Abstract
Objective: To analyze the factors influencing recurrent urinary tract infections (UTIs) in children and explore predictive factors and intervention measures. Methods: Data of 158 children with UTIs treated at the Longyan First Affiliated Hospital of Fujian Medical University from January 2020 to June 2023 were analyzed. Among them, 122 children without recurrent UTIs were included in a non-recurrent group, while the remaining 36 were included in a recurrent group. The quality of life prior to treatment and six weeks after treatment, the immunoglobulin A (IgA) and immunoglobulin G (IgG) levels after treatment, the relationship between the quality of life after six weeks of treatment and the levels of IgA and IgG were analyzed. Multivariate logistic regression analysis was conducted to identify factors impacting the recurrence of UTIs, and receiver operating characteristic (ROC) curves were generated to predict recurrent UTIs based on independent risk factors. Results: Before treatment, no notable difference was observed in Short Form 36 Health Survey (SF-36) scores between the non-recurrent group and the recurrent group (P>0.05). After treatment, the SF-36 scores notably increased in the non-recurrent group (P<0.0001), while there was no notable increase in the recurrent group (P>0.05). However, the difference in SF-36 scores after treatment was significant between the two groups (P<0.0001). In addition, there was a significantly positive correlation between IgA levels and quality of life after 6 weeks of treatment (P<0.05). The recurrent group showed significantly lower IgA and IgG levels than the non-recurrent group (P<0.05). Multivariate logistic regression analysis identified anemia, urinary system malformation, constipation, decreased IgA level, and decreased IgG level as independent risk factors for recurrent UTIs in children. ROC curves-based analysis of independent risk factors demonstrated that urinary system malformation had a better performance in predicting recurrent UTIs in children than the other four factors. Conclusion: Urinary system malformation, constipation, anemia, decreased IgA and IgG levels are all identified as independent risk factors for recurrent UTIs in children, and urinary system malformation is a better predictor for recurrent UTIs in children than the other four factors.
Keywords: Recurrent urinary tract infections, urinary system malformation, constipation, anemia, IgA, IgG
Introduction
Urinary tract infections (UTIs) are a general term for diseases such as pyelonephritis, cystitis, and urethritis caused by bacteria. They occur when bacteria invade the urinary tract, multiply in the urine, and cause damage to the urinary tract mucosa or tissues. UTIs are one of the most common bacterial infections in children [1,2]. UTIs in children are linked to alterations in intestinal flora, anomalies in the anatomical structure and functionality of the urinary system, unique physiological configurations, and variations in immune function [3]. Due to the immaturity of organ functions and weak resistance in children, the incidence of UTIs is increased [4]. In addition to potentially causing severe sepsis in children, UTIs can also trigger chronic kidney diseases such as renal scars, hypertension, and chronic renal insufficiency. The risk of developing these chronic kidney diseases is even higher in individuals who experience recurrent UTIs [5].
Recurrent UTIs are relatively common in children, especially among girls between the ages of 2 and 8 years [5]. In children with recurrent UTIs, clinical manifestations may not be readily apparent, often presenting with nonspecific symptoms such as vomiting, fever, abdominal distension, and abdominal pain. Diagnosis typically involves urinalysis and urine culture to confirm the presence of a UTI [6,7]. Recurrent UTIs are often the result of multiple factors, typically including anatomical abnormalities in the urinary system, urinary stasis, persistent presence of urethral infections, and immune system dysfunction [8,9]. Due to the nonspecific symptoms associated with recurrent UTIs, there is a relatively high rate of misdiagnosis, which can impact the treatment of the condition [10]. If high-risk children with recurrent UTIs are not identified early and provided with appropriate treatment, it can lead to prolonged illness and even result in permanent renal scarring. This result, in turn, can lead to conditions such as impaired kidney development, chronic pyelonephritis, renal glomerular dysfunction, hypertension, and end-stage renal failure [9]. Therefore, the prevention of recurrent UTIs holds significant clinical importance. Existing literature has identified various influencing factors such as urinary system malformations, and altered immune markers like decreased immunoglobulin A (IgA) and immunoglobulin G (IgG) levels as potential contributors to recurrent UTIs in children [11].
This study analyzed the influencing factors of recurrent UTIs in children to reduce the recurrence rate of UTIs in pediatric patients, and explored predictive factors and intervention measures to enable targeted prevention. The innovative aspect of this study, compared to others, lies in its comprehensive approach to explore a wider range of potential influencing factors for predicting UTIs in children. By analyzing data from 158 children with UTIs, the study delves deeper into the roles of factors such as anemia, urinary system malformations, constipation, and immune marker levels.
Methods and data
Sample source and processing
With approval from the Medical Ethics Committee of the Longyan First Affiliated Hospital of Fujian Medical University, this retrospective analysis collected the clinical data of 200 children with UTIs treated at the Longyan First Affiliated Hospital of Fujian Medical University between January 2020 and June 2023. After the initial screening of the 200 children, 158 children who met the inclusion and exclusion criteria were included. Inclusion criteria: (1) Children with symptoms of UTI with more than 5 white blood cells/high power field in centrifugal urinary sediment; (2) Children who were diagnosed with UTI by laboratory examinations, and met the diagnostic criteria of “Chinese expert consensus on diagnosis and treatment of urinary tract infection (2015 version)” [12]; (3) Children who were younger than 12 years old; (4) Children with required follow-up and clinical data. Exclusion criteria: (1) Children with other systemic infections; (2) Children with immunodeficiency; (3) Children with underlying diseases such as diabetes.
Grouping
Based on the follow-up results, 122 patients who did not experience recurrent UTIs were assigned to the non-recurrent group, while 36 patients who experienced recurrent UTIs were assigned to the recurrent group. Recurrence of UTI refers to the reappearance of symptoms in the child within 6 weeks, with a urine bacterial colony count ≥105/mL, and the presence of the same or different bacterial species compared to the previous infection [5]. The non-recurrent group consisted of 79 females and 43 males, ranging in age from 2 months to 12 years. The recurrent group was comprised of 27 females and 9 males, with ages ranging from 3 months to 11 years. No significant difference was found in the baseline characteristics between the two groups, indicating comparability between the two groups (P>0.05).
Data collection
Relevant data of the children were collected from the electronic medical record system in the Longyan First Affiliated Hospital of Fujian Medical University, including age, sex, anemia status, allergic constitution, constipation status, urinary system malformation, IgA levels, IgG levels, and distribution of pathogens.
Outcome measures
Primary outcome measures
(1) IgA and IgG levels after treatment were compared between the two groups. (2) The relationship between the quality of life after six weeks of treatment and the levels of IgA and IgG in the two groups was analyzed. (3) Based on the recurrence status of UTIs, the clinical data of the children were included in a univariate analysis. Factors showing significant differences were then subjected to a multivariate logistic regression analysis.
Secondary outcome measures
(1) The clinical data of patients were compared. (2) The quality of life was compared between the two groups prior to treatment and six weeks after treatment using Short Form 36 Health Survey (SF-36) scores. A higher SF-36 score indicates higher quality of life. (3) Based on the independent risk factors, we plotted ROC curves to predict the recurrence of UTIs in children, and the predictive efficacy of the independent risk factors was explored.
Statistical analyse
Statistical analyses in this study were performed using SPSS 20.0 (IBM Corp, Armonk, NY, USA). Categorical data were described as [n (%)] and compared between the two groups using the chi-square test. Mean ± standard deviation (SD) was used to describe the continuous variables, and the t-test was used to compare the continuous variables between the two groups. Pearson correlation coefficient was adopted for correlation analysis. The predictive performance of independent risk factors for UTI recurrence in children was assessed using ROC curve analysis, and a corresponding Nomogram was generated on the website https://shiny.medsta.cn/coxpre1/ to visualize the risk factor predictive model. Graphs were generated using GraphPad Prism 7 (GraphPad Software, San Diego, USA). P<0.05 suggests a significant difference.
Results
Baseline data of patients
According to comparison of baseline data of the non-recurrent group and the recurrent group, the two groups did not differ notably in terms of age, sex, BMI, course of disease, etc. (Table 1).
Table 1.
Baseline data of patients
| Non-recurrent group (n=122) | Recurrent group (n=36) | χ2 | P value | |
|---|---|---|---|---|
| Age | 0.493 | 0.483 | ||
| ≥6 years old | 79 | 21 | ||
| <6 years old | 43 | 15 | ||
| Sex | 3.035 | 0.082 | ||
| Male | 50 | 9 | ||
| Female | 72 | 27 | ||
| BMI | 3.382 | 0.068 | ||
| ≥20 kg/m2 | 72 | 15 | ||
| <20 kg/m2 | 50 | 21 | ||
| Course of disease | 0.250 | 0.617 | ||
| 1-20 days | 62 | 20 | ||
| 20-40 days | 60 | 16 | ||
| Place of incidence | 0.047 | 0.829 | ||
| Urban areas | 35 | 11 | ||
| Rural areas | 87 | 25 |
Comparison of IgA and IgG levels between the two groups
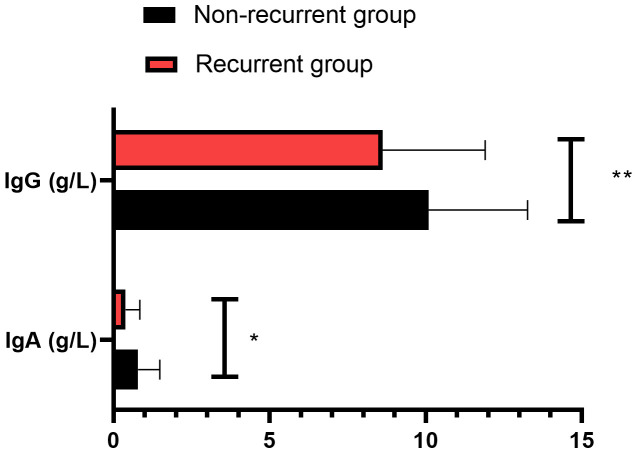
As shown in Figure 1, the IgA and IgG levels were markedly lower in the recurrent group when compared to those in the non-recurrent group (P<0.05).
Figure 1.
Comparison of IgA and IgG levels between the two groups. Notes: *P<0.05; **P<0.01. IgA: immunoglobulin A; IgG: immunoglobulin G.
Comparison of quality of life between the two groups
Prior to treatment, the two groups did not differ significantly in SF-36 scores (P>0.05). After treatment, the SF-36 scores significantly increased in the non-recurrent group (P<0.0001), while there was no notable increase in the recurrent group (P>0.05). However, a significant difference was observed in SF-36 scores between the two groups after treatment (P<0.0001, Table 2).
Table 2.
Comparison of quality of life between the two groups
| Group | SF-36 score | |
|---|---|---|
|
| ||
| Before treatment | After treatment | |
| Non-recurrent group (n=122) | 97.20±10.36 | 111.26±8.29a |
| Recurrent group (n=36) | 96.73±9.25 | 99.37±9.62b |
indicates P<0.0001 vs. Before treatment.
indicates P<0.0001 vs. non-recurrent group.
Note: SF-36: Short Form 36 Health Survey.
Relationship between IgA and IgG levels and the quality of life in patients with UTIs
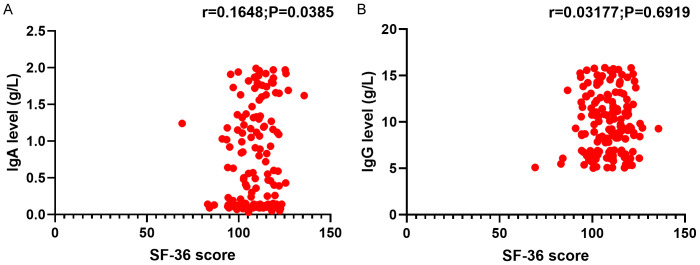
The study found a strong positive correlation between IgA levels and the quality of life after 6 weeks of treatment (P<0.05). However, no significant relationship was observed between IgG levels and the quality of life after 6 weeks of treatment (P>0.05, Figure 2).
Figure 2.
Relationship between IgA (A) and IgG (B) levels and the quality of life in patients with urinary tract infections. Notes: IgA: immunoglobulin A; IgG: immunoglobulin G; SF-36: Short Form 36 Health Survey.
Univariate analysis of recurrent UTIs in children
A comparative analysis was conducted on several factors including age, sex, anemia, allergic constitution, constipation, urinary system malformation, as well as IgA and IgG levels, in the two groups. The results revealed that anemia, allergic constitution, constipation, urinary system malformation, and decreased levels of IgA and IgG were significant risk factors influencing recurrent UTIs (P<0.05, Table 3).
Table 3.
Univariate analysis of factors affecting recurrent urinary tract infections in the two groups
| Non-recurrent group (n=122) | Recurrent group (n=36) | χ2 | P value | |
|---|---|---|---|---|
| Age | 0.493 | 0.483 | ||
| ≥6 years old | 79 | 21 | ||
| <6 years old | 43 | 15 | ||
| Sex | 3.035 | 0.082 | ||
| Male | 50 | 9 | ||
| Female | 72 | 27 | ||
| Anemia | 19.601 | <0.0001 | ||
| Yes | 35 | 25 | ||
| No | 87 | 11 | ||
| Allergic constitution | 10.021 | 0.002 | ||
| Yes | 36 | 21 | ||
| No | 86 | 15 | ||
| Urinary system malformation | 17.371 | <0.0001 | ||
| Yes | 37 | 23 | ||
| No | 85 | 13 | ||
| Constipation | 26.411 | <0.0001 | ||
| Yes | 31 | 26 | ||
| No | 91 | 10 | ||
| IgA level | 13.961 | 0.001 | ||
| Normal | 80 | 11 | ||
| Decreased | 42 | 25 | ||
| IgG level | 12.321 | 0.001 | ||
| Normal | 92 | 16 | ||
| Decreased | 30 | 20 | ||
| Pathogenic bacteria | 2.392 | 0.122 | ||
| Escherichia coli | 50 | 20 | ||
| Non-Escherichia coli | 72 | 16 |
Notes: IgA: immunoglobulin A; IgG: immunoglobulin G.
Assignment
Logistic regression analysis was carried out on the indicators with significant differences in Table 3, with the occurrence of recurrent UTIs as the dependent variable, and anemia, allergic constitution, urinary system malformation, constipation, IgA level, and IgG level as independent variables (Table 4).
Table 4.
Assignment
| Covariates | Assignment | |
|---|---|---|
| 0 | 1 | |
| Anemia | No | Yes |
| Allergic constitution | No | Yes |
| Urinary system malformation | No | Yes |
| Constipation | No | Yes |
| IgA level | Normal | Decreased |
| IgG level | Normal | Decreased |
| Dependent variable | ||
| Recurrent urinary tract infection | No | Yes |
Notes: IgA: immunoglobulin A; IgG: immunoglobulin G.
Multivariate logistic regression analysis of recurrent UTIs in children
Multivariate logistic regression analysis identified anemia, urinary system malformation, constipation, and decreased levels of IgA and IgG as independent risk factors influencing recurrent UTIs (Table 5).
Table 5.
Multivariate logistics regression analysis
| Factors | B | S.E. | Wald | df | Sig. | Exp (B) | 95% C.I. for EXP (B). | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lower limit | Upper limit | |||||||
| Anemia | 2.157 | 0.546 | 15.598 | 1 | <0.001 | 8.648 | 2.965 | 25.229 |
| Allergic constitution | 0.832 | 0.507 | 2.697 | 1 | 0.101 | 2.299 | 0.851 | 6.207 |
| Urinary system malformation | 1.909 | 0.519 | 13.505 | 1 | 0.000 | 6.746 | 2.437 | 18.673 |
| Constipation | 1.670 | 0.545 | 9.406 | 1 | 0.002 | 5.314 | 1.827 | 15.455 |
| IgA level | 1.487 | 0.520 | 8.188 | 1 | 0.004 | 4.424 | 1.598 | 12.249 |
| IgG level | 1.464 | 0.517 | 8.019 | 1 | 0.005 | 4.322 | 1.569 | 11.901 |
Notes: IgA: immunoglobulin A; IgG: immunoglobulin G.
Predictive efficacy of independent risk factors for recurrent UTIs in children
ROC curve-based analysis results in Figure 3 revealed the prediction of recurrent UTIs based on anemia, urinary system malformation, constipation, IgA, and IgG levels. It was found that urinary system malformation has a better performance in predicting recurrent UTIs in children than the other four factors (Figure 3 and Table 6). Based on the logistic regression analysis results, we also constructed a Nomogram prediction model incorporating five independent factors to predict the risks of recurrent UTIs in children (Figure 4).
Figure 3.
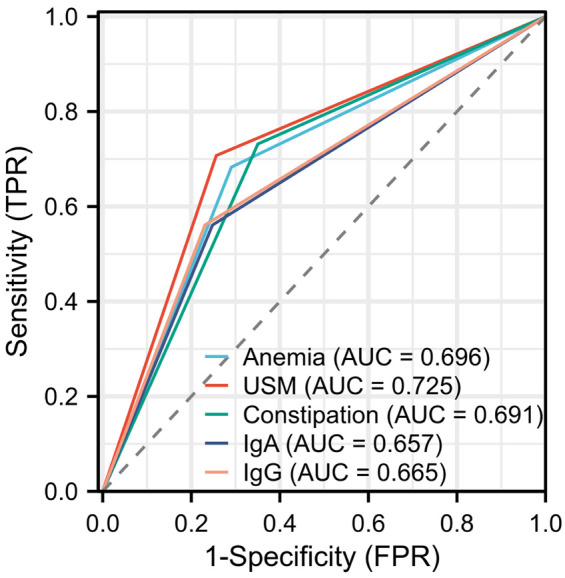
ROC curves illustrating the predictive efficacy of independent risk factors for recurrent UTIs. Notes: USM: Urinary system malformation; UTIs: urinary tract infections; IgA: immunoglobulin A; IgG: immunoglobulin G; ROC: receiver operating characteristic.
Table 6.
Related parameters of ROC curves illustrating the predictive efficacy of independent risk factors for recurrent UTIs
| Sensitivity | Specify | Accuracy | AUC | |
|---|---|---|---|---|
| Anemia | 68.29% | 70.94% | 70.25% | 0.696 |
| USM | 70.73% | 74.340 | 73.42% | 0.725 |
| Constipation | 73.17% | 64.96 | 67.09% | 0.691 |
| IgA | 56.10% | 75.21% | 70.25% | 0.657 |
| IgG | 56.10% | 76.92% | 71.52% | 0.665 |
Notes: ROC: receiver operating characteristic; USM: Urinary system malformation; UTIs: urinary tract infections; IgA: immunoglobulin A; IgG: immunoglobulin G.
Figure 4.
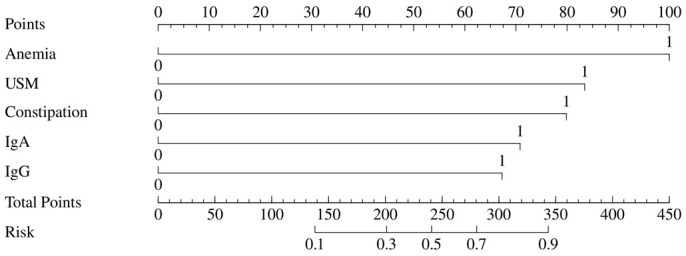
Nomogram for predicting recurrent UTIs. Notes: USM: Urinary system malformation; UTIs: urinary tract infections; IgA: immunoglobulin A; IgG: immunoglobulin G.
Discussion
This study explored the relevant influencing factors and intervention measures for recurrent UTIs in children and found that urinary system malformation, constipation, anemia, and decreased IgA and IgG levels are independent risk factors for recurrent UTIs in children, and the prediction using these risk factors demonstrates good efficacy.
UTI refers to a common inflammatory condition caused by the invasion of pathogens into the urinary tract mucosa or tissues, which occurs in individuals of all ages and both genders [13-15]. Children are predisposed to recurrent UTIs because of their relatively shorter urethra, the proximity of the urethral opening to the anus, and the incomplete maturation of their immune system [16]. Recurrent UTIs can trigger abdominal pain, frequent urination, urgency of urination, and dysuria in children, affecting their appetite, sleep, and daily activities, and can even trigger complications such as pyelonephritis and renal scarring in severe cases [17].
In this study, it was observed that after treatment, the SF-36 scores significantly increased in the non-recurrent group (P<0.0001), while there was no significant increase in the recurrent group. However, there was a significant difference in SF-36 scores between the two groups. This indicates that recurrent UTIs have a significant impact on the quality of life of patients.
Furthermore, this study found a positive correlation between IgA levels in patients and the quality of life after 6 weeks of treatment. This may be attributed to the fact that IgA is primarily present on mucosal surfaces and takes part in protecting mucosal membranes from infection [18]. In patients with UTIs, the presence of inflammation during the treatment process can result in a decline in immune function, including a decrease in mucosal IgA levels. However, as the treatment progresses and inflammation subsides, immune function is restored, and mucosal IgA levels increase, thereby reducing the occurrence of infections and improving the quality of life. Additionally, this study found no significant relationship between IgG levels and the quality of life after 6 weeks of treatment, which may be due to the limited sample size. We did find significantly lower levels of IgA and IgG in children with recurrent UTIs compared to those in children without recurrent UTIs. This finding suggests a potential association between IgA and IgG levels and the occurrence of recurrent UTIs in children.
The influencing factors for initial and recurrent UTIs differ significantly. Initial UTIs may be influenced by anatomical factors, sexual activity, hygiene practices, voiding habits, and hormonal changes like those in menopause. In contrast, recurrent UTIs can be linked to structural abnormalities in the urinary tract, incomplete treatment of previous infections, and urinary tract obstructions such as kidney stones [16,17]. In this study, through logistic multivariable analysis, we identified anemia, urinary system malformation, constipation, decreased IgA levels, and decreased IgG levels as independent risk factors for recurrent UTIs in children. Anemia may increase susceptibility to infections by weakening the immune system, particularly in the context of UTIs, where decreased immune function due to anemia may heighten the risk of recurrent infections [19]. Additionally, tissue hypoxia caused by anemia, including in the urinary tract tissues, can impact the body’s ability to fight infections and maintain urinary system health [20,21]. The identification of anemia as an independent risk factor for recurrent UTIs in children is a significant finding, indicating a substantial association between anemia and recurrent UTIs in children. Anemia may raise the risk of recurrent infections in children through mechanisms such as compromised immune function and reduced tissue oxygen supply. Therefore, addressing anemia could be a key strategy in reducing the recurrence of UTIs in children. This research finding, highlighting the impact of anemia on UTIs in children, is innovative and provides important insights for further clinical practice. Lee et al. [22] found that compared to non-anemic individuals, anemic patients showed elevated levels of plasma neutrophil gelatinase-associated lipocalin (NGAL), and the increased levels of plasma NGAL may be associated with febrile UTIs in anemic children and the presence of renal scars, which supports the conclusions of this study.
Furthermore, constipation was also found to be a factor that increased the risk of recurrent UTIs. Constipation can decrease the normal vaginal bacterial population, raising the risk of bacterial infections. Bacterial infections in the vagina can spread to the urinary tract via the urethra, triggering UTIs [23]. Furthermore, constipation can result in incomplete emptying of the bladder during urination, allowing bacteria to reside and multiply in the residual urine, increasing the risk of infection [24]. For children with UTIs, it is important to focus on dietary adjustments to ensure an adequate intake of dietary fiber and foods rich in iron, vitamin B12, and folic acid. It is also imperative to encourage children to drink plenty of water to maintain adequate hydration. Additionally, children should engage in regular physical activity to promote bowel movements. If necessary, iron supplements can be prescribed to improve constipation and anemia, thereby preventing recurrent UTIs.
Urinary system malformation in children refers to the structural or functional abnormalities of the urinary system present at birth or during the developmental process. These abnormalities can result in obstructed or refluxed urine flow, making it easier for bacteria to enter the urinary tract and cause infection [25]. Furthermore, urinary system malformation can lead to urine retention during the voiding process, preventing complete emptying of the bladder. The stagnant urine provides a favorable environment for bacterial growth and reproduction, increasing the likelihood of infection [26,27]. Visuri et al. [28] have found that children with ureterocele or double ureter collection system related to giant ureter without reflux face a high risk of UTI, which is consistent with the results in this study. Therefore, for children who experience UTIs, it is important to promptly conduct imaging examinations to determine if there is any urinary system malformation. If malformation is detected, timely treatment is necessary to prevent recurrent UTIs.
Decreased levels of IgA and IgG increase the likelihood of recurrent UTIs in children. IgA is an immunoglobulin that is primarily found on the surface of mucous membranes, including the mucosa of the urinary tract. It plays a vital role in defending against infections by preventing the invasion and attachment of pathogens, thus protecting the mucosal surfaces from infection. In the cause of decreased IgA levels, the immune defense capacity of the mucosa weakens, making the urinary tract more susceptible to infection [29]. IgG is another important immunoglobulin that primarily functions in the humoral immune response. It is involved in neutralizing pathogens and promoting inflammatory reactions within the body. In the case of decreased IgG levels, the body’s ability to fight infections is weakened, making it easier for pathogens to proliferate and cause infections in the urinary tract [11]. When both IgA and IgG levels are reduced, the immune defense of the urinary tract is weakened, making it more susceptible to bacterial and other pathogenic infections. This can lead to the recurrence of UTIs. For patients with decreased levels of IgA and IgG, further immunological evaluation and treatment are necessary to enhance immune defense to reduce the recurrence of UTIs.
The study explored the predictive efficacy of anemia, urinary system malformation, constipation, IgA, and IgG levels in predicting the recurrence of UTIs, and it was found that urinary system malformation has a better performance in predicting recurrent UTIs in children than the other four factors.
This study has several limitations. Firstly, the sample size was relatively small, which may introduce potential bias into the results. Future research should aim to include a larger sample size to enhance the generalizability of the findings and minimize bias. Secondly, as this study employed a retrospective design, there may be limitations in terms of incomplete or biased information retrieval. Moreover, the absence of a validation set to verify the model’s performance and the lack of evaluation of the model, including discrimination and calibration analyses, limit reliability and generalizability of the predictive model developed in this study. Future research with larger sample sizes, prospective designs, and comprehensive assessments of potential influencing factors, along with validation procedures and thorough model evaluation, are warranted to enhance the validity and utility of the study findings.
Conclusion
In summary, urinary system malformation, constipation, anemia, and decreased IgA and IgG levels are independent risk factors for recurrent UTIs in children, and urinary system malformation has a better performance in predicting recurrent UTIs than the other four factors. Therefore, it is important to systematically evaluate and address the presence of malformations, anemia, constipation, and decreased IgA and IgG levels in children with UTIs to prevent recurrence.
Acknowledgements
This work was supported by the Startup Fund for scientific research, Fujian Medical University (Grant number: 2020QH1334).
Disclosure of conflict of interest
None.
References
- 1.Byron JK. Urinary tract infection. Vet Clin North Am Small Anim Pract. 2019;49:211–221. doi: 10.1016/j.cvsm.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Millner R, Becknell B. Urinary tract infections. Pediatr Clin North Am. 2019;66:1–13. doi: 10.1016/j.pcl.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Mattoo TK, Shaikh N, Nelson CP. Contemporary management of urinary tract infection in children. Pediatrics. 2021;147:e2020012138. doi: 10.1542/peds.2020-012138. [DOI] [PubMed] [Google Scholar]
- 4.Brandström P, Hansson S. Urinary tract infection in children. Pediatr Clin North Am. 2022;69:1099–1114. doi: 10.1016/j.pcl.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Williams G, Craig JC. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst Rev. 2019;4:CD001534. doi: 10.1002/14651858.CD001534.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sihra N, Goodman A, Zakri R, Sahai A, Malde S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat Rev Urol. 2018;15:750–776. doi: 10.1038/s41585-018-0106-x. [DOI] [PubMed] [Google Scholar]
- 7.Peck J, Shepherd JP. Recurrent urinary tract infections: diagnosis, treatment, and prevention. Obstet Gynecol Clin North Am. 2021;48:501–513. doi: 10.1016/j.ogc.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Hughes T, Juliebø-Jones P, Saada L, Saeed K, Somani BK. Recurrent urinary tract infections in adults: a practical guide. Br J Hosp Med (Lond) 2021;82:1–11. doi: 10.12968/hmed.2021.0337. [DOI] [PubMed] [Google Scholar]
- 9.Pigrau C, Escolà-Vergé L. Recurrent urinary tract infections: from pathogenesis to prevention. Med Clin (Barc) 2020;155:171–177. doi: 10.1016/j.medcli.2020.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Tullus K, Shaikh N. Urinary tract infections in children. Lancet. 2020;395:1659–1668. doi: 10.1016/S0140-6736(20)30676-0. [DOI] [PubMed] [Google Scholar]
- 11.Pirdel L, Pirdel M. A differential immune modulating role of vitamin d in urinary tract infection. Immunol Invest. 2022;51:531–545. doi: 10.1080/08820139.2020.1845723. [DOI] [PubMed] [Google Scholar]
- 12.Chang SJ, Lin CD, Hsieh CH, Liu YB, Chiang IN, Yang SS. Reliability and validity of a Chinese version of urinary tract infection symptom assessment questionnaire. Int Braz J Urol. 2015;41:729–738. doi: 10.1590/S1677-5538.IBJU.2014.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waller TA, Pantin SAL, Yenior AL, Pujalte GGA. Urinary tract infection antibiotic resistance in the United States. Prim Care. 2018;45:455–466. doi: 10.1016/j.pop.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Kwok M, McGeorge S, Mayer-Coverdale J, Graves B, Paterson DL, Harris PNA, Esler R, Dowling C, Britton S, Roberts MJ. Guideline of guidelines: management of recurrent urinary tract infections in women. BJU Int. 2022;130(Suppl 3):11–22. doi: 10.1111/bju.15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jent P, Berger J, Kuhn A, Trautner BW, Atkinson A, Marschall J. Antibiotics for preventing recurrent urinary tract infection: systematic review and meta-analysis. Open Forum Infect Dis. 2022;9:ofac327. doi: 10.1093/ofid/ofac327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson P, Dudley AG, Rowe CK. Contemporary management of urinary tract infections in children. Curr Treat Options Pediatr. 2022;8:192–210. doi: 10.1007/s40746-022-00242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hum S, Liu H, Shaikh N. Risk factors for the development of febrile recurrences in children with a history of urinary tract infection. J Pediatr. 2022;243:152–157. doi: 10.1016/j.jpeds.2021.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil HP, Rane PS, Shrivastava S, Palkar S, Lalwani S, Mishra AC, Arankalle VA. Antibody (IgA, IgG, and IgG subtype) responses to SARS-CoV-2 in severe and nonsevere COVID-19 patients. Viral Immunol. 2021;34:201–209. doi: 10.1089/vim.2020.0321. [DOI] [PubMed] [Google Scholar]
- 19.Adewale B, Mafe MA, Mogaji HO, Balogun JB, Sulyman MA, Ajayi MB, Akande DO, Balogun EO. Urinary schistosomiasis and anemia among school-aged children from southwestern Nigeria. Pathog Glob Health. 2024;118:325–333. doi: 10.1080/20477724.2024.2322800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dassah S, Asiamah GK, Harun V, Appiah-Kubi K, Oduro A, Asoala V, Amenga-Etego L. Urogenital schistosomiasis transmission, malaria and anemia among school-age children in Northern Ghana. Heliyon. 2022;8:e10440. doi: 10.1016/j.heliyon.2022.e10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sousa-Figueiredo JC, Gamboa D, Pedro JM, Fançony C, Langa AJ, Magalhães RJ, Stothard JR, Nery SV. Epidemiology of malaria, schistosomiasis, geohelminths, anemia and malnutrition in the context of a demographic surveillance system in northern Angola. PLoS One. 2012;7:e33189. doi: 10.1371/journal.pone.0033189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Yim HE, Yoo KH. Associations of plasma neutrophil gelatinase-associated lipocalin, anemia, and renal scarring in children with febrile urinary tract infections. J Korean Med Sci. 2020;35:e65. doi: 10.3346/jkms.2020.35.e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meling Stokland AE, Dahle AL, Kloster VL, Nedrebø T, Nedrebø BG. Myxedema coma complicated by bilateral hygromas. Endocrinol Diabetes Metab Case Rep. 2021;2021:21-0067. doi: 10.1530/EDM-21-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayaweera JAAS, Reyes M, Joseph A. Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci Rep. 2019;9:12637. doi: 10.1038/s41598-019-49122-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Alp EK, Dönmez Mİ, Alp H, Elmacı AM. The association between the congenital heart diseases and congenital anomalies of the kidney and the urinary tract in nonsyndromic children. Congenit Anom (Kyoto) 2022;62:4–10. doi: 10.1111/cga.12443. [DOI] [PubMed] [Google Scholar]
- 26.Delforge X, Kongolo G, Cauliez A, Braun K, Haraux E, Buisson P. Transient pseudohypoaldosteronism: a potentially severe condition affecting infants with urinary tract malformation. J Pediatr Urol. 2019;15:265.e261–265.e267. doi: 10.1016/j.jpurol.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Nigam A, Knoers NVAM, Renkema KY. Impact of next generation sequencing on our understanding of CAKUT. Semin Cell Dev Biol. 2019;91:104–110. doi: 10.1016/j.semcdb.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Visuri S, Jahnukainen T, Taskinen S. Prenatal complicated duplex collecting system and ureterocele-Important risk factors for urinary tract infection. J Pediatr Surg. 2018;53:813–817. doi: 10.1016/j.jpedsurg.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Koenen MH, van Montfrans JM, Sanders EAM, Bogaert D, Verhagen LM. Immunoglobulin A deficiency in children, an undervalued clinical issue. Clin Immunol. 2019;209:108293. doi: 10.1016/j.clim.2019.108293. [DOI] [PubMed] [Google Scholar]