Abstract
Objective: This study aimed to explore the efficacy of neoadjuvant chemotherapy plus programmed death-1 (PD-1) inhibitor camrelizumab for the treatment of locally advanced esophageal cancer. Methods: This was a retrospective analysis. 87 patients with locally advanced esophageal cancer were included who received neoadjuvant chemotherapy plus immunotherapy between June 2018 and April 2021 in our oncology department. The postoperative clinical outcomes and varying expressions of PD-1 were evaluated in all enrolled patients. Results: The post-treatment disease control rate (DCR) was 83.91%, and the objective response rate (ORR) was 59.77%. Cancer tissues were categorized based on PD-1 expression into PD-1 negative (39 cases) and PD-1 positive (33 cases), with a PD-1 positive rate of 45.83%. Patients with PD-1-positive tumors exhibited a significantly higher ORR compared to those with PD-1-negative tumors, although DCRs did not differ significantly between the groups. The 12-month progression-free survival rate was significantly higher in PD-1-positive patients. In contrast, no significant difference was found in the 12-month overall survival rate between the two groups. The incidence of grade III adverse events was 10.34%, and no grade IV or higher adverse events were observed. Conclusion: In patients with locally advanced esophageal cancer, neoadjuvant chemotherapy plus immunotherapy demonstrates good efficacy and safety, especially for PD-1-positive patients, and significantly improves prognosis.
Keywords: Locally advanced esophageal cancer, immunotherapy, neoadjuvant chemotherapy, programmed death-1
Introduction
Esophageal cancer is a common malignant tumor of the gastrointestinal tract, with high invasiveness and lethality, and its main histologic subtypes include esophageal adenocarcinoma and squamous carcinoma [1,2]. Approximately half a million people worldwide are diagnosed with esophageal cancer each year, and it ranks eighth in global tumor incidence and sixth in mortality [3,4]. Locally advanced esophageal cancer is defined as stage IIb to IIIc esophageal cancer that invades regional lymph nodes (N1-3) without distant metastasis [5]. It is partially resectable, but the postoperative outcome is poor due to its predisposition to local infiltration and the difficulty of complete resection of the tumor lesions [6]. Research has shown that the 5-year survival rate of patients with locally advanced esophageal cancer after surgical treatment is only 20.64%-34%, and most patients develop metastases or local recurrence within 3 years after surgery [7].
Neoadjuvant chemotherapy has transformed the treatment landscape by shrinking tumors preoperatively, thereby rendering inoperable cancers treatable [8], and significantly enhancing survival by eradicating metastatic cells [9]. Furthermore, immunotherapy, particularly agents targeting programmed death-1 (PD-1), has become a cornerstone of both adjuvant postoperative therapy and first-line treatment for advanced cases of esophageal and esophagogastric junction cancer [10]. In recent years, regulators of gastrointestinal tumors, such as epigenetic modifications, have gradually received recognition, but little has been reported on the biologic functions and mechanisms of N 6-methyladenosine (m6A) in malignant tumors. A recent study has shown that the efficacy of anti-PD-1 therapy in colorectal cancer can be enhanced by inhibiting m6A methyltransferase. Research has demonstrated that immunotherapy plus chemotherapy can provide better survival benefit than chemotherapy alone for patients receiving first-line treatment for esophageal cancer [11]. A phase I trial found that camrelizumab demonstrated good antitumor activity and manageable adverse effects in patients with recurrent or metastatic esophageal squamous carcinoma who were refractory to chemotherapy, achieving an objective efficiency of 33.3% and a median progression-free survival (PFS) of 3.6 months [12]. However, data on neoadjuvant chemotherapy plus immunotherapy in patients with locally advanced esophageal cancer, particularly in the context of data from China, remain scarce.
The current study was performed to explore the efficacy of neoadjuvant chemotherapy plus PD-1 inhibitor camrelizumab for the treatment of locally advanced esophageal cancer. In addition, patients with different PD-1 expressions were compared.
Materials and methods
Ethical review
The study was approved by the Ethics Committee of the First Affiliated Hospital of USTC, and all procedures were in accordance with the Helsinki Declaration on Ethical Guidelines for Clinical Research [13]. Signed informed consent was obtained from all patients before enrollment.
Study population
We selected 87 patients with locally advanced esophageal cancer who received neoadjuvant chemotherapy plus immunotherapy between January 2017 and January 2020 in the oncology department of the First Affiliated Hospital of USTC were collected for this retrospective analysis. The inclusion criteria were as follows: 1) Patients aged 18-80 years, with complete medical data including PD-1 expression and follow-up data; 2) Patients with locally advanced esophageal cancer confirmed by histologic or cytologic examination (AJCC 8th) [14]; 3) Patients who received surgery and met the indications of neoadjuvant chemotherapy plus immunotherapy, including: Karnofsky Performance Status (KPS) score of at least 80 points, suggesting adequate physical condition to undergo surgery and handle potential risks associated with anesthesia and surgical complications [15]; patients who completed the treatment regimen and subsequent assessments, including PD-1 expression analysis. The exclusion criteria were as follows: 1) Patients with other malignant tumors or known distant metastases; 2) Patients who were pregnant or breastfeeding; 3) Patients with a history of recurrence after surgical treatment or previous immunotherapy; 4) Patients with severe insufficiency of organs, such as heart, brain, liver, or kidney; 5) Patients suffering from immune or hematologic disorders; 6) Women and men with reproductive potential who were expecting to conceive or father children.
Treatment interventions
All participants received the same treatment including neoadjuvant chemotherapy and surgical intervention.
Neoadjuvant chemotherapy: patients received 25 mg/m2 of vinorelbine through intravenous injection on days 1 and 8 of a 21-day cycle, 75 mg/m2 cisplatin through intravenous infusion on day 1 of a 21-day cycle (or 25 mg/m2 of cisplatin through intravenous infusion on days 1-4 of a 21-day cycle), and 200 mg/d of camrelizumab on day 1 of a 21-day cycle through intravenous injection. A total of 2 cycles of neoadjuvant chemotherapy were administered. The patient’s vital signs were monitored under close observation during dosing and 5 mg of tropisetron was administered through intravenous infusion to prevent gastrointestinal reactions. The treatment was discontinued if disease progression or unacceptable toxicity occurred.
Surgical interventions: Radical esophagectomy for esophageal cancer was performed 4-6 weeks after the chemotherapy, and the procedure was selected according to individual circumstances, including McKeown or Ivor Lewis esophagectomy with bilateral OD lymph node dissection and total mediastinal lymph node dissection. After surgical resection, the PD-1 expression of cancer tissues was determined by immunohistochemistry.
Detection of PD-1 expression level and follow-up data
Immunohistochemical staining was conducted to determine PD-1 expression in tumor tissue. Tissue samples were fixed in 4% neutral formaldehyde solution, embedded in paraffin, and sectioned. After deparaffinization, endogenous peroxidase activity was blocked, and antigen retrieval was performed under high pressure. Sections were incubated with a primary antibody for 1 hour at room temperature, followed by a secondary antibody for 30 min. Color development was achieved using DAB and monitored microscopically. Hematoxylin was applied for counterstaining. Sections were dehydrated and mounted. PD-1 expression was evaluated using a mouse anti-human monoclonal antibody (Catalog: MAB-0734, Fuzhou Maixin Biotechnology Development Co.). Positive staining in cytoplasm and cell membrane indicated PD-1 positivity. Each section was divided into quadrants for counting positive cells under high magnification, and the average positive expression rate of each quadrant was calculated separately to obtain the PD-1 positive expression rate of the tumor. PD-1 positivity was defined as ≥ 5% positive cells, based on established literature [15]. Appropriate negative and positive controls were included in each batch of immunohistochemistry.
The patients were followed up until April 2023, with data on PFS and overall survival (OS) collected through telephone and outpatient reexamination. PFS was defined as the time from treatment initiation to tumor progression or death (any cause), and OS was defined as the time from the treatment initiation to death (any cause).
Outcome measures
Adverse reactions were recorded within 1 month after surgery, and all treatment-related adverse events (AEs) were defined and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) [16]. In terms of efficacy, all patients were evaluated by imaging 6 weeks after the surgery, and responses were classified as complete remission (CR), partial remission (PR), stable disease (SD), and progressed disease (PD). Objective remission rate (ORR) = CR + PR, and disease control rate (DCR) = CR + PR + SD. In addition, patients were observed for their postoperative OS and PFS. The baseline data were compared between PD-1 positive and negative groups.
Statistical analysis
Survival analyses, including median overall survival, progression-free survival, and duration of response, were performed using the Kaplan-Meier method. A stratified Cox proportional hazards regression model was constructed to estimate hazard ratios (HRs) and 95% confidence interval (CI). Survival curves were plotted using the survival package in R. Categorical data were presented as frequencies and percentages (n, %) and analyzed using the chi-square test. A p-value of less than 0.05 was considered significant for all analyses.
Results
Patient characteristics
The study included 87 participants: 56 males and 31 females. Age range included 31 patients aged ≤ 60 years and 56 aged > 60 years. Regarding tumor size, 22 patients had a maximum tumor diameter ≤ 3 cm, and 65 had a diameter > 3 cm. By tumor node metastasis (TNM) stage, there were 17 patients with stage II, 27 with stage IIIA, and 43 with stage IIIB. Tumor locations included 14 in the cervical segment, 13 in the upper segment, 29 in the middle segment, and 31 in the lower segment. There were 39 cases with high PD-1 expression and 48 with low expression (Table 1).
Table 1.
Patient characteristics
| Index | Number (n=87) | % |
|---|---|---|
| Sex | ||
| Male | 56 | 64.37 |
| Female | 31 | 35.63 |
| Age | ||
| ≤ 60 years old | 31 | 35.63 |
| > 60 years old | 56 | 64.37 |
| Largest diameter of lesion | ||
| ≤ 3 cm | 22 | 25.29 |
| > 3 cm | 65 | 74.71 |
| TNM stage | ||
| Stage II | 17 | 19.54 |
| Stage IIIA | 27 | 31.03 |
| Stage IIIB | 43 | 49.43 |
| Origin location | ||
| Neck segment | 14 | 16.09 |
| Upper segment | 13 | 14.94 |
| Middle segment | 29 | 33.33 |
| Lower segment | 31 | 35.63 |
| PD-1 status | ||
| Positive | 39 | 44.83 |
| Negative | 48 | 55.17 |
Note: TNM = Tumor Node Metastasis, PD-1 = Programmed death-1.
Relationship between PD-1 and clinical data
Patients were categorized based on PD-1 status into PD-1 negative (n=39) and PD-1 positive (n=48) groups. There were no significant differences in clinical data between the two groups (P > 0.05, Table 2).
Table 2.
The relationship between PD-1 and baseline data of patients
| Index | Number (n=87) | PD-1 Negative (n=39) | PD-1 Positive (n=48) | x2 value | P value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 56 | 27 | 29 | 0.728 | 0.393 |
| Female | 31 | 12 | 19 | ||
| Age | |||||
| ≤ 60 years old | 31 | 16 | 15 | 0.896 | 0.343 |
| > 60 years old | 56 | 23 | 33 | ||
| Largest diameter of lesion | |||||
| ≤ 3 cm | 22 | 8 | 14 | 0.852 | 0.355 |
| > 3 cm | 65 | 31 | 34 | ||
| TNM stage | |||||
| Stage II | 17 | 7 | 12 | ||
| Stage IIIA | 27 | 15 | 12 | 1.934 | 0.380 |
| Stage IIIB | 43 | 17 | 24 | ||
| Origin location | |||||
| Neck segment | 14 | 7 | 7 | ||
| Upper segment | 13 | 6 | 7 | 5.519 | 0.137 |
| Middle segment | 29 | 17 | 12 | ||
| Lower segment | 31 | 9 | 22 |
Note: TNM = Tumor Node Metastasis, PD-1 = Programmed death-1.
Short-term clinical efficacy
Among the 87 patients, 12 achieved CR, 40 PR, 21 SD, and 14 PD, with a DCR of 83.91% and an ORR of 59.77%. When stratified by PD-1 expression, PD-1 negative patients had a DCR of 81.20% and an ORR of 50.00%, while PD-1 positive patients had a DCR of 87.18%, and an ORR of 71.19%. The ORR was significantly higher in PD-1-positive patients compared to PD-1-negative patients, although no significant differences were observed in DCR (Table 3).
Table 3.
Short-term clinical efficacy
| n | CR | PR | SD | PD | DCR | ORR | |
|---|---|---|---|---|---|---|---|
| Total | 87 | 12 | 40 | 21 | 14 | 83.91% | 59.77% |
| PD-1 Negative | 48 | 5 | 19 | 15 | 9 | 81.25% | 50.00% |
| PD-1 Positive | 39 | 7 | 21 | 6 | 5 | 87.18% | 71.79% |
| χ2 | 0.293 | 4.251 | |||||
| P | 0.588 | 0.039 |
Note: PD-1 = Programmed death-1, CR = Complete Remission, PR = Partial Remission, SD = Stable Disease, PD = Progressed Disease, DCR = Disease Control Rate, ORR = Objective Remission Rate.
A total of 72 patients were followed for more than 12 months, during which 13 died and 36 experienced disease progression. The 12-month OS rate was 81.94% (59/72) and the 12-month PFS rate was 50.00% (36/72). Among the PD-1 positive patients (n=33), 3 died and 11 experienced PD, resulting in a 12-month OS rate of 90.91% (30/33) and a 12-month PFS rate of 66.67% (22/33). Among PD-1 negative patients (n=39), 10 died and 25 experienced PD, with a 12-month OS rate of 74.36% (29/39) and a 12-month PFS rate of 35.90% (14/39). The 12-month PFS rate was significantly higher in PD-1-positive patients, but there was no significant difference in the 12-month OS rate between the two groups (Table 4).
Table 4.
PFS and OS after 12 months
| n | 12 months OS rate | 12 months PFS rate | |
|---|---|---|---|
| Total | 72 | 13 (18.06%) | 36 (50.00%) |
| PD-1 Negative | 39 | 10 (25.64%) | 25 (64.10%) |
| PD-1 Positive | 33 | 3 (9.09%) | 11 (33.33%) |
| χ2 | 3.309 | 6.769 | |
| P | 0.069 | 0.009 |
Note: PD-1 = Programmed death-1, OS = Overall Survival, PFS = Progression-free survival.
Long-term clinical efficacy
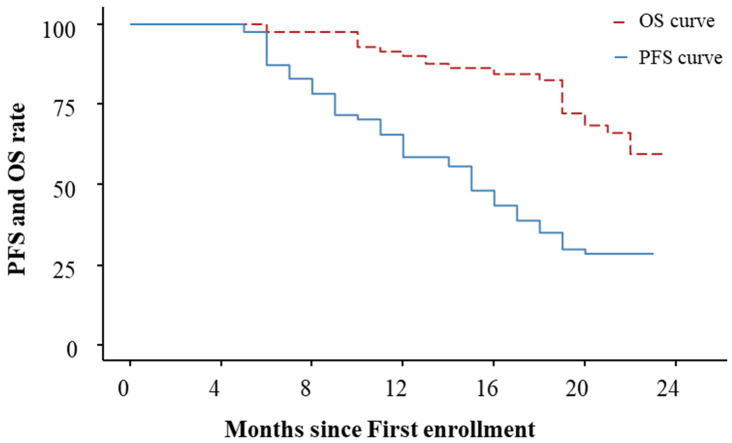
The median follow-up for all participants was 18 months, with an interquartile range of 13 to 21 months. The median PFS was 15 months (95% CI: 12 to 18) and the median OS was not calculable (NC) (95% CI: 22 to NC) in all patients (Figure 1).
Figure 1.
PFS and OS curves.
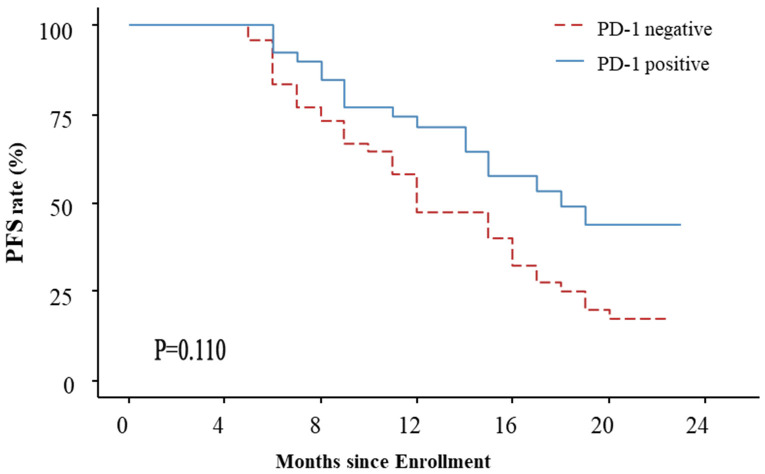
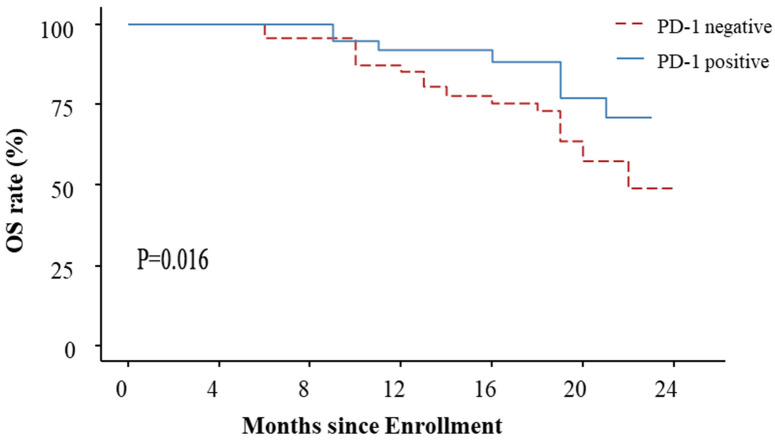
For PD-1 positive patients, the median follow-up was 18.5 months (interquartile range of 12.75 to 22 months), with a median PFS of 18 months (95% CI: 15-NC) and a median OS that was NC. For PD-1 negative patients, the median follow-up was 17.5 months (interquartile range of 13.25 to 21 months), with a median PFS of 12 months (95% CI: 11 to 16), and a median OS of 22 months (95% CI: 20 to NC). There was no significant difference in PFS between the PD-1 negative and positive groups (HR=1.992, 95% CI: 0.9148-4.336, P=0.110). However, OS was significantly longer in PD-1-positive patients compared to PD-1-negative patients (HR=1.921, 95% CI: 1.132-3.260, P=0.016). See Figures 2 and 3.
Figure 2.
PFS curve of PD-1 negative and positive patients.
Figure 3.
OS curve of PD-1 negative and positive patients.
Adverse events
No grade IV or higher adverse reactions were observed. The incidence of grade I AEs was 82.76% (72/87), grade II AEs was 27.59% (24/87), and grade III AEs was 10.34% (9/87) (Table 5).
Table 5.
Occurrence of adverse events
| Grade I | Grade II | Grade III | Grade IV | |
|---|---|---|---|---|
| Total | 72 | 24 | 9 | 0 |
| Decreased white blood cell count | 21 | 7 | 4 | 0 |
| Decreased platelet count | 11 | 7 | 2 | 0 |
| Anemias | 14 | 4 | 0 | 0 |
| Nausea and vomiting | 8 | 2 | 1 | 0 |
| Impaired liver function | 22 | 11 | 3 | 0 |
| Impaired kidney function | 12 | 4 | 2 | 0 |
| Hypothyroidism | 14 | 5 | 1 | 0 |
| Rash | 11 | 7 | 0 | 0 |
Discussion
Neoadjuvant chemotherapy combined with immunotherapy yielded promising results here for the treatment of locally advanced esophageal cancer, achieving a DCR of 81.20% and an ORR of 50.00%. Additionally, the 12-month OS and PFS rates were 81.94% and 50.00%, respectively. Despite advancements in multidisciplinary treatments such as surgery, radiotherapy, and chemotherapy, the prognosis for esophageal cancer patients remains unsatisfactory [17]. Neoadjuvant therapy improves the likelihood of complete surgical resection (R0) by reducing tumor volume, size, and the number of affected lymph nodes preoperatively, and decreases recurrence rates by targeting micrometastases. Immune checkpoints play a crucial role by boosting the tumor-killing activity of immune cells through mitigating the body’s inhibitory effects on them. Recently, therapies involving immune checkpoint inhibitors have emerged as a focus of research in the treatment of solid tumors, achieving significant breakthroughs in esophageal cancer [18]. These inhibitors are now approved for use in first-line, second-line, and postoperative adjuvant therapy in esophageal cancer. However, the specific benefits of neoadjuvant immunotherapy in esophageal cancer remain less explored [19].
Adding immunotherapy to neoadjuvant chemotherapy and surgery has proven effective to enhance tumor removal and patient survival in locally advanced esophageal cancer. However, surgical R0 resection rates remain suboptimal, and many patients still experience a recurrence pattern predominantly characterized by distant metastases. A study by Shapiro et al. suggested that this pattern of recurrence may be attributed to an insufficient intensity of systemic therapy [20]. Therefore, integrating systemic immunotherapy may improve outcomes following traditional neoadjuvant therapy, which is often plagued by high rates of distant metastases. The KEYNOTE-590 study, a pioneering phase III clinical trial of first-line immunotherapy for locally advanced or metastatic esophageal cancer, reported an ORR of 45% in patients receiving a combination of pembrolizumab and chemotherapy [21]. These findings align closely with those of our study. Camrelizumab, a high-affinity, selective IgG4-type PD-1 monoclonal antibody that targets T cells, B lymphocytes, natural killer cells, and dendritic cells expressing CD4+ and CD8+, disrupts the interaction between these immune cells and cancer cells. By blocking PD-1 mediated T-cell immunosuppression, camrelizumab exerts significant antitumor effects [22]. It has been shown to improve R0 resection rate, and, when combined with chemotherapy, to significantly extend median survival in patients with locally advanced esophageal cancer [22].
With growing interest in racial and ethnic disparities in healthcare, research indicates that different races and populations may respond differently to specific treatment regimens. For example, Taylor [23] and Wright [24] discussed the significance of racial and ethnic considerations in clinical trials, highlighting findings from studies like the African-American Heart Failure Trial (A-HeFT), the African-American Study of Kidney Disease and Hypertension (AASK), and the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). These studies underscore existing disparities within the U.S. healthcare system and the necessity of addressing them. Additionally, Owusu et al. [25] evaluated the effectiveness of mixed care therapy across different races and ethnicities, finding it generally effective, though with some variation among groups. Based on these insights, it is imperative to undertake studies within China using real data to better understand possible treatment response variations across different racial and ethnic backgrounds.
According to a multicenter, single-arm, phase II trial, the combination of camrelizumab and chemotherapy as neoadjuvant therapy for locally advanced esophageal squamous carcinoma showed promising results: 91.7% of patients completed the full 2 cycles of treatment, achieving a 98.0% R0 resection rate, with 39.2% of patients reaching pathologic complete remission of both the primary tumor and lymph nodes, although 9.8% had complete remission in the primary tumor but residual lesions in the lymph nodes [26]. In our study, PD-1 expression in resected cancer tissues was used to classify patients into PD-1 positive and PD-1 negative groups. PD-1 positive patients (45.83%) demonstrated significantly higher 12-month PFS rates and longer OS compared to PD-1 negative patients. Approximately 40% of esophageal cancer patients exhibit PD-1 overexpression, associated with poorer OS. PD-1 inhibitors block the upregulation of PD-1 in tumor cells, enhancing the T cells’ ability to kill esophageal cancer cells [27,28]. Our findings indicate superior efficacy in PD-1 positive patients, suggesting that neoadjuvant chemotherapy combined with immunotherapy yields better outcomes for this group.
This study has several limitations. First, the follow-up period was relatively short, with a median follow-up of 18 months and a maximum of only 24 months, insufficient to ascertain the long-term efficacy of immunotherapy in patients with locally advanced esophageal cancer. Second, this retrospective study lacked a control group, which means comparisons between the efficacy of immunotherapy and conventional chemotherapy could not be made.
With the growing interest in the prognosis of esophageal cancer patients, several predictive tools and models have been developed. These tools aim to predict patient survival and disease progression by considering factors such as clinical characteristics, biomarkers, and immune expression, including PD-1. In our study, we specifically examined PD-1 expression and discovered that patients with positive PD-1 exhibited a significantly better prognosis compared to PD-1-negative patients. If simulations are considered in future studies, we would recommend to use predictive models based on PD-1 expression and other relevant clinical characteristics. By combining these data, we can better understand the relationship between PD-1 expression and the prognosis of esophageal cancer and provide patients with more personalized treatment recommendations.
Conclusion
In patients with locally advanced esophageal cancer, neoadjuvant chemotherapy combined with immunotherapy demonstrates good efficacy and safety, as well as significant improvement in patient prognosis, especially for PD-1-positive patients.
Disclosure of conflict of interest
None.
References
- 1.Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg. 2018;41:210–215. doi: 10.1016/j.asjsur.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933–7943. doi: 10.3748/wjg.v21.i26.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bollschweiler E, Plum P, Monig SP, Holscher AH. Current and future treatment options for esophageal cancer in the elderly. Expert Opin Pharmacother. 2017;18:1001–1010. doi: 10.1080/14656566.2017.1334764. [DOI] [PubMed] [Google Scholar]
- 4.Jayaprakasam VS, Yeh R, Ku GY, Petkovska I, Fuqua JL 3rd, Gollub M, Paroder V. Role of imaging in esophageal cancer management in 2020: update for radiologists. AJR Am J Roentgenol. 2020;215:1072–1084. doi: 10.2214/AJR.20.22791. [DOI] [PubMed] [Google Scholar]
- 5.Thakur B, Devkota M, Chaudhary M. Management of locally advanced esophageal cancer. JNMA J Nepal Med Assoc. 2021;59:409–416. doi: 10.31729/jnma.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borggreve AS, Kingma BF, Domrachev SA, Koshkin MA, Ruurda JP, van Hillegersberg R, Takeda FR, Goense L. Surgical treatment of esophageal cancer in the era of multimodality management. Ann N Y Acad Sci. 2018;1434:192–209. doi: 10.1111/nyas.13677. [DOI] [PubMed] [Google Scholar]
- 7.Shah MA, Kennedy EB, Catenacci DV, Deighton DC, Goodman KA, Malhotra NK, Willett C, Stiles B, Sharma P, Tang L, Wijnhoven BPL, Hofstetter WL. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J. Clin. Oncol. 2020;38:2677–2694. doi: 10.1200/JCO.20.00866. [DOI] [PubMed] [Google Scholar]
- 8.Leng XF, Daiko H, Han YT, Mao YS. Optimal preoperative neoadjuvant therapy for resectable locally advanced esophageal squamous cell carcinoma. Ann N Y Acad Sci. 2020;1482:213–224. doi: 10.1111/nyas.14508. [DOI] [PubMed] [Google Scholar]
- 9.Griffin Y. Esophageal cancer: role of imaging in primary staging and response assessment post neoadjuvant therapy. Semin Ultrasound CT MR. 2016;37:339–351. doi: 10.1053/j.sult.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Zhou N, Hofstetter WL. Prognostic and therapeutic molecular markers in the clinical management of esophageal cancer. Expert Rev Mol Diagn. 2020;20:401–411. doi: 10.1080/14737159.2020.1731307. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe M, Otake R, Kozuki R, Toihata T, Takahashi K, Okamura A, Imamura Y. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today. 2020;50:12–20. doi: 10.1007/s00595-019-01878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura K, Kato K, Igaki H, Ito Y, Mizusawa J, Ando N, Udagawa H, Tsubosa Y, Daiko H, Hironaka S, Fukuda H, Kitagawa Y Japan Esophageal Oncology Group/Japan Clinical Oncology Group. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study) Jpn J Clin Oncol. 2013;43:752–755. doi: 10.1093/jjco/hyt061. [DOI] [PubMed] [Google Scholar]
- 13.Halonen JI, Erhola M, Furman E, Haahtela T, Jousilahti P, Barouki R, Bergman A, Billo NE, Fuller R, Haines A, Kogevinas M, Kolossa-Gehring M, Krauze K, Lanki T, Vicente JL, Messerli P, Nieuwenhuijsen M, Paloniemi R, Peters A, Posch KH, Timonen P, Vermeulen R, Virtanen SM, Bousquet J, Anto JM. The Helsinki Declaration 2020: Europe that protects. Lancet Planet Health. 2020;4:e503–e505. doi: 10.1016/S2542-5196(20)30242-4. [DOI] [PubMed] [Google Scholar]
- 14.Sudo N, Ichikawa H, Muneoka Y, Hanyu T, Kano Y, Ishikawa T, Hirose Y, Miura K, Shimada Y, Nagahashi M, Sakata J, Kobayashi T, Bamba T, Nakagawa S, Kosugi SI, Wakai T. ASO author reflections: ypTNM stage grouping in the 8th edition of the AJCC cancer staging manual refines the prognostic prediction for patients with esophageal squamous cell carcinoma undergoing neoadjuvant chemotherapy. Ann Surg Oncol. 2021;28:661–662. doi: 10.1245/s10434-020-09184-0. [DOI] [PubMed] [Google Scholar]
- 15.Ge F, Huo Z, Cai X, Hu Q, Chen W, Lin G, Zhong R, You Z, Wang R, Lu Y, Wang R, Huang Q, Zhang H, Song A, Li C, Wen Y, Jiang Y, Liang H, He J, Liang W, Liu J. Evaluation of clinical and safety outcomes of neoadjuvant immunotherapy combined with chemotherapy for patients with resectable esophageal cancer: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:e2239778. doi: 10.1001/jamanetworkopen.2022.39778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal AN, Gadgeel SM, Girshman J, Kris MG, Riely GJ, Yu HA, Hellmann MD. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol. 2019;30:839–844. doi: 10.1093/annonc/mdz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto K, Nishimura S, Ito T, Kakinoki R, Akagi M. Immunohistochemical expression and clinicopathological assessment of PD-1, PD-L1, NY-ESO-1, and MAGE-A4 expression in highly aggressive soft tissue sarcomas. Eur J Histochem. 2022;66:3393. doi: 10.4081/ejh.2022.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Ma S. History and current situation of neoadjuvant treatment for locally advanced esophageal cancer. Thorac Cancer. 2021;12:2293–2299. doi: 10.1111/1759-7714.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu YM, Zhuo Y, Chen LQ, Yuan Y. The clinical application of neoantigens in esophageal cancer. Front Oncol. 2021;11:703517. doi: 10.3389/fonc.2021.703517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Li J, Lin W, Shao D, Depypere L, Zhang Z, Li Z, Cui F, Du Z, Zeng Y, Jiang S, He P, Gu X, Chen H, Zhang H, Lin X, Huang H, Lv W, Cai W, Liang W, Liang H, Jiang W, Wang W, Xu K, Cai W, Wu K, Lerut T, Fu J, He J. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): a multicenter, phase 2 study. Int J Cancer. 2022;151:128–137. doi: 10.1002/ijc.33976. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro J, ten Kate FJ, van Hagen P, Biermann K, Wijnhoven BP, van Lanschot JJ. Residual esophageal cancer after neoadjuvant chemoradiotherapy frequently involves the mucosa and submucosa. Ann Surg. 2013;258:678–688. doi: 10.1097/SLA.0b013e3182a6191d. discussion 688-679. [DOI] [PubMed] [Google Scholar]
- 22.Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K KEYNOTE-590 Investigators. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 23.Markham A, Keam SJ. Camrelizumab: first global approval. Drugs. 2019;79:1355–1361. doi: 10.1007/s40265-019-01167-0. [DOI] [PubMed] [Google Scholar]
- 24.Taylor AL, Wright JT Jr. Should ethnicity serve as the basis for clinical trial design? Importance of race/ethnicity in clinical trials: lessons from the African-American heart failure trial (A-HeFT), the African-American study of kidney disease and hypertension (AASK), and the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) Circulation. 2005;112:3654–3660. doi: 10.1161/CIRCULATIONAHA.105.540443. discussion 3666. [DOI] [PubMed] [Google Scholar]
- 25.Derman BA, Jasielec J, Langerman SS, Zhang W, Jakubowiak AJ, Chiu BC. Racial differences in treatment and outcomes in multiple myeloma: a multiple myeloma research foundation analysis. Blood Cancer J. 2020;10:80. doi: 10.1038/s41408-020-00347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owusu JT, Wang P, Wickham RE, Cottonham DP, Varra AA, Chen C, Lungu A. Blended care therapy for depression and anxiety: outcomes across diverse racial and ethnic groups. J Racial Ethn Health Disparities. 2023;10:2731–2743. doi: 10.1007/s40615-022-01450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Yan C, Gao X, Li X, Cao F, Zhao G, Zhao J, Er P, Zhang T, Chen X, Wang Y, Jiang Y, Wang Q, Zhang B, Qian D, Wang J, Zhou D, Ren X, Yu Z, Zhao L, Yuan Z, Wang P, Pang Q. Safety and feasibility of radiotherapy plus camrelizumab for locally advanced esophageal squamous cell carcinoma. Oncologist. 2021;26:e1110–e1124. doi: 10.1002/onco.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vrana D, Matzenauer M, Neoral C, Aujesky R, Vrba R, Melichar B, Rusarova N, Bartouskova M, Jankowski J. From Tumor immunology to immunotherapy in gastric and esophageal cancer. Int J Mol Sci. 2018;20:13. doi: 10.3390/ijms20010013. [DOI] [PMC free article] [PubMed] [Google Scholar]