (Cr), urea nitrogen (UN), and B-type natriuretic peptide, as well as lipid parameters including LDL-C, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A1 (Apo A1), and apolipoprotein B (Apo B), were measured using a fully automated biochemical production line.
Genetic sequencing
Similar to the laboratory tests, the study subjects fasted for 8-12 hours overnight before blood collection. 4 mL of peripheral venous blood was collected using ethylene diamine tetraacetic acid (EDTA) anticoagulant tubes. Samples were processed using the same centrifugation method and stored at -80°C until examination. Blood samples were sent to Guangzhou Huayin Medical Testing Center for targeted gene sequencing using second-generation high-throughput sequencing technology (next-generation sequencing, NGS) (Thermo Fisher Scientific Inc.), which covers all exons of known FH-related genes.
Echocardiography examination
Left ventricular end-diastolic dimension (LVd) and left ventricular ejection fractions (LVEF) were measured using the GE Voluson E10 ultrasound imaging system.
Observation indicators
The primary outcomes were the FH incidence rate, clinical phenotypes, and genetic typing in all early-onset CAD patients. The secondary outcomes included differences in biochemical parameters, lipid indicators, and echocardiographic indicators between the FH group and non-FH group.
Statistical analysis
Data analysis was conducted using IBM SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, N.Y., USA). Frequency data were presented as n (%), and the chi-square test was employed. Normally distributed measurement data were presented as (mean ± SD) and analyzed using the t-test, while non-normally distributed data were presented as the median and interquartile range [M (P25, P75)] and analyzed using Kruskal-Wallis test. A P values less than 0.05 was considered statistically significant.
Results
Incidence rate, clinical phenotypes, and genetic typing of FH
The incidence rate, clinical phenotypes, and genetic typing of FH provide precise genetic information for early diagnosis and prevention of the disease. This study included a total of 167 early-onset CAD Hakka patients, among whom 22 patients developed FH, resulting in an FH incidence rate of 13.17%. Only 1 FH patient had a homozygous type, accounting for 4.55%, while 21 patients had a heterozygous type, accounting for 95.45%. The main gene mutation was in the low-density lipoprotein receptor (LDL-R) gene, accounting for 54.17%, followed by Apo B gene mutation, accounting for 16.67%. Mutations in the Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) gene, adenosine triphosphate-binding cassette transporter G5 (ABCG5) gene, ATP binding cassette subfamily C member 6 (ABCC6) gene, ATP-binding cassette transporter A1 (ABCA1) gene, and Low-density lipoprotein receptor-related protein 6 (LRP6) gene accounted for 8.33%, 4.17%, 8.33%, 4.17%, and 4.17%, respectively. The study results indicate a high incidence rate of FH, mostly in the heterozygous type, with mutations in LDL-R and Apo B genes being the most common (Table 1).
Table 1.
Clinical phenotype and genotyping of FH
| Indicator | Number of Cases | Proportion (%) | |
|---|---|---|---|
| Clinical Phenotype | Homozygous | 1 | 4.55 |
| Heterozygous | 21 | 95.45 | |
| Genotyping | LDL-R Gene Variation | 13 | 54.17 |
| Apo B Gene Variation | 4 | 16.67 | |
| PCSK9 Gene Variation | 2 | 8.33 | |
| ABCG5 Gene Variation | 1 | 4.17 | |
| ABCC6 Gene Variation | 2 | 8.33 | |
| ABCA1 Gene Variation | 1 | 4.17 | |
| LRP6 Gene Variation | 1 | 4.17 | |
LDL-R: Low-Density Lipoprotein Receptor; Apo B: Apolipoprotein B; PCSK9: Proprotein Convertase Subtilisin/Kexin 9; ABCG5: ATP-Binding Cassette Transporter G5; ABCC6: ATP Binding Cassette Subfamily C Member 6; ABCA1: ATP-Binding Cassette Transporter A1; LRP6: Low-Density Lipoprotein Receptor-Related Protein 6.
Comparison of general data between the HF and non-HF group
There were no significant differences in the clinical data (including gender, age, BMI, blood pressure, smoking history, past medical history, family medical history) between the two groups (all P>0.05) (Table 2).
Table 2.
Comparison of demographic data between the FH and non-FH group
| Clinical Data | Number of Cases | FH Group (n = 22) | Non-FH Group (n = 145) | x2/t/Z | P | |
|---|---|---|---|---|---|---|
| Gender | Male | 92 | 10 | 82 | 0.951 | 0.330 |
| Female | 75 | 12 | 63 | |||
| Age (years) | - | 50.50±7.20 | 52.77±6.87 | 1.435 | 0.153 | |
| BMI (kg/m2) | - | 24.270 (22.60, 27.60) | 24.900 (23.30, 26.90) | -0.054 | 0.957 | |
| Blood Pressure | Systolic (mmHg) | - | 126.73±16.34 | 134.19±23.20 | 1.453 | 0.148 |
| Diastolic (mmHg) | - | 79.18±11.64 | 82.68±16.84 | 0.941 | 0.349 | |
| Smoking History | 50 | 4 | 46 | 1.670 | 0.196 | |
| Past Medical History | Hypertension | 83 | 5 | 78 | 7.295 | 0.399 |
| Diabetes | 42 | 4 | 38 | |||
| Coronary Heart Disease | 16 | 2 | 14 | |||
| Acute Myocardial Infarction | 2 | 1 | 1 | |||
| Cerebrovascular Accident (Stroke) | 8 | 0 | 8 | |||
| Gout | 2 | 0 | 2 | |||
| Renal Insufficiency/Failure | 3 | 0 | 3 | |||
| Carotid Artery Stenosis | 1 | 0 | 1 | |||
| Family History | Coronary Heart Disease | 24 | 5 | 19 | 0.982 | 0.612 |
| Myocardial Infarction | 10 | 1 | 9 | |||
| Stroke | 3 | 1 | 2 | |||
Comparison of biochemical parameters between the HF and non-HF group
Biochemical parameters are important indicators for assessing the risk, severity, and prognosis of coronary artery disease. FPG is a predictive risk factor for coronary artery disease, Cr levels are closely associated with the development of kidney disease, UN is an indirect indicator of renal impairment, UA is an independent risk factor for predicting coronary artery disease, and B-type natriuretic peptide is one of the indicators for clinical diagnosis and screening of myocardial disease and heart failure. As shown in Table 3, there were no differences in FPG, Cr, UN, UA, and B-type natriuretic peptide levels between the two groups (all P>0.05), indicating no significant changes in blood sugar, renal function levels, and other biochemical indicators between the two groups.
Table 3.
Comparison of biochemical parameters between the FH and non-FH group
| Group | FPG (mmol/L) | Cr (umol/L) | UN (mmol/L) | UA (umol/L) | B-type Natriuretic Peptide (pg/ml) |
|---|---|---|---|---|---|
| FH Group (n = 22) | 6.74±1.89 | 84.82±22.69 | 5.81±1.09 | 412.61±58.96 | 198.24±29.16 |
| Non-FH Group (n = 145) | 6.21±2.17 | 90.98±26.74 | 5.53±1.21 | 384.47±67.15 | 203.05±25.47 |
| t | 1.084 | 1.025 | 1.024 | 1.859 | 0.810 |
| P | 0.280 | 0.307 | 0.308 | 0.065 | 0.419 |
FPG: Fasting Plasma Glucose; Cr: Blood Creatinine; UN: Blood Urea Nitrogen; UA: Urinary Acid.
Comparison of lipid parameters between the HF and non-HF group
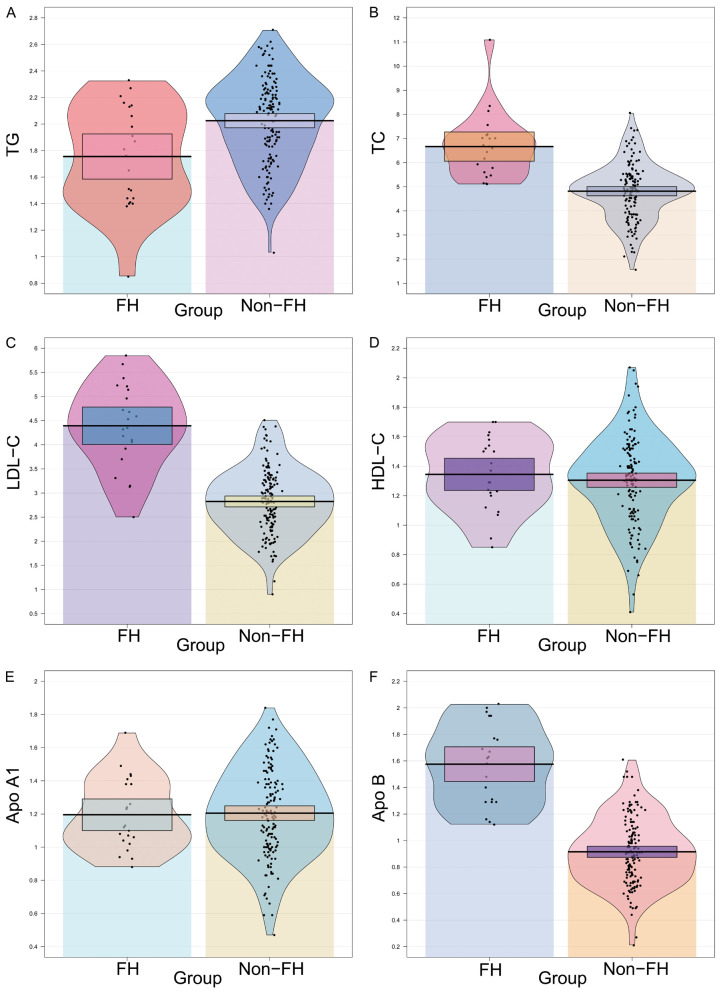
Lipid parameters are key indicators for assessing cardiovascular disease and risk. TG is a commonly used indicator for assessing the risk of cardiovascular disease such as atherosclerosis and CAD. LDL-C levels are closely related to cardiovascular disease, HDL-C is a protective factor for cardiovascular disease, and Apo A1 and Apo B can reflect lipid metabolism well. As shown in Figure 2, compared to the non-FH group, the FH group exhibited lower TG levels [1.785 (1.40, 2.10) mmol/L vs. 2.090 (1.80, 2.30) mmol/L, P = 0.002], higher TC levels [6.635 (5.60, 7.10) mmol/L vs. 4.830 (4.00, 5.40) mmol/L, P<0.001], higher LDL-C levels [4.440 (3.90, 5.20) mmol/L vs. 2.820 (2.40, 3.30) mmol/L, P<0.001], and higher Apo B levels [1.600 (1.30, 1.80) g/L vs. 0.910 (0.70, 1.10) g/L, P<0.001]. There were no differences in HDL-C and Apo A levels between the two groups (all P>0.05).
Figure 2.
Comparison of blood lipid parameters and apolipoprotein between the FH and non-FH group. A. TG: Triglycerides; B. TC: Total Cholesterol; C. LDL-C: Low-Density Lipoprotein Cholesterol; D. HDL-C: High-Density Lipoprotein Cholesterol; E. Apo A1: Apolipoprotein A1; F. Apo B: Apolipoprotein B. **P<0.01, ***P<0.001, ns: no significant difference.
Comparison of echocardiographic parameters between the HF and non-HF group
Echocardiographic parameters are important for assessing the cardiac structure and function. LVd quantifies left ventricular volume, while LVEF is a key indicator for assessing left ventricular systolic function. As shown in Table 4, there were no differences in LVd and LVEF between the two groups (all P>0.05). The results indicate no significant changes in cardiac structure and systolic function between the HF and non-HF group. See Figures 3, 4.
Table 4.
Comparison of echocardiographic parameters between the FH and non-FH group
| Group | LVd (mm) | LVEF (%) |
|---|---|---|
| FH Group (n = 22) | 47.000 (42.00, 49.00) | 62.000 (57.00, 65.00) |
| Non-FH Group (n = 145) | 46.000 (43.00, 50.50) | 61.000 (57.50, 64.00) |
| Z | -0.187 | -0.396 |
| P | 0.851 | 0.692 |
LVd: left ventricular end-diastolic diameter; LVEF: Left ventricular ejection fraction.
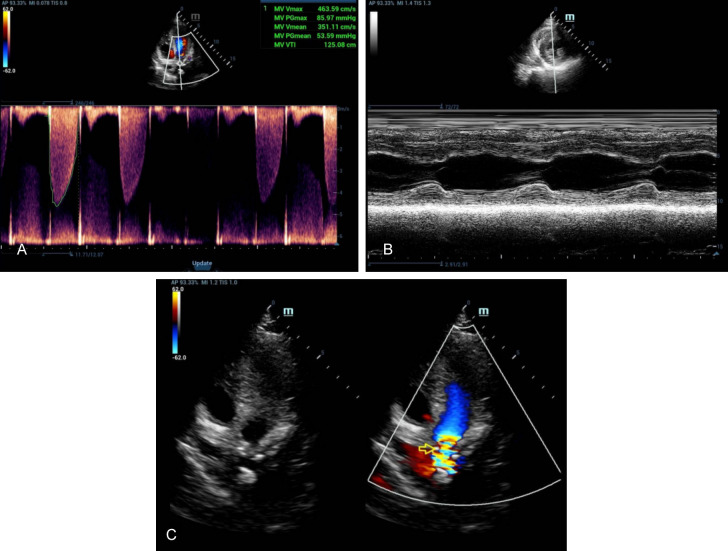
Figure 3.
Ultrasound reveals complete IVS and no PE in the case of IAS. A. Tissue Doppler imaging: Mitral annular septal tissue movement velocity: S’: 65 mm/s, E’: 74 mm/s, A’: 72 mm/s. B. Left atrial enlargement, MV focal thickening, ventricular septal thickening, suggesting AS as the cause, MI (moderate); TI (mild); AI (mild). C. The left ventricular systolic function is normal, and the diastolic function is normal. IVS: inter ventricular septum; IAS: atrial septum; AS: aortic stenosis; MI: mitral insufficiency; TI: tricuspid incompetence; AI: aortic incompetence.
Figure 4.
Ultrasound reveals complete IVS, no PEMV thickening, reverse movement of bimodal, and the anterior and posterior lobes with normal opening as well as normal AV thickness and opening in the case of IAS. A. Tissue Doppler imaging: Tissue Doppler imaging: Mitral annulus septal lateral tissue motion velocity: S: 102 mm/s, E: 88 mm/s, A: 110 mm/s. B. Consider conforming to ultrasound changes in coronary heart disease. C. Left heart enlargement; MI (moderate), TI (mild), decreased left ventricular systolic function and decreased diastolic function. IVS: inter ventricular septum; IAS: atrial septum; AS: aortic stenosis; MI: mitral insufficiency; TI: tricuspid incompetence; AI: aortic incompetence.
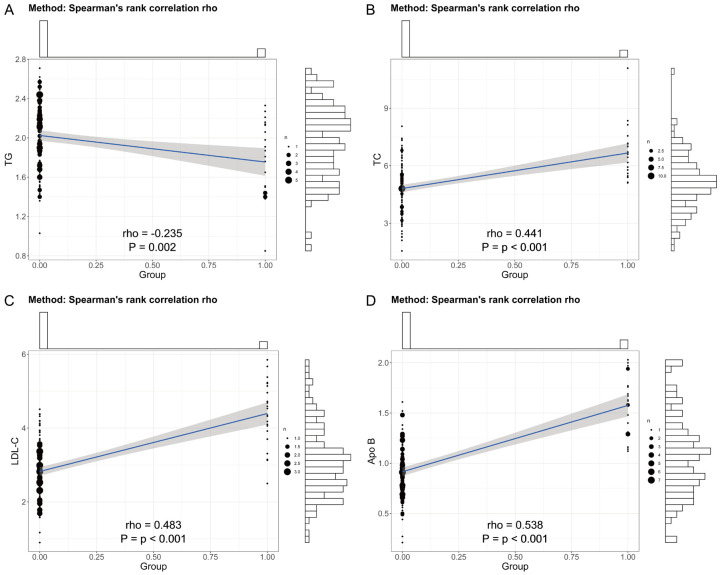
Correlation between FH and TG, TC, LDL-C, and Apo B levels
The correlation between FH and TG, TC, LDL-C, and Apo B levels can provide important evidence for the differential diagnosis of FH and hold potential value in assessing cardiovascular disease risk. As shown in Table 5 and Figure 5, there were correlations between FH and TG, TC, LDL-C, and Apo B levels (r1 = -0.235; r2 = 0.441; r3 = 0.483; r4 = 0.538). The study results indicate that TG levels were negatively correlated with FH, while TC, LDL-C, and Apo B levels were positively correlated with FH.
Table 5.
Correlation between FH and levels of TG, TC, LDL-C, and Apo B
| Indicator | FH | |
|---|---|---|
|
| ||
| r | P | |
| TG | -0.235 | <0.001 |
| TC | 0.441 | <0.001 |
| LDL-C | 0.483 | <0.001 |
| Apo B | 0.538 | <0.001 |
TG: Triglycerides; TC: Total Cholesterol; LDL-C: Low-Density Lipoprotein Cholesterol; Apo B: Apolipoprotein B.
Figure 5.
Correlation between FH and TG, TC, LDL-C and Apo B levels. A. TG: Triglycerides; B. TC: Total Cholesterol; C. LDL-C: Low-Density Lipoprotein Cholesterol; D. Apo B: Apolipoprotein B.
Logistic regression analysis
As shown in Table 6, the OR values for TC (OR = 0.893, 95% CI: 0.322-1.323; P<0.001), LDL-C (OR = 0.642, 95% CI: 0.081-0.968; P<0.001), and Apo B (OR = 0.699, 95% CI: 0.045-1.574; P<0.001) were all less than 1, indicating their protective effect on the risk of developing FH. The OR value of TG (OR = 2.104, 95% CI: 1.024-2.392; P = 0.001) was greater than 1, highlighting their role in increasing the risk of FH. These results underscore the significant influence of lipid status on FH and confirm that these lipid parameters are independent influencing factors for the development of FH.
Table 6.
Logistic regression analysis
| Indicator | S.E. | Wald | OR | 95% CI | P |
|---|---|---|---|---|---|
| TG | 0.699 | 3.232 | 2.104 | 1.024-2.392 | 0.001 |
| TC | 0.273 | 4.884 | 0.893 | 0.322-1.323 | <0.001 |
| LDL-C | 0.495 | 5.308 | 0.642 | 0.081-0.968 | <0.001 |
| Apo B | 1.653 | 5.076 | 0.699 | 0.045-1.574 | <0.001 |
TG: Triglycerides; TC: Total Cholesterol; LDL-C: Low-Density Lipoprotein Cholesterol; Apo B: Apolipoprotein B.
Discussion
Atherosclerosis (AS) serves as the main pathological basis for coronary artery disease (CAD), cerebrovascular accidents, and peripheral vascular diseases [15]. Familial hypercholesterolemia (FH), characterized by significantly elevated levels of TC and LDL-C, can prompt early-onset AS and consequently accelerate the occurrence of early-onset CAD [16]. The trend towards a younger age at CAD onset is a significant characteristic threatening public health [17]. Conducting genetic testing for early-onset CAD patients is of paramount importance in the early detection and pharmaceutical treatment of FH to prevent the onset and progression of CAD [18]. Several studies have suggested that the Hakka population in Meizhou has relatively unique genetic characteristics [19,20]. However, there have been no reports on the correlations of epidemiological characteristics and FH in early-onset CAD patients of Hakka population. Therefore, considering the limitations of genetic diagnostic applications, exploring the correlation between early-onset coronary artery disease and FH in the Hakka population of Meizhou, Guangdong, can effectively fill the research gap in the epidemiological characteristics and the diagnosis and treatment of early-onset CAD patients with FH.
This study revealed that among the 167 included early-onset CAD Hakka patients, 22 patients had FH, resulting in an FH incidence rate of 13.17%, with only 1 case being homozygous for FH and 21 cases being heterozygous for FH, mainly with LDL-R gene mutations. A meta-analysis involving 11 million participants in 2020 indicated that the incidence rate of FH in the general population was approximately 0.32%, around 0.19% in the Asian region, and the incidence rate of FH in CAD patients was approximately 3.2%, which increased to 6.7% in early-onset CAD patients, lower than the findings of this study [21]. Another survey indicated that among 285 CAD patients with LDL-C levels ≥160 mg/dL, the prevalence of FH was as high as 14.4%, exceeding the results of this study, and FH mutation carriers were more likely to have early-onset CAD [22]. Genetic mutations are important factors in the pathogenesis of FH. Loss-of-function mutations in the LDL-R gene, Apo B gene mutations, and gain-of-function mutations in the PCSK9 gene are closely associated with FH, leading to impairment of low-density lipoprotein (LDL) receptor function, Apo B defects, impaired binding of LDL to receptors, and PCSK9 gain-of-function mutations causing LDL receptor degradation, exacerbating the early onset of AS and showing high expression levels [23,24]. Meizhou is located in the northeastern mountainous region of Guangdong, with a concentration of Hakka population and relatively low population mobility, resulting in fewer introductions of foreign genes in rural areas [25]. The genetic background is relatively homogeneous, leading to a relatively high incidence of FH in the Hakka population [26]. The clinical phenotypes and mutation genotypes detected in the FH cases are consistent with the reported pathogenic gene types of FH both domestically and abroad [27]. However, there are still some FH patients with undetectable genetic mutation sites and unclear clinical relevance, necessitating further improvement of genetic mutation detection technology to provide more accurate diagnostic evidence for clinical practice.
The FH group exhibited lower TG levels and higher levels of TC, LDL-C, and Apo B than the non-FH group. Furthermore, TG levels were inversely correlated with FH, while TC, LDL-C, and Apo B levels were positively associated with FH. These variations in levels hold significant value in clinically predicting the risk of developing FH in CAD patients. Previous study proposed that lipids, as a category of organic macromolecules in the body, may be involved in the biological mechanisms of various diseases, potentially affecting endothelial cells and influencing the production, release, and biological functions of nitric oxide, thus triggering AS [28]. It may also exacerbate smooth muscle cell hypertrophy, collagen deposition, renal capillary injury, sympathetic nervous system hyperactivity, and insulin resistance, thereby accelerating AS progression [29]. Research suggests that TG expression levels are closely related to lipid disorders, where high TC levels and low TG levels of lipoprotein remnants can penetrate the arterial intima, selectively binding to the connective tissue matrix such as collagen and elastin, and be cleared by subendothelial resident macrophages, inducing endothelial dysfunction, promoting foam cell and plaque formation, and accelerating AS disease progression [30,31]. Multiple studies have established a close association between LDL-C and the occurrence and development of AS and cardiovascular disease (CVD) [32,33]. LDL-C can transport TC from peripheral tissues to the liver circulation, inhibit the expression of endothelial adhesion molecules and the recruitment of monocytes within arterial walls, exerting antioxidant effects, suppressing vascular inflammation and thrombosis while regulating vascular function [34]. However, high levels of LDL-C may impair cardiovascular endothelial function and be engulfed by subendothelial macrophages, forming oxidized low-density lipoprotein and depositing it in vessel walls to cause stenosis and thereby inducing the onset of cardiovascular diseases [35]. It has been found that increased levels of Apo-B in plasma are directly related to the development of CAD [36]. Besides, Apo-B may not only be a better predictor of risk but also a better monitor of therapy than LDL-C alone [37].
In several clinical conditions, a series of lipid markers such as TC, LDL-c, HDL-c, and non-HDL-c have been utilized in predicting the risk of CVD. In addition, non-HDL-c and HDL-c can predict the severity of coronary artery lesions. Non-HDL-c indicates total cholesterol within all the apolipoprotein B (Apo B) particles, including LDL-c and remnant cholesterol. For FH patients with abnormal blood lipids and accompanying cardiovascular metabolic risk factors, the risk assessment role of the single protein molecule Apo B is superior to TC and LDL-C, making it an ideal indicator for assessing CAD risk in FH patients. It is deemed to hold important value in predicting clinical prognosis, similar to the results of this study. Furthermore, it has been found that the Apo B/Apo A1 ratio can serve as a risk marker for predicting cardiovascular diseases [38]. The concentration of Apo B is an intuitive indicator reflecting AS risk, whereas the expression level of Apo A in serum represents its potential in resisting AS. The higher the Apo B/Apo A1 ratio, the greater the risk of AS occurrence. In addition, this study found no significant differences in biochemical indicator levels and echocardiography results between FH patients and non-FH patients, including fasting plasma glucose (FPG), uric acid (UA), creatinine (Cr), urea nitrogen (UN), B-type natriuretic peptide, high-density lipoprotein cholesterol (HDL-C), apolipoprotein A1 (Apo A1), left ventricular end-diastolic dimension (LVd), and left ventricular ejection fraction (LVEF). This suggests that the occurrence of FH in early-onset CAD patients is not significantly correlated with blood sugar, renal function, HDL-C, Apo A1 levels, cardiac structure, or left ventricular systolic function. Genetic pathogenic mutation detection is the gold standard for diagnosing FH, but whole-genome sequencing and whole-exome sequencing are expensive, time-consuming, and not yet widely practiced in clinical settings domestically [39]. In contrast, lipid testing is more cost-effective and simpler, and the combined use of commonly used lipid indicators such as TC, TG, LDL-C, in conjunction with Apo B and the Apo B/Apo A1 ratio, holds promise as an ideal indicator for differentiating and diagnosing FH in early-onset CAD patients.
The present study has several limitations. We only included early-onset CAD patients from the Hakka population in the Meizhou region, which limits the geographical scope and uniqueness of the genetic background of the samples. Additionally, as this was a retrospective study, it was unable to completely eliminate potential confounding factors and information bias. Therefore, we made efforts to ensure the completeness and diversity of the patient data collection to ensure comparability between the two groups. When choosing the observational indicators and interpreting the research results, we also considered the relevant indicators and the aforementioned limitations in the evaluation of the research results to ensure the objectivity of the conclusions. Despite these limitations, this study effectively fills the gap in the epidemiological characteristics of FH in early-onset CAD patients in the Hakka population in Meizhou, Guangdong. The aim was to explore the correlation between early-onset CAD and FH with the goal of providing early diagnosis and prevention strategies for clinical practice.
Conclusion
The incidence of FH is higher in early-onset CAD patients in the Hakka population in Meizhou, with heterozygous and LDL-R gene variations being predominant. FH patients showed decreased TG levels and increased TC, LDL-C, and Apo B levels, and these parameters were correlated with FH, demonstrating their potential value in clinically differentiating and diagnosing FH in CAD patients. This study provides a theoretical basis for the early screening and diagnosis of FH among early-onset CAD patients in the Hakka population in Meizhou, Guangdong. Subsequent efforts should focus on conducting large-sample, multicenter, randomized controlled trials to further validate the effectiveness of this research approach.
Acknowledgements
This study was supported by the Fourth Batch of Scientific Research and Cultivation Projects of Meizhou People’s Hospital in 2022.
Disclosure of conflict of interest
None.
References
- 1.Ziaee S, Hosseindokht M, Cheraghi S, Pourgholi L, Ahmadi A, Sadeghian S, Abbasi SH, Davarpasand T, Boroumand M. Predictive inflammation-related microRNAs for cardiovascular events following early-onset coronary artery disease. Arch Med Res. 2021;52:69–75. doi: 10.1016/j.arcmed.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Zheng PF, Chen LZ, Pan HW, Liu P, Zheng ZF. Effects of USF1 SNPs and SNP-environment interactions on serum lipid profiles and the risk of early-onset coronary artery disease in the Chinese population. Front Cardiovasc Med. 2022;9:882728. doi: 10.3389/fcvm.2022.882728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang SY, Xuan C, Wang Y, Zhang SQ, Li H, He GW, Tian QW. Association between ALMS 1 variants and early-onset coronary artery disease: a case-control study in Chinese population. Biosci Rep. 2020;40:BSR20193637. doi: 10.1042/BSR20193637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao K, Dai Y, Shen J, Wang Y, Yang H, Wu R, Liao Q, Wu H, Fang X, Shali S, Xu L, Hao M, Lin C, Sun Z, Liu Y, Li M, Wang Z, Gao Q, Zhang S, Li C, Gao W, Ge L, Zou Y, Sun A, Qian J, Jin L, Hong S, Zheng Y, Ge J. Exome sequencing identifies rare mutations of LDLR and QTRT1 conferring risk for early-onset coronary artery disease in Chinese. Natl Sci Rev. 2022;9:nwac102. doi: 10.1093/nsr/nwac102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xuan C, Tian QW, Li H, Guo JJ, He GW, Lun LM. Serum fatty acids profile and association with early-onset coronary artery disease. Ther Adv Chronic Dis. 2021;12:20406223211033102. doi: 10.1177/20406223211033102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xuan C, Li J, Liu RH, Guo JJ, Zhao C, Zhou TT, Wang Y, He GW, Lun LM. Association between serum gamma-glutamyltransferase and early-onset coronary artery disease: a retrospective case-control study. Ann Med. 2023;55:2289606. doi: 10.1080/07853890.2023.2289606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xuan C, Li H, Tian QW, Guo JJ, He GW, Lun LM, Wang Q. Quantitative assessment of serum amino acids and association with early-onset coronary artery disease. Clin Interv Aging. 2021;16:465–474. doi: 10.2147/CIA.S298743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu TT, Zheng YY, Ma X, Xiu WJ, Yang HT, Hou XG, Yang Y, Chen Y, Ma YT, Xie X. Mutated CYP17A1 promotes atherosclerosis and early-onset coronary artery disease. Cell Commun Signal. 2023;21:155. doi: 10.1186/s12964-023-01061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Li H, Wang Y, Zhao C, Tian QW, Wang Q, He GW, Lun LM, Xuan C. Hematological parameters and early-onset coronary artery disease: a retrospective case-control study based on 3366 participants. Ther Adv Chronic Dis. 2023;14:20406223221142670. doi: 10.1177/20406223221142670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkataraman P, Stanton T, Liew D, Huynh Q, Nicholls SJ, Mitchell GK, Watts GF, Tonkin AM, Marwick TH. Coronary artery calcium scoring in cardiovascular risk assessment of people with family histories of early onset coronary artery disease. Med J Aust. 2020;213:170–177. doi: 10.5694/mja2.50702. [DOI] [PubMed] [Google Scholar]
- 11.Tudurachi BS, Anghel L, Tudurachi A, Sascău RA, Stătescu C. Assessment of inflammatory hematological ratios (NLR, PLR, MLR, LMR and monocyte/HDL-cholesterol ratio) in acute myocardial infarction and particularities in young patients. Int J Mol Sci. 2023;24:14378. doi: 10.3390/ijms241814378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu G, Fang Z, Zhao Y, Wu Q. Association of +138I/D and Lys198Asn polymorphisms in the endothelin-1 gene with early onset of coronary artery disease among the Chinese Han population. Med Sci Monit. 2020;26:e921542. doi: 10.12659/MSM.921542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam To B, Roy R, Melikian N, Gaughran FP, O’Gallagher K. Coronary artery disease in patients with severe mental illness. Interv Cardiol. 2023;18:e16. doi: 10.15420/icr.2022.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takashima A, Yagi S, Yamaguchi K, Kurahashi K, Kojima Y, Zheng R, Ise T, Kusunose K, Yoshida S, Yamada H, Soeki T, Wakatsuki T, Aihara KI, Akaike M, Sata M. Congenital hypogonadotropic hypogonadism with early-onset coronary artery disease. J Med Invest. 2021;68:189–191. doi: 10.2152/jmi.68.189. [DOI] [PubMed] [Google Scholar]
- 15.Stone NJ, Smith SC Jr, Orringer CE, Rigotti NA, Navar AM, Khan SS, Jones DW, Goldberg R, Mora S, Blaha M, Pencina MJ, Grundy SM. Managing atherosclerotic cardiovascular risk in young adults: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:819–836. doi: 10.1016/j.jacc.2021.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro D, Lee K, Asmussen J, Bourquard T, Lichtarge O. Evolutionary action-machine learning model identifies candidate genes associated with early-onset coronary artery disease. J Am Heart Assoc. 2023;12:e029103. doi: 10.1161/JAHA.122.029103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shali S, Luo L, Yao K, Sun X, Wu H, Zhang S, Xu L, Gao W, Li J, Qian J, Zheng Y, Dai Y, Ge J the GRAND investigators. Triglyceride-glucose index is associated with severe obstructive coronary artery disease and atherosclerotic target lesion failure among young adults. Cardiovasc Diabetol. 2023;22:283. doi: 10.1186/s12933-023-02004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pauli N, Puchałowicz K, Kuligowska A, Krzystolik A, Dziedziejko V, Safranow K, Rać M, Chlubek D, Ewa Rać M. Associations between IL-6 and echo-parameters in patients with early onset coronary artery disease. Diagnostics (Basel) 2019;9:189. doi: 10.3390/diagnostics9040189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muniyappa R, Narayanappa SBK. Disentangling dual threats: premature coronary artery disease and early-onset type 2 diabetes mellitus in South Asians. J Endocr Soc. 2023;8:bvad167. doi: 10.1210/jendso/bvad167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mousavi I, Suffredini J, Virani SS, Ballantyne C, Michos ED, Misra A, Saeed A, Jia X. Early onset atherosclerotic cardiovascular disease. Eur J Prev Cardiol. 2024 doi: 10.1093/eurjpc/zwae240. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Montarello N, Chan WPA. Coronary artery disease in women. Aust Prescr. 2022;45:193–199. doi: 10.18773/austprescr.2022.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazawa K, Ito K. Genetic analysis for coronary artery disease toward diverse populations. Front Genet. 2021;12:766485. doi: 10.3389/fgene.2021.766485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihăilă RG. Pragmatic analysis of dyslipidemia involvement in coronary artery disease: a narrative review. Curr Cardiol Rev. 2020;16:36–47. doi: 10.2174/1573403X15666190522100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menezes Fernandes R, Mota T, Costa H, Bispo J, Azevedo P, Bento D, Guedes J, Carvalho D, Marques N, Santos W, Mimoso J, de Jesus I Portuguese Registry of Acute Coronary Syndromes (ProACS) investigators. Premature acute coronary syndrome: understanding the early onset. Coron Artery Dis. 2022;33:456–464. doi: 10.1097/MCA.0000000000001141. [DOI] [PubMed] [Google Scholar]
- 25.Manolis AA, Manolis TA, Manolis AS. Early-onset or premature coronary artery disease. Curr Med Chem. 2024 doi: 10.2174/0109298673303891240528114755. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Manikpurage HD, Eslami A, Perrot N, Li Z, Couture C, Mathieu P, Bossé Y, Arsenault BJ, Thériault S. Polygenic risk score for coronary artery disease improves the prediction of early-onset myocardial infarction and mortality in men. Circ Genom Precis Med. 2021;14:e003452. doi: 10.1161/CIRCGEN.121.003452. [DOI] [PubMed] [Google Scholar]
- 27.Maino A, Sadeghian S, Mancini I, Abbasi SH, Poorhosseini H, Boroumand MA, Lotfi-Tokaldany M, Jalali A, Pagliari MT, Rosendaal FR, Peyvandi F. Opium as a risk factor for early-onset coronary artery disease: results from the Milano-Iran (MIran) study. PLoS One. 2023;18:e0283707. doi: 10.1371/journal.pone.0283707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnani G, Bricoli S, Ardissino M, Maglietta G, Nelson A, Malagoli Tagliazucchi G, Disisto C, Celli P, Ferrario M, Canosi U, Cernetti C, Negri F, Merlini PA, Tubaro M, Berzuini C, Manzalini C, Ignone G, Campana C, Moschini L, Ponte E, Pozzi R, Fetiveau R, Buratti S, Paraboschi E, Asselta R, Botti A, Tuttolomondo D, Barocelli F, Biagi A, Bonura R, Moccetti T, Crocamo A, Benatti G, Paoli G, Solinas E, Notarangelo MF, Moscarella E, Calabrò P, Duga S, Niccoli G, Ardissino D. Long-term outcomes of early-onset myocardial infarction with non-obstructive coronary artery disease (MINOCA) Int J Cardiol. 2022;354:7–13. doi: 10.1016/j.ijcard.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Lali R, Cui E, Ansarikaleibari A, Pigeyre M, Paré G. Genetics of early-onset coronary artery disease: from discovery to clinical translation. Curr Opin Cardiol. 2019;34:706–713. doi: 10.1097/HCO.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 30.Koenig SN, Sucharski HC, Jose EM, Dudley EK, Madiai F, Cavus O, Argall AD, Williams JL, Murphy NP, Keith CBR, El Refaey M, Gumina RJ, Boudoulas KD, Milks MW, Sofowora G, Smith SA, Hund TJ, Wright NT, Bradley EA, Zareba KM, Wold LE, Mazzaferri EL Jr, Mohler PJ. Inherited variants in SCARB1 cause severe early-onset coronary artery disease. Circ Res. 2021;129:296–307. doi: 10.1161/CIRCRESAHA.120.318793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kayıkçıoğlu M, Tetik Vardarlı A. How to generate unbiased data in molecular genetic studies in patients with early onset coronary artery disease or premature myocardial infarction? Turk Kardiyol Dern Ars. 2022;50:404–406. doi: 10.5543/tkda.2022.22586. [DOI] [PubMed] [Google Scholar]
- 32.Kaw K, Chattopadhyay A, Guan P, Chen J, Majumder S, Duan XY, Ma S, Zhang C, Kwartler CS, Milewicz DM. Smooth muscle α-actin missense variant promotes atherosclerosis through modulation of intracellular cholesterol in smooth muscle cells. Eur Heart J. 2023;44:2713–2726. doi: 10.1093/eurheartj/ehad373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahya Eren N, Karaca E, Şirin FB, Levent F, Gündüz C, Özdemir E, Nazlı C, Cogulu O, Ergene AO. Does microRNA profile differ in early onset coronary artery disease? Turk Kardiyol Dern Ars. 2022;50:407–414. doi: 10.5543/tkda.2022.22408. [DOI] [PubMed] [Google Scholar]
- 34.Jiao W, Wei L, Jiao F, Pjetraj D, Feng J, Wang J, Catassi C, Gatti S. Very early onset of coronary artery aneurysm in a 3-month infant with Kawasaki disease: a case report and literature review. Ital J Pediatr. 2023;49:60. doi: 10.1186/s13052-023-01478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo H, Yu X, Liu Y, Paik DT, Justesen JM, Chandy M, Jahng JWS, Zhang T, Wu W, Rwere F, Zhao SR, Pokhrel S, Shivnaraine RV, Mukherjee S, Simon DJ, Manhas A, Zhang A, Chen CH, Rivas MA, Gross ER, Mochly-Rosen D, Wu JC. SGLT2 inhibitor ameliorates endothelial dysfunction associated with the common ALDH2 alcohol flushing variant. Sci Transl Med. 2023;15:eabp9952. doi: 10.1126/scitranslmed.abp9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coto E, Pascual I, Avanzas P, Cuesta-Lavona E, Lorca R, Martín M, Vázquez-Coto D, Díaz-Corte C, Morís C, Rodríguez-Reguero J, Gómez J. IL17RA in early-onset coronary artery disease: total leukocyte transcript analysis and promoter polymorphism (rs4819554) association. Cytokine. 2020;136:155285. doi: 10.1016/j.cyto.2020.155285. [DOI] [PubMed] [Google Scholar]
- 37.Chuapakdee O, Layangkool T, Theerasuwipakorn N. Pyridostigmine-induced coronary artery spasm in early-onset myasthenia gravis: a case presentation and review of the literature. BMJ Case Rep. 2022;15:e249819. doi: 10.1136/bcr-2022-249819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Y, Nie L, Ma W, Zheng B. Early onset acute coronary artery occlusion after pembrolizumab in advanced non-small cell lung cancer: a case report. Cardiovasc Toxicol. 2021;21:683–686. doi: 10.1007/s12012-021-09664-z. [DOI] [PubMed] [Google Scholar]
- 39.Benschop L, Brouwers L, Zoet GA, Meun C, Boersma E, Budde RPJ, Fauser BCJM, de Groot CMJ, van der Schouw YT, Maas AHEM, Velthuis BK, Linstra KM, Kavousi M, Duvekot JJ, Franx A, Steegers E, van Rijn BB, Roeters van Lennep JE CREW Consortium. Early onset of coronary artery calcification in women with previous preeclampsia. Circ Cardiovasc Imaging. 2020;13:e010340. doi: 10.1161/CIRCIMAGING.119.010340. [DOI] [PubMed] [Google Scholar]