Abstract
Objectives: This study aimed to assess the interaction between metoprolol and Ginkgo tablets during their co-administration to provide a reference for clinical prescribing. Methods: The co-administration of metoprolol (20 mg/kg) and Ginkgo tablets (2.4 mg/kg) was conducted in adult Sprague Dawley (SD) rats (n = 8). An optimized liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed for the analysis of plasma metoprolol to evaluate its pharmacokinetics. In vitro, the rat liver microsomes were employed to assess the effect of Ginkgo tablets on the metabolic stability of metoprolol and the activity of Cytochrome P450 2D6 (CYP2D6). Results: The developed LC-MS/MS method was demonstrated of high sensitivity, accuracy, and precision. When co-administered with Ginkgo tablets, it increased the area under the curve (AUC, 59.01 ± 10.11 vs. 39.19 ± 10.21 μg/mL × min), the maximum plasma concentration (Cmax, 461.72 ± 44.64 vs. 276.35 ± 118.09 ng/mL), and the half-life (t1/2, 302.83 ± 91.52 vs. 262.34 ± 111.12 min) of metoprolol in rats and reduced the clearance rate (0.346 ± 0.057 vs. 0.539 ± 0.145 L/min/kg). In vitro, Ginkgo tablets improved the metabolic stability of metoprolol and suppressed the activity of CYP2D6 in a concentration-dependent manner with the IC50 value of 11.17 μM. Conclusion: Co-administration of metoprolol with Ginkgo tablets resulted in increasing its systemic exposure through inhibiting CYP2D6 activity.
Keywords: Metoprolol, Ginkgo tablets, pharmacokinetics, CYP2D6, IC50
Introduction
β blockers are commonly used in the clinical therapy of cardiovascular diseases, such as hypertension, heart failure, coronary heart disease, arrhythmia, etc. [1,2]. Metoprolol is a selective β blocker that can lower blood pressure and reduce decreased sinus rhythm, which makes it widely used in the treatment of hypertension, angina pectoris, myocardial infarction, aortic dissection, arrhythmia, and relieve the symptoms like palpitations and tachycardia [3,4]. Metoprolol is metabolized by cytochrome P450 enzymes (CYP450s) in the liver and absorbed by the gut with > 90% absorption. The in vivo biotransformation of metoprolol mainly involved the O-demethylation, α-hydroxylation, and N-dealkylation mediating by CYP2D6, which showed a significant first-pass effect [5].
The Ginkgo tablet, developed from extracting effective components of Ginkgo biloba leaves, plays a crucial role in treating cardiovascular diseases. It is mainly composed of flavonoids and terpene lactones. The combination of metoprolol with Ginkgo tablets has shown significant effects on hypertension and its complications [6-8]. This co-administration also benefits patients with coronary heart disease and heart failure. It is particularly effective for hypertensive patients with hemodynamic changes. However, it is unclear whether an interaction occurs during their co-administration.
Previous investigations have identified numerous interactions between co-administered drugs. The drug-drug interactions sometimes induced adverse reactions influencing drug efficacy and even resulting in toxicity. Pharmacokinetic or pharmacodynamic drug-drug interactions often relate to the activity of CYP450s. While a former study indicated that the Ginkgo biloba showed a weak inhibitory effect on the activity of CYP2D6, it is also was able to induce its pharmacokinetic interaction with metoprolol due to the deep involvement of CYP2D6 in the metabolism of metoprolol [9]. Therefore, the pharmacokinetics of metoprolol during its co-administration with Ginkgo tablets was investigated in the present study, aiming to disclose a possible risk during drug combination and provide a reference for the co-prescription of metoprolol and Ginkgo tablets.
Materials and methods
Animal experiments
This study was approved by the Ethics Committee of the Zhejiang Chinese Medical University (No. IACUC-20201123-07). All institutional and national guidelines regarding the care and use of laboratory animals were followed. Adult Sprague Dawley rats weighing 200-240 g were procured from the Sino-British SIPPR/BK Lab and housed at 20-24°C with 40-60% humidity. After maintaining for 10 d with free access to drink and food, the rats were fasted overnight for experiments.
The rats were randomly divided into single administration and co-administration treatments. Each group consistent of 8 rats for each treatment (4 male rats and 4 female rats). Both metoprolol (20 mg/kg) and Ginkgo tablets (2.4 mg/kg) were orally gavaged after powdering and dissolving in water. For the co-administration treatment, the rats were pre-treated with Ginkgo for 10 d to avoid its chemical interaction with metoprolol during their co-existence. Blood samples were collected after 0 (before administration), 8, 15, 30, 45, 60, 90, 120, 180, 240, 360, 480, 720, 960, and 1440 min of drug administration through intubation of the jugular vein. Blood samples were collected in the ethylene diamine tetraacetic acid (EDTA) anticoagulation tubes, and the plasma was obtained after centrifugation at 104 r/min for 5 min and stored at -20°C.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) conditions
Chromatographic analysis was conducted with the Agilent 1290 series HPLC system. Sample separation was conducted on a 100 mm × 3.0 mm C18 column with 0.2% formic acid-acetonitrile (68:32, v/v) as the mobile phase. The system operated at a flow rate of 0.3 mL/min and a column temperature of 30°C.
The mass spectrometry analysis was performed with the Agilent 6470 Triple Quad MS system. An electrospray ion source served as the ion source and the capillary voltage was set to 3500 V. N2 was used as the drying gas with a flow rate of 10 L/min and a temperature of 350°C. The nebulizer pressure was set at 40 psi, while the sheath gas temperature was 400°C with a flow rate of 11 L/min. The multiple-reaction monitoring mode was applied with m/z 268.2→116.0 for metoprolol and m/z 237.2→194.0 for the internal standard (carbamazepine). The collision energy was 35 eV and the fragmentation voltage was 120 V.
Quality control and standards calibration
A stock solution of metoprolol was prepared in methanol solution and then diluted to prepare the final standard solution. Calibration standards were established from a series of working solutions (3.42-7000 ng/mL) that were then added to blank rat plasma. The low-quality control (LQC), medium-quality control (MQC), and high-quality control (HQC) were prepared with 10, 100, and 3500 ng/mL metoprolol.
Method validation
Selectivity
The blank plasma with corresponding concentrations of metoprolol and internal standard were analyzed. The signal detected in the blank calibrators should be < 20% of the lower limit of quantification (LLOQ, the metoprolol concentration with the signal-noise ratio of 10) during its retention time, and the peak signal should be < 5% relative to the IS signal response.
Sensitivity
The response signals should exceed the blank matrix signal by over five times, and the accuracy should be within ± 20% of the nominal concentration. The maximum relative standard deviation (RSD) or variation coefficient should be 20%.
Calibration curve and linearity
The calibration curve was established with the peak-area ratio between metoprolol and the internal standard (IS, y) and nominal concentration of metoprolol (x). This was achieved using a 1/x2 weighted linear least-squares regression model. The acceptance criteria included a correlation coefficient (r2) > 0.99 and a deviation within ± 15% of the nominal concentration.
Accuracy and precision
The QC samples at LQC, MQC, and HQC were employed with six replicates to evaluate the intra- and inter-day precision and accuracy over three consecutive days. Accuracy, defined as having a difference between nominal and measured concentrations of less than 15%, and precision, defined as having a relative standard deviation (RSD) of no more than ± 15%, were deemed acceptable.
Stability
The stability was assessed with LQC, MQC, and HQC under various conditions, including three freeze-thaw cycles (ranging from -20°C to room temperature as a cycle), short-term stability at room temperature for 3 h, and long-term stability at -20°C for 2 weeks.
Extraction recovery and matrix effect
The extraction recovery was assessed by the comparison between the peak area of blank plasma and samples. The matrix effect was estimated by comparing the blank processed matrix solution and the solution with LQC, MQC, and HQC.
Metabolic stability evaluation
The metabolic stability of metoprolol was evaluated in the pooled rat liver microsomes. Briefly, rat liver microsomes were mixed with phosphate buffered saline (PBS) buffer and a nicotinamide adenine dinucleotide phosphate (NADPH) generating system containing MgCl2, glucose-6-phosphate dehydrogenase, NADP+, and sodium citrate. The mixture was incubated for 5 min and then metoprolol was added. The mixture was pre-incubated with Ginkgo before adding metoprolol to assess its effect on the metabolic stability of metoprolol. After incubating for 0, 1, 5, 15, 30, and 60 min, the mixture was analyzed by LC-MS/MS as described above.
CYP2D6 activity evaluation
The evaluation of CYP2D6 activity was also performed in rat liver microsomes. The reaction system was prepared as described above, with the addition of dextromethorphan, a typical substrate of CYP2D6. This system was incubated with 0, 5, 10, 25, 50, and 100 μM Ginkgo to evaluate its effect on CYP2D6 activity and estimate its IC50.
Statistical analysis
The DAS 3.0 software was employed to calculate the pharmacokinetic parameters of metoprolol, both with and without co-administration. A difference comparison was conducted by one-way ANOVA using SPSS 26.0 software (P < 0.05).
Results
Method validation
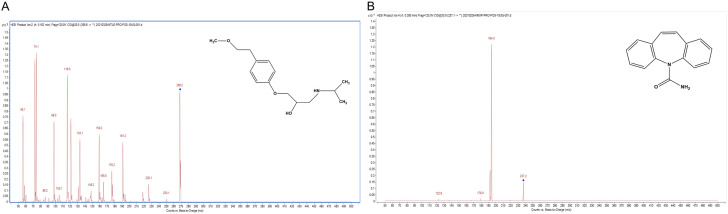
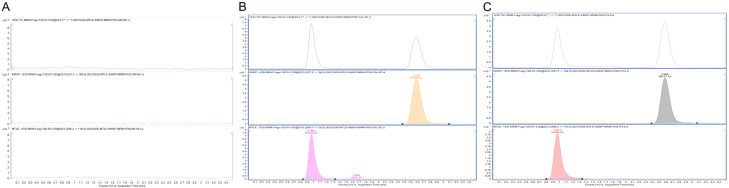
The mass spectra of metoprolol typically showed its m/z transformed from 268.2 to 116.0 (Figure 1A), while the m/z of IS changed from 237.2 to 194.0 (Figure 1B). Internal standard showed no significant peaks (Figure 2A). The chromatograms showed that the retention time of metoprolol in the blank plasma was 0.964 min and the retention time of IS was 2.586 min (Figure 2B). In rat plasma, the retention time of metoprolol and IS was 0.971 min and 2.586 min, respectively (Figure 2C). The LLOQ of metoprolol was 0.1 ng/mL with the signal-noise ratio over 10. In addition, the weighted linear and quadratic analysis revealed the r of the calibration curve was 0.993.
Figure 1.
Chemical structure and product ion mass spectra of metoprolol (A) and internal standard (B).
Figure 2.
Chromatograms of blank plasma (A), blank plasma with metoprolol and internal standard (B), and rat plasma after oral administration of metoprolol (C).
For intra- and inter-day accuracy and precision, the intra-day accuracy and precision ranged from 1.23 to 2.51% and -8.33 to 3.49%, respectively, while the intra-day accuracy and precision were from 1.27 to 2.46% and -6.45 to 5.79%, respectively, which were acceptable (Table 1).
Table 1.
Accuracy and precision of metoprolol in rat plasma
| Concentration | Intra-day | Inter-day | ||
|---|---|---|---|---|
|
|
|
|||
| RSD (%) | RE (%) | RSD (%) | RE (%) | |
| 10 | 1.23 | -8.33 | 2.46 | -6.45 |
| 100 | 1.67 | 3.49 | 1.27 | 4.74 |
| 3500 | 2.51 | -6.02 | 1.98 | 5.79 |
RSD: relative standard deviation indicating precision; RE: relative error indicating accuracy.
Both the matrix effect and recovery of metoprolol were over 85% ranged 92.34-98.71% and 89.78-101.33%, respectively, and no significant difference was observed among LQC, MQC, and HQC (Table 2).
Table 2.
Matrix effect and recovery of metoprolol in rat plasma
| Concentration (ng/mL) | Matrix effect (%) | RSD (%) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 10 | 94.56 | 3.53 | 89.78 | 6.24 |
| 100 | 98.71 | 4.26 | 101.33 | 1.43 |
| 3500 | 92.34 | 1.78 | 97.46 | 1.59 |
RSD: relative standard deviation indicating precision.
The stability was evaluated under different conditions. It was found that the variation of metoprolol concentration under freeze-thaw, short-term, and long-term were within 10%, indicating qualified stability (Table 3).
Table 3.
Stability outcomes of metoprolol in rat plasma
| Concentration (ng/mL) | Three freeze-thaw cycles | Short-term stability | Long-term stability | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Measured | RE | Measured | RE | Measured | RE | |
| 10 | 10.12 | 4.35 | 10.22 | 3.14 | 12.83 | 4.44 |
| 100 | 99.78 | 2.17 | 101.37 | 2.56 | 104.56 | 7.68 |
| 3500 | 3502.10 | 3.43 | 3501.09 | 4.12 | 3507.13 | 2.54 |
Pharmacokinetic study
As shown in Figure 3, metoprolol reached the maximum concentration (276.35 ± 118.09 ng/mL) at 55.71 ± 37.46 min, while the co-administration of Ginkgo significantly improved the plasma concentration of metoprolol, where the Cmax elevated to 461.72 ± 44.64 ng/mL at 30.26 ± 13.89 min accompanied by the increasing AUC0-t (from 39.19 ± 10.21 to 59.01 ± 10.11 μg/mL × min). The pharmacokinetics of metoprolol showed no significant difference in male and female rats (Figure S1 and Table S1). Additionally, the co-administered drugs also induced the prolonged half-life (302.83 ± 91.52 vs. 262.34 ± 111.12 min), decreased Cl (0.346 ± 0.057 vs. 0.539 ± 0.145 L/min/kg) and Vd (0.154 ± 0.059 vs. 0.188 ± 0.048 L/kg), indicating an increased systemic exposure of metoprolol (Table 4). Affected by hormones, the metabolic ability was different between male and female rats. Comparing the pharmacokinetics of metoprolol between male and female rats, we found no significant differences.
Figure 3.
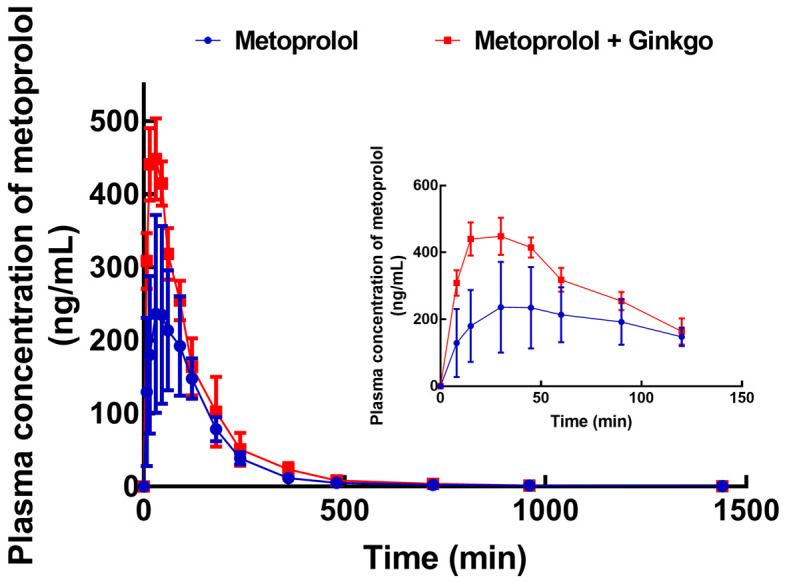
Plasma concentration-time curve of metoprolol (20 mg/kg) in the presence or absence of Ginkgo (2.4 mg/kg) in rats. n = 8.
Table 4.
Pharmacokinetic measurements of metoprolol with or without co-administration of Ginkgo
| Parameter | Units | Metoprolol | Metoprolol + Ginkgo |
|---|---|---|---|
| AUC0-t | μg/mL × min | 39.19 ± 10.21 | 59.01 ± 10.11 |
| t1/2 | min | 262.34 ± 111.12 | 302.83 ± 91.52 |
| Tmax | min | 55.71 ± 37.46 | 30.26 ± 13.89 |
| Cmax | ng/mL | 276.35 ± 118.09 | 461.72 ± 44.64 |
| MRT0-t | min | 163.18 ± 41.13 | 132.14 ± 24.96 |
| Cl | L/min/kg | 0.539 ± 0.145 | 0.346 ± 0.057 |
| Vd | L/kg | 0.188 ± 0.048 | 0.154 ± 0.059 |
AUC0-t: area under the curve; t1/2: half-life; Tmax: time reached the maximum concentration; Cmax: maximum concentration; MRT0-t: mean residence time; Cl: clearance rate; Vd: apparent volume of distribution at steady state.
In vitro metabolic stability of metoprolol
The in vitro metabolism of metoprolol in rat liver microsomes showed that the half-life of metoprolol was 28.76 ± 7.34 min with the intrinsic clearance rate of 48.19 ± 8.59 μL/min/mg protein. In the presence of Ginkgo, the half-life of metoprolol was prolonged to 46.54 ± 4.84 min and the clearance rate decreased to 29.78 ± 7.17 μL/min/mg protein (Table 5).
Table 5.
Metabolic stability of metoprolol in rat liver microsomes
| Metoprolol | Metoprolol + Ginkgo | P-value | |
|---|---|---|---|
| Half-life (min) | 28.76 ± 7.34 | 46.54 ± 4.84 | < 0.0001 |
| Intrinsic clearance (μL/min/mg protein) | 48.19 ± 8.59 | 29.78 ± 7.17 | < 0.0001 |
Effect of Ginkgo on the activity of CYP2D6
In rat liver microsomes, Ginkgo was found to serve as an inhibitor of CYP2D6 and its inhibitory effect was enhanced with the increase in concentration. The concentration-dependent manner was demonstrated by an IC50 of 11.17 μM (Figure 4).
Figure 4.
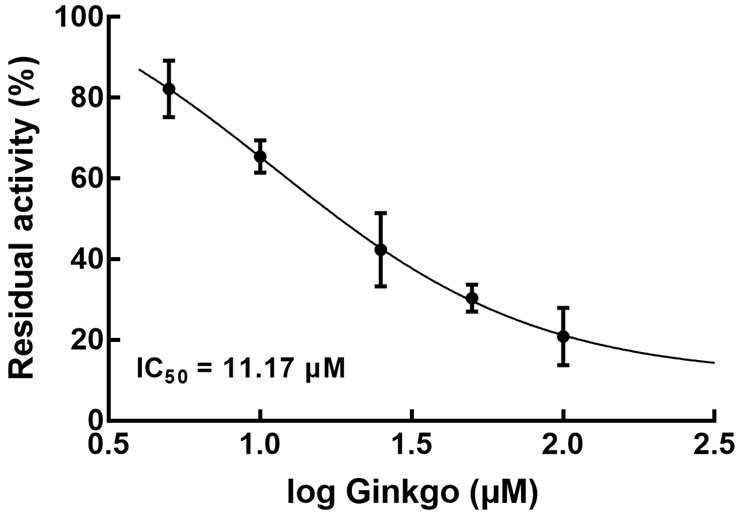
Effect of Ginkgo (0, 5, 10, 25, 50, and 100 μM) on the activity of CYP2D6 in rat liver microsomes.
Discussion
Both metoprolol and Ginkgo tablets are commonly prescribed to treat cardiovascular diseases such as angina pectoris, myocardial infarction, coronary heart disease, and stroke [7,8,10-13]. The combination of metoprolol and Ginkgo tablets has been applied in the treatment of heart failure and coronary heart disease, resulting in significant therapeutic effects. While no adverse reactions have been reported to data, it is still necessary to assess their interaction during co-administration.
An optimized LC-MS/MS quantitative analysis method of metoprolol was developed in the present study. Through a series of validation indicators, the method was considered to have high selectivity, accuracy, and precision and showed satisfactory stability under different measuring conditions. With the help of this analysis method, the interaction between metoprolol and Ginkgo tablets was assessed.
Previously, the combination of Ginkgo and drugs with similar indications was known to induce drug-drug interactions [14-16]. For example, combining Ginkgo tablets with atorvastatin improved the therapeutic efficiency of coronary heart disease, but Ginkgo tablets notably boosted the metabolism of atorvastatin [17]. The use of Ginkgo tablets with rosiglitazone could be applied in the treatment of 2 diabetes mellitus, which accelerates rosiglitazone metabolism by regulating CYP2C8 and CYP2C9 activity [18]. Metoprolol was co-administrated with Ginkgo at concentrations of 20 mg/kg and 2.4 mg/kg derived from the clinical dosage of these two drugs. The pharmacokinetic profile of metoprolol showed that the co-administration induced significant changes in the in vivo metabolism of metoprolol (Figure 3). Compared to a single administration of metoprolol, its co-administration with Ginkgo resulted in an increasing AUC, and Cmax, and prolonged the half-life, suggesting an increasing systemic exposure and reduced clearance of metoprolol (Table 4). Interestingly, the plasma concentration of metoprolol reached a similar level after 100 min of corresponding administration strategies between the single administration and co-administration group. The metabolic rate and plasma concentration would stabilize after the Tmax, which was considered mainly a result of excretion. Therefore, it was hypothesized that the co-administration exerted stronger effect on the early stage of the pharmacokinetics of metoprolol and slightly influenced the excretion process. On the other hand, in vitro metabolism of metoprolol was also investigated in rat liver microsomes in the present study. Consistently, Ginkgo improved the in vitro metabolic stability of metoprolol by increasing in vitro half-life and decreasing the clearance rate. The co-administration was performed by prolonging the interval between the administration of Ginkgo and metoprolol according to previous studies on in vivo drug-drug interaction evaluation, which could avoid adverse chemical interactions and ensure the observation result was from pharmacokinetic interactions. However, this method might prolong the experimental period. Sequential administration can be considered for the future studies, and the appropriate time intervals are a research focus.
The activity of CYP450s has been accepted as a major risk factor for drug-drug interactions. The inhibitory effect of Ginkgo tablets on the activity of CYP3A4 was revealed to lead to an inhibition of amlodipine biotransformation and therefore induced drug-drug interaction [15]. It is well known that metoprolol is metabolized by CYP2D6, and the effect of Ginkgo tablets on CYP2D6 activity has also been disclosed. Hellum et al. found that Ginkgo significantly suppressed CYP2D6 activity with low concentration and showed a dramatically enhanced effect with high concentration [19]. Herein, the concentration-dependent inhibition of CYP2D6 by the Ginkgo tablet was observed with an IC50 of 11.17 μM (Figure 4). The co-administered concentration of Ginkgo in the present study is lower than the induced concentration in the previous study. Hence, it was speculated that the interaction between metoprolol and Ginkgo tablets was mediated by CYP2D6. This study provided direct evidence demonstrating the inhibition of CYP2D6 by Ginkgo, which was hypothesized as the mechanism mediating its interaction with metoprolol. However, there was a lack of reverse verification for the involvement of CYP2D6, and a rescue experiment with the knockout or knockdown of CYP2D6 would be an effective means to test this. Further investigation should focus on the high co-administration concentration of Ginkgo and confirm the involvement of CYP2D6 with the help of molecular biologic means to complete various combining conditions and completely disclose the interaction mechanism. Moreover, the results might be distinct in humans. A previous study reported that the effect of Ginkgo tablets on CYP2C9 activity did not influence the clearance of flurbiprofen due to its relatively lower administrated amounts [20]. Therefore, clinical validation is also necessary for drug-drug interaction assessment.
Conclusions
The developed optimized LC-MS/MS method, which offers high sensitivity and accuracy, can be used for the measurement of metoprolol. Co-administration of metoprolol and Ginkgo tablets inhibited metabolism of metoprolol due to the inhibition of CYP2D6 by Ginkgo.
Acknowledgements
This project was supported by Zhejiang Medical and Health Technology Project (2022KY259), Zhejiang Traditional Chinese Medicine Science and Technology Project (2022ZB279), Project of Medical and Health Technology Development Program in Shandong Province (2019WS366), and Hangzhou Special Fund for Supporting Science and Technology in the Development of Biomedical and Health Industries (2021WJCY143 and 2021WJCY145).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ogrodowczyk M, Dettlaff K, Jelinska A. Beta-blockers: current state of knowledge and perspectives. Mini Rev Med Chem. 2016;16:40–54. doi: 10.2174/1389557515666151016125948. [DOI] [PubMed] [Google Scholar]
- 2.Prijic S, Buchhorn R. Mechanisms of beta-blockers action in patients with heart failure. Rev Recent Clin Trials. 2014;9:58–60. doi: 10.2174/1574887109666140908125402. [DOI] [PubMed] [Google Scholar]
- 3.Ripley TL, Saseen JJ. β-blockers: a review of their pharmacological and physiological diversity in hypertension. Ann Pharmacother. 2014;48:723–733. doi: 10.1177/1060028013519591. [DOI] [PubMed] [Google Scholar]
- 4.Heusch G, Kleinbongard P. Is metoprolol more cardioprotective than other beta-blockers? Eur Heart J. 2020;41:4441–4443. doi: 10.1093/eurheartj/ehaa764. [DOI] [PubMed] [Google Scholar]
- 5.Lee CM, Kang P, Cho CK, Park HJ, Lee YJ, Bae JW, Choi CI, Kim HS, Jang CG, Lee SY. Physiologically based pharmacokinetic modelling to predict the pharmacokinetics of metoprolol in different CYP2D6 genotypes. Arch Pharm Res. 2022;45:433–445. doi: 10.1007/s12272-022-01394-2. [DOI] [PubMed] [Google Scholar]
- 6.Wikstrand J, Warnold I, Tuomilehto J, Olsson G, Barber HJ, Eliasson K, Elmfeldt D, Jastrup B, Karatzas NB, Leer J, et al. Metoprolol versus thiazide diuretics in hypertension. Morbidity results from the MAPHY Study. Hypertension. 1991;17:579–588. doi: 10.1161/01.hyp.17.4.579. [DOI] [PubMed] [Google Scholar]
- 7.Yang WM, Wang H, Sun SL, Zhang YL, Chen XH, Lu JQ, Wu BS, Sun JN, Chen W, Tang LL The Editorial Team Represented. Expert consensus on clinical application of GBE50 Dispersible Tablets for ischemic cardiovascular and cerebrovascular diseases. Zhongguo Zhong Yao Za Zhi. 2022;47:301–305. doi: 10.19540/j.cnki.cjcmm.20210809.501. [DOI] [PubMed] [Google Scholar]
- 8.Issing W, Klein P, Weiser M. The homeopathic preparation Vertigoheel versus Ginkgo biloba in the treatment of vertigo in an elderly population: a double-blinded, randomized, controlled clinical trial. J Altern Complement Med. 2005;11:155–160. doi: 10.1089/acm.2005.11.155. [DOI] [PubMed] [Google Scholar]
- 9.Yale SH, Glurich I. Analysis of the inhibitory potential of Ginkgo biloba, Echinacea purpurea, and Serenoa repens on the metabolic activity of cytochrome P450 3A4, 2D6, and 2C9. J Altern Complement Med. 2005;11:433–439. doi: 10.1089/acm.2005.11.433. [DOI] [PubMed] [Google Scholar]
- 10.Haria M, Plosker GL, Markham A. Felodipine/metoprolol: a review of the fixed dose controlled release formulation in the management of essential hypertension. Drugs. 2000;59:141–157. doi: 10.2165/00003495-200059010-00011. [DOI] [PubMed] [Google Scholar]
- 11.Prakash A, Markham A. Metoprolol: a review of its use in chronic heart failure. Drugs. 2000;60:647–678. doi: 10.2165/00003495-200060030-00011. [DOI] [PubMed] [Google Scholar]
- 12.Meng TT, Tian ZY, Xie XL, Li TT, Liu WD, Gao Y. Systematic review and Meta-analysis of clinical efficacy and safety of Ginkgo Leaf Tablets in treatment of acute cerebral infarction. Zhongguo Zhong Yao Za Zhi. 2021;46:1537–1546. doi: 10.19540/j.cnki.cjcmm.20200903.501. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Zhou S, Bronks R, Graham J, Myers S. Supervised exercise training combined with ginkgo biloba treatment for patients with peripheral arterial disease. Clin Rehabil. 2007;21:579–586. doi: 10.1177/0269215507075205. [DOI] [PubMed] [Google Scholar]
- 14.Dong B, Yuan S, Hu J, Yan Y. Effects of Ginkgo leaf tablets on the pharmacokinetics of losartan and its metabolite EXP3174 in rats and its mechanism. Pharm Biol. 2018;56:333–336. doi: 10.1080/13880209.2018.1481107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R, Zhang H, Sun S, Wang Y, Chai Y, Yuan Y. Effect of Ginkgo Leaf Tablets on the pharmacokinetics of amlodipine in rats. Eur J Drug Metab Pharmacokinet. 2016;41:825–833. doi: 10.1007/s13318-015-0312-3. [DOI] [PubMed] [Google Scholar]
- 16.Wolf HR. Does Ginkgo biloba special extract EGb 761 provide additional effects on coagulation and bleeding when added to acetylsalicylic acid 500 mg daily? Drugs R D. 2006;7:163–172. doi: 10.2165/00126839-200607030-00003. [DOI] [PubMed] [Google Scholar]
- 17.Ren Y, Li H, Liu X. Effects of Ginkgo leaf tablets on the pharmacokinetics of atovastatin in rats. Pharm Biol. 2019;57:403–406. doi: 10.1080/13880209.2019.1622569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xing X, Kong M, Hou Q, Li J, Qian W, Chen X, Li H, Yang C. Effects of ginkgo leaf tablet on the pharmacokinetics of rosiglitazone in rats and its potential mechanism. Pharm Biol. 2022;60:1190–1197. doi: 10.1080/13880209.2022.2087688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellum BH, Hu Z, Nilsen OG. The induction of CYP1A2, CYP2D6 and CYP3A4 by six trade herbal products in cultured primary human hepatocytes. Basic Clin Pharmacol Toxicol. 2007;100:23–30. doi: 10.1111/j.1742-7843.2007.00011.x. [DOI] [PubMed] [Google Scholar]
- 20.Greenblatt DJ, von Moltke LL, Luo Y, Perloff ES, Horan KA, Bruce A, Reynolds RC, Harmatz JS, Avula B, Khan IA, Goldman P. Ginkgo biloba does not alter clearance of flurbiprofen, a cytochrome P450-2C9 substrate. J Clin Pharmacol. 2006;46:214–221. doi: 10.1177/0091270005283465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.