Abstract
Objective: To study the effectiveness and safety of abdominal active balloon closure during cesarean sections for patients with pernicious placenta previa (PPP). Methods: A retrospective analysis was conducted on 140 patients with PPP and placenta accreta who were hospitalized and gave birth at the Third Affiliated Hospital of Zhengzhou University from June 2022 to December 2023. The patients were divided into two groups based on whether intervention was applied. The intervention group [intraoperative abdominal aortic balloon occlusion (IAABO) group] included 118 patients who received prophylactic abdominal aortic balloon occlusion during cesarean section. The routine group, without balloon occlusion, consisted of 22 patients. Results: Compared to the routine group, the IAABO group had significantly lower intraoperative blood loss, blood transfusion volume, and hysterectomy rate (all P<0.05). Additionally, the IAABO group showed lower postoperative pain scores, shorter ambulation time, and higher patient satisfaction (all P<0.05). The neonatal intensive care unit admission rate and incidence of complications were also significantly lower in the IAABO group (both P<0.05). Conclusion: Abdominal aortic balloon closure during cesarean sections can reduce bleeding and the need for blood transfusions in cases of PPP, thereby lowering the hysterectomy rate and the incidence of related complications.
Keywords: Pernicious placenta previa, intraoperative abdominal aortic balloon occlusion, cesarean section
Introduction
Pernicious placenta previa (PPP) refers to a condition where the placenta is associated with a previous cesarean section scar in a current pregnancy [1,2]. With the implementation of the two-child policy in China, the cesarean section rate has increased, consequently raising the risk of PPP [3].
Currently, there are few reports on the incidence of PPP, with most statistics focusing on placenta previa in general. According to studies encompassing data from multiple hospitals between 1982 and 2018, the incidence of PPP was 0.56% [4]. Cesarean hysterectomy is often considered the preferred method by many national societies. Various conservative treatments can be employed, such as uterine artery ligation, external compression with different uterine suture techniques, uterine cavity compression using various balloons, preoperative placement of abdominal aortic balloons, or combinations of these methods [5]. These approaches, however, may not always be effective, particularly in severe cases of placental adherence and invasion into the myometrium or serosa, which are more common in PPP cases [6].
The increased likelihood of placental and villous invasion into the myometrium or even serosa is a significant concern. Some researchers suggest that the abnormal expression of factors promoting angiogenesis and trophoblast cell activity is a major contributor to implantation [7,8]. This aligns with Illsley’s view that factors secreted by trophoblast cells, which facilitate endometrial invasion, are a key cause of placental implantation [9]. Postpartum hemorrhage can occur due to the invasion of the placenta into the myometrium, leading to the loss of normal myometrial tissue [10,11]. Following placental detachment, poor uterine contraction and the ineffectiveness of uterotonic agents can result in the failure to close blood sinuses, causing intractable postpartum hemorrhage [12,13]. In such cases, obstetricians often resort to hysterectomy to save the lives of patients suffering from severe postpartum hemorrhage [14-16]. However, the loss of reproductive function significantly impacts the affected women and their families.
Interventional therapy, specifically intraoperative abdominal aortic balloon occlusion (IAABO), presents a novel approach to managing excessive bleeding associated with PPP during cesarean sections. By preemptively occluding the abdominal aorta, IAABO aims to significantly reduce blood flow to the pelvic region, thereby minimizing intraoperative blood loss and potentially decreasing the need for hysterectomy. This study investigates the efficacy and safety of IAABO in PPP patients undergoing cesarean sections, aiming to establish its clinical significance as a potential standard protocol for managing PPP.
Materials and methods
Research subjects
The study was approved by the Ethics Committee of West China Second University Hospital (No. 2023[018]), and all patients provided oral informed consent. A retrospective analysis was conducted on 140 patients with PPP complicated by placental implantation, who gave birth at the Third Affiliated Hospital of Zhengzhou University between June 2022 and December 2023. These patients were divided into two groups based on whether they opted for intervention during delivery. The intervention group (IAABO group) included 118 patients who received prophylactic intraoperative abdominal aortic balloon occlusion during cesarean section. The routine group consisted of 22 patients who underwent standard cesarean section procedures and received other bleeding control treatments as necessary.
Inclusion criteria: (1) Gestational age ≥28 weeks. (2) Age 18-45 years old. (3) Diagnosis of PPP implantation by color ultrasound or magnetic resonance imaging (MRI) before and during operation, according to the clinical diagnosis and treatment guidelines for placenta previa [17]. (4) Complete general information.
Exclusion criteria: (1) Incomplete clinical data. (2) Abnormal coagulation function. (3) Inability to cooperate with follow-up.
Data collection
Clinical data from the IAABO and routine groups during hospitalization were collected, including:
Patient information: name, age, hypertension, diabetes, smoking history, alcohol consumption, Body Mass Index (BMI), gestational age, contact information, number of pregnancies, previous deliveries, previous cesarean sections.
Surgical details: duration of surgery, intraoperative blood loss volume, blood transfusion volume.
Postoperative outcomes: complication rates (fever, infection, pain, thrombus, puerperal infection, etc.), hysterectomy rate, bladder injury rate, hospitalization time, opioid consumption, time to ambulation, patient satisfaction, duration of malodor, menstrual rehydration, rehospitalization rate.
Neonatal outcomes: newborn weight, neonatal intensive care unit (NICU) admission rate, complications.
Apgar scores at 1 and 5 minutes after birth, assessed by neonatologists, obstetricians, and midwives.
Operation modes
IAABO group
Before the cesarean section, after completing epidural anesthesia, the patient lay supine in the digital subtraction angiography (DSA) equipment, receiving oxygen inhalation and electrocardiographic (ECG) monitoring. The patient’s abdomen and bilateral inguinal areas were disinfected. After disinfection, 2% lidocaine was used for local anesthesia, and the right femoral artery was punctured. An 8F sheath (Medtronic, USA) was placed, and a 0.035-inch guide wire and a balloon catheter were introduced through the sheath. The balloon’s diameter was selected based on preoperative magnetic resonance measurements of the abdominal aorta and renal artery. With the guide wire’s assistance, the balloon was positioned on the abdominal aorta, with its distal end located below the renal artery level.
Subsequently, the obstetrician began the cesarean section. Once the fetus was delivered, the interventional physician filled the balloon with 0.9% sodium chloride solution to block blood flow in the abdominal aorta, preventing fresh blood supply to the pelvic cavity. To avoid tissue and organ ischemia and reduce the risk of ischemia-reperfusion injury, the balloon was deflated for 1 minute every 10 minutes to restore blood flow. The balloon remained inflated until the uterine wound was sutured and there was no fresh bleeding. After the uterus and abdomen were closed, the interventional physician exchanged the 5F pigtail catheter and the 5F Cobra catheter to perform angiography of the abdominal aorta, bilateral internal iliac artery, and external iliac artery, checking for vascular abnormalities and any contrast medium overflow in the uterine blood supply artery. If no abnormalities or fresh bleeding were detected, the operation was concluded, instruments were withdrawn, and the femoral artery puncture site was treated for hemostasis. The patient was then transferred to postoperative monitoring. Hysterectomy was performed if bleeding could not be controlled under the blocked state.
Routine group
During the cesarean section, bleeding and placental implantation were managed using local sutures, uterine artery ligation, uterine cavity tamponade, and other methods. Emergency hysterectomy was performed when necessary to control bleeding.
Blood tests
A 3 mL fasting venous blood sample was collected on the morning of the second day of hospitalization. After serum separation, hemoglobin, platelet count, prothrombin time, fibrinogen, and D-dimer levels were measured using a fully automated biochemical analyzer (BS-280, Mindray, China).
Visual analog scale (VAS)
Postoperative pain levels at 24 and 48 hours were assessed using the VAS for pain, ranging from 0 to 10 points, where a higher score indicates more severe pain. The Cronbach’s alpha for the VAS was 0.94 [18].
Quality of life score
Quality of life was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30), which includes five functional dimensions: physical, role, emotional, social, and cognitive functions. Each item was rated on a 1-7 Likert scale, with total scores ranging from 0 to 100, where higher scores indicate better quality of life. The Cronbach’s alpha for this questionnaire was 0.927 [19].
Statistical analysis
In conducting our analysis, we utilized G*Power 3.1.9.7, specifically the “Means: Difference between two independent means (two groups)” option for t-tests, to perform a post hoc analysis. We set the analysis to two-tailed mode with an effect size (d) of 0.7 and a significance level (α-error probability) of 0.05. Upon entering the sample sizes for both groups, we calculated the statistical power (1 - β error probability), achieving a power value of 0.849.
Data analysis was conducted using SPSS version 22.0. Normal data were presented as Mean ± SD, while non-normal data were presented as medians (P25-P75). For comparisons between measurement data groups, either the T-test or the Mann-Whitney U test was employed. Categorical data were analyzed using the χ2 test or Fisher’s exact probability method. The significance threshold was set at α=0.05. A P-value below 0.05 was regarded as statistically significant.
Results
Comparison of general data
As shown in Table 1, the results indicated no significant differences between the two groups in terms of age, hypertension, diabetes, smoking history, alcohol consumption, BMI, gestational age, gestational week, or number of pregnancies (all P>0.05).
Table 1.
Comparison of general data
| General information | Iaabo group (n=118) | Routine group (n=22) | t/χ2 | P | Power |
|---|---|---|---|---|---|
| Age | 31.96 ± 4.76 | 31.23 ± 4.41 | 0.697 | 0.491 | 0.10 |
| Hypertension | 6 (5.08%) | 2 (9.09%) | 0.059 | 0.808 | 0.11 |
| Diabetes | 12 (10.17%) | 3 (13.64%) | 0.012 | 0.915 | 0.08 |
| Smoking history | 4 (3.39%) | 2 (9.09%) | None | 0.238 | 0.22 |
| Alcohol consumption | 3 (2.54%) | 2 (9.09%) | 0.799 | 0.371 | 0.32 |
| BMI (kg/m2) | 23.85 ± 5.12 | 24.25 ± 3.51 | 0.450 | 0.655 | 0.06 |
| Gestational age | 36.75 ± 1.53 | 36.62 ± 1.84 | 0.301 | 0.765 | 0.06 |
| Gestational week <36 W | 36 (30.51%) | 10 (45.45%) | 0.28 | ||
| Gestational week ≥36 W | 82 (69.49%) | 12 (54.55%) | 1.261 | 0.261 | |
| Number of pregnancies ≤2 | 21 (17.80%) | 1 (4.55%) | 1.560 | 0.212 | 0.34 |
| Number of pregnancies ≥3 | 97 (82.20%) | 21 (95.45%) | |||
| Previous production: once | 76 (64.41%) | 13 (59.09%) | 0.08 | ||
| Previous production: 2 times | 38 (32.20%) | 8 (36.36%) | |||
| Previous production times: 3 times | 4 (3.39%) | 1 (4.55%) | None | 0.762 | |
| Previous cesarean section (1 time) | 77 (65.25%) | 13 (59.09%) | 0.06 | ||
| Previous cesarean section (2 times) | 37 (31.36%) | 9 (40.91%) | |||
| Previous cesarean section (3 times) | 4 (3.39%) | 0 (0.00%) | None | 0.658 |
Comparison of hemostatic parameters
As shown in Table 2, there were no significant differences in hemostatic parameters between the two groups, including hemoglobin, platelet count, prothrombin time, fibrinogen, and D-dimer levels (P>0.05).
Table 2.
Comparison of hemostatic parameters
| Parameter | IAABO Group (n=118) | Routine Group (n=22) | t | p Value | Power |
|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 10.78 ± 2.32 | 10.03 ± 2.18 | 1.469 | 0.152 | 0.29 |
| Platelet count (×109/L) | 238.61 ± 43.87 | 242.13 ± 43.26 | 0.350 | 0.729 | 0.06 |
| Prothrombin time (seconds) | 13.85 ± 3.24 | 14.48 ± 3.12 | 0.862 | 0.396 | 0.13 |
| Fibrinogen level (g/L) | 4.52 ± 1.04 | 4.41 ± 1.15 | 0.419 | 0.679 | 0.07 |
| D-dimer (ug/mL) | 0.72 ± 0.26 | 0.84 ± 0.31 | 1.669 | 0.107 | 0.45 |
Comparison of intraoperative conditions
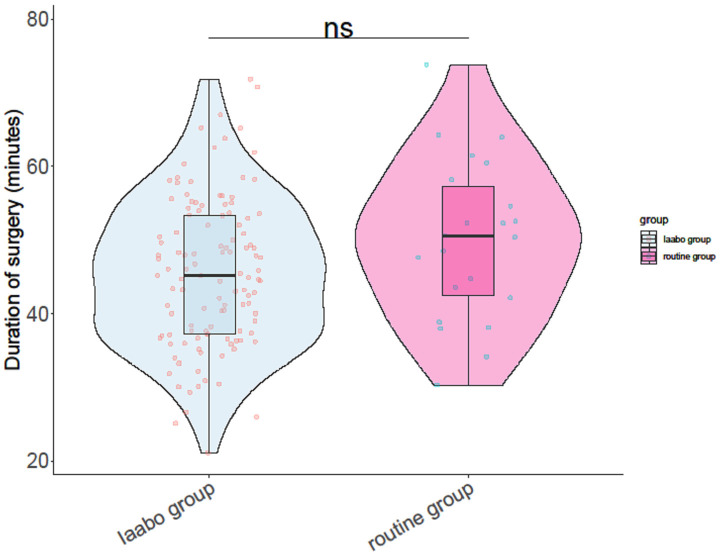
As shown in Table 3, the IAABO group experienced significantly reduced blood loss (P=0.037) and blood transfusion volumes (P=0.004). The hysterectomy rate in the IAABO group was also lower than in the routine group (P=0.02). There were no significant differences in the duration of surgery (Figure 1) or bladder injury rate between the two groups (P>0.05).
Table 3.
Comparison of intraoperative condition
| Intraoperative condition | Iaabo group | Routine group | t/χ2 | P | Power |
|---|---|---|---|---|---|
| Intraoperative blood loss (ML) | 980.24 ± 230.15 | 1105.26 ± 248.27 | 2.193 | 0.037 | 0.64 |
| Blood transfusion volume (ML) | 425.38 ± 109.15 | 503.24 ± 108.27 | 3.093 | 0.004 | 0.86 |
| Hysterectomy rate | 0 (0%) | 2 (9.09%) | 5.384 | 0.02 | 0.92 |
| Bladder injury rate | 9 (7.63%) | 1 (4.55%) | 0.004 | 0.949 | 0.08 |
Figure 1.
Duration of surgery.
Comparison of postoperative conditions
As shown in Table 4, there were no significant differences between the two groups in hospital stay, postoperative complication rate, or opioid consumption (all P>0.05). However, there were significant differences in VAS scores at 24 hours (3.54 ± 1.05 vs. 4.15 ± 1.23, t=2.429, P=0.016) and 48 hours (2.82 ± 0.73 vs. 3.55 ± 1.05, t=3.124, P=0.004) after surgery (Figure 2). Additionally, the IAABO group had shorter time to ambulation (P=0.042) and higher patient satisfaction (P=0.003).
Table 4.
Comparison of postoperative conditions
| Postoperative condition | Iaabo group | Routine group | t/χ2 | P | Power |
|---|---|---|---|---|---|
| Hospital stay (d) | 10.09 ± 3.95 | 9.55 ± 5.79 | 0.421 | 0.677 | 0.09 |
| Postoperative complication rate | 9 (7.63%) | 1 (4.55%) | 0.004 | 0.949 | 0.08 |
| Opioid consumption (mg) | 10.35 ± 5.14 | 12.37 ± 6.25 | 1.428 | 0.165 | 0.37 |
| Time to ambulation (hrs) | 12.57 ± 3.25 | 14.58 ± 4.17 | 2.141 | 0.042 | 0.71 |
| Patient satisfaction (%) | 87.25 ± 9.62 | 80.24 ± 9.26 | 3.24 | 0.003 | 0.88 |
Figure 2.
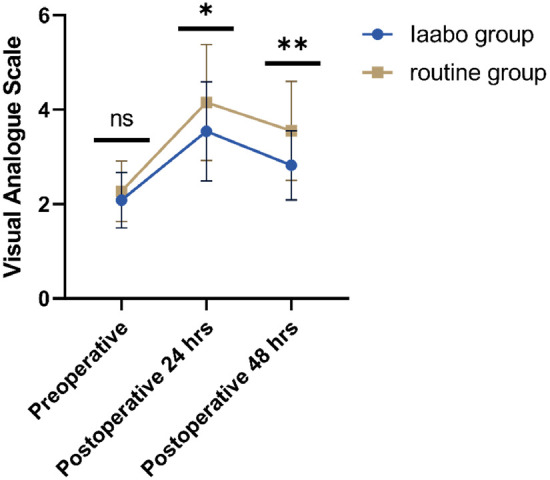
The preoperative and postoperative visual analogue scale (VAS) at 24 hrs and 48 hrs.
Comparison of follow-up after discharge
As shown in Table 5, there were no significant differences between the IAABO group and the routine group in the duration of malodor, time to menstrual rehydration, rehospitalization rate, or quality of life (all P>0.05).
Table 5.
Comparison of follow-up after discharge
| Follow up after discharge | Iaabo group | Routine group | t/χ2 | P | Power |
|---|---|---|---|---|---|
| Duration of malodor (d) | 30.77 ± 2.83 | 30.51 ± 3.17 | 0.357 | 0.724 | 0.07 |
| Menstrual rehydration time (d) | 108.07 ± 12.31 | 109.14 ± 13.63 | 0.344 | 0.734 | 0.07 |
| Rehospitalization | 12 (10.17%) | 3 (13.64%) | 0.012 | 0.915 | 0.08 |
| Physical Function | 70.17 ± 4.81 | 68.18 ± 5.03 | 1.710 | 0.098 | 0.42 |
| Role Function | 68.32 ± 5.66 | 67.54 ± 6.34 | 0.536 | 0.596 | 0.09 |
| Emotional Function | 71.29 ± 4.58 | 69.38 ± 4.25 | 1.911 | 0.065 | 0.43 |
| Cognitive Function | 83.56 ± 4.83 | 82.22 ± 5.06 | 1.155 | 0.258 | 0.22 |
| Social Function | 76.82 ± 4.56 | 76.33 ± 4.26 | 0.493 | 0.626 | 0.08 |
Comparison of newborn condition
As shown in Table 6, there were no significant differences between the IAABO group and the routine group in birth weight or Apgar scores at 1 minute and 5 minutes (all P>0.05). However, the IAABO group had significantly lower NICU admission rates (11.86% vs. 31.82%, χ2=4.331, P=0.037) and fewer complications (8.47% vs. 31.82%, χ2=7.410, P=0.006) compared to the routine group.
Table 6.
Comparison of newborn condition
| Neonatal condition | Iaabo group | Routine group | t/χ2 | P | Power |
|---|---|---|---|---|---|
| Newborn weight (kg) | 2.96 ± 1.34 | 2.64 ± 1.13 | 1.187 | 0.244 | 0.19 |
| Apgar 1 min score | 8.13 ± 1.24 | 8.32 ± 1.16 | 0.704 | 0.487 | 0.11 |
| Apgar 5 min score | 9.15 ± 1.68 | 9.26 ± 1.25 | 0.354 | 0.726 | 0.06 |
| NICU admission | 14 (11.86%) | 7 (31.82%) | 4.331 | 0.037 | 0.68 |
| Neonatal complications | 10 (8.47%) | 7 (31.82%) | 7.410 | 0.006 | 0.88 |
Discussion
PPP refers to a condition where the placenta is attached to a previous cesarean section scar during a subsequent pregnancy, posing a high risk of placental implantation [20]. The risk of placental implantation in cases of PPP is about 50%, making it a leading indication for emergency hysterectomy [21-23].
The incidence of placenta previa in women with a history of cesarean section is five times higher than in those without such a history, with recurrence rates of placental implantation in subsequent pregnancies reaching approximately 50% [24,25]. Placental implantation is often associated with repeated painless vaginal bleeding during pregnancy, with some cases of severe hemorrhage potentially leading to hemorrhagic shock which threatens the lives of both mother and child [26]. The low position of the placenta in PPP, often covering the internal cervical os, increases the risk of placental detachment and bleeding due to poor stretchability in the lower uterine segment during the third trimester. Additionally, the poor contractile ability of the lower uterus in PPP patients prevents the closure of blood sinuses post-delivery, elevating the risk of postpartum hemorrhage [27]. Placental attachment on the anterior wall is particularly hazardous.
Previous studies have demonstrated that most women with placenta previa and placental implantation experience significant perioperative bleeding, averaging over 1000 mL [28]. This substantial blood loss often results in hemorrhagic shock, endangering maternal and fetal safety [29]. Consequently, PPP combined with placental implantation is classified as a very high-risk pregnancy, necessitating timely transfer to a facility capable of emergency interventions. Close monitoring, preoperative blood preparation, and readiness for transfusions are essential during delivery.
To reduce intraoperative bleeding and the rate of hysterectomy, various techniques have been employed by obstetricians, including the use of carboprost tromethamine, B-Lynch sutures, uterine cavity tamponade, “8” suture hemostasis, and uterine artery ligation. Li et al. reported the successful use of a low abdominal aortic balloon during cesarean section to reduce intraoperative bleeding in a patient with placenta previa [30]. They suggested that the balloon could be expanded if heavy bleeding was anticipated. Given the recognized clinical efficacy of this method, IAABO technology has been successfully implemented in clinical settings, becoming a crucial approach for managing patients with placenta previa, placental implantation, and similar conditions [31].
Some scholars believe that selecting appropriate surgical methods is crucial for reducing intraoperative bleeding and preserving reproductive function [32,33]. In cases of PPP complicated by implantation, the placental position is typically low and often adheres to or penetrates the bladder, making the operation challenging and increasing the risk of major bleeding. During cesarean sections for PPP patients, hysterectomy should be considered under the following conditions to save the patient’s life: (1) The patient has extensive placental implantation or penetration of the uterus, reaching the bladder or adjacent organs, making separation difficult. (2) The placenta previa is central, with placental tissue covering the entire lower uterine segment and cervical myometrium. (3) Conservative treatments fail to effectively stop bleeding during the operation, and the significant bleeding endangers the patient’s life.
To prevent the deterioration of the condition, obstetricians must evaluate whether timely hysterectomy is necessary. However, with the implementation of China’s two-child policy and improvements in quality of life, many pregnant women wish to preserve their uterus. To reduce intraoperative bleeding and the rate of hysterectomy, obstetricians have developed new surgical methods. Huang et al. proposed using rubber catheters to temporarily ligate the low abdominal aorta to block the pelvic blood supply, with intermittent ligation as needed during the operation to reduce bleeding and hysterectomy rates [34]. Other scholars suggested using 100 mg of gelatin sponge dissolved in 20 ml of sterile physiological saline for internal iliac artery embolization to achieve hemostasis during the operation, with restoration 2-3 weeks postoperatively [35].
In recent years, interventional technology for obstetric acute hemorrhage has advanced significantly. Balloon occlusion, widely recognized, involves angiography of the main blood supply artery via femoral artery puncture. A balloon catheter is placed to block the blood supply, reducing intraoperative bleeding. This technique has gradually been used to treat PPP, mainly targeting the internal iliac artery and abdominal aorta. The use of interventional technology for cesarean sections in patients with high-risk pregnancies caused by placenta previa or placental implantation has been included in radiology guidelines. As interventional technology matures, IAABO is increasingly used to treat PPP patients with placenta implantation.
In this study, among the 118 patients in the IAABO group, 4 underwent bladder repair due to bladder injury, while in the routine group, 2 out of 22 patients underwent hysterectomy, and 1 underwent bladder repair. The results indicate that abdominal aortic balloon occlusion can reduce intraoperative bleeding, blood transfusion, and hysterectomy rates. Previous literature has reported that IAABO-assisted cesarean sections significantly reduce bleeding volume and hysterectomy rates compared to conventional cesarean sections [36]. The advantage of abdominal aortic balloon occlusion is its ability to block most of the pelvic blood supply, which is more effective than uterine artery embolization. Short-term blood supply blockage (40-60 minutes) does not lead to lower limb defects. Wang et al. found that IAABO reduced postoperative bleeding but not the hysterectomy rate [37]. The reason may be that blocking most pelvic blood supply during balloon release reduces bleeding, providing a clear surgical field and facilitating the surgeon’s work.
Currently, balloons primarily block the abdominal aorta and bilateral internal iliac arteries. The advantages and disadvantages of these approaches are still debated. Some scholars report that bilateral internal iliac artery balloon blocking reduces intraoperative bleeding and hysterectomy rates [38]. However, other studies indicate that temporary internal iliac artery balloon occlusion does not reduce the hysterectomy rate in implanted PPP patients, although it does reduce intraoperative bleeding [39]. Wang et al. showed that both bilateral internal iliac artery and abdominal aortic balloon occlusion significantly reduce intraoperative bleeding and hysterectomy rates [40]. However, compared to internal iliac artery occlusion, IAABO has better effects and higher safety. This study did not compare abdominal aortic balloon occlusion with internal iliac artery balloon occlusion. Further research is needed to demonstrate the advantages and disadvantages of these two interventional occlusion methods.
The interventional technique for cesarean sections offers several advantages, including a simple operation, short radiation exposure time, and a low blocking plane. Organs below the blocking plane generally tolerate temporary ischemia well. The technique blocks most of the pelvic blood supply when the balloon is inflated, reducing bleeding and providing a relatively clear surgical field, which facilitates the surgeon’s work [20]. However, this intervention can lead to complications such as pain, infection, arterial thrombosis, and acute renal failure. Renal failure is the most serious complication and may be related to the balloon’s high position and renal artery variations. Preoperative vascular examinations and accurate intraoperative positioning are essential to minimize the risk of acute renal failure. If the procedure is complex and prolonged, the balloon can be intermittently deflated to restore blood flow. Wang et al. highlighted renal failure as a possible complication of this treatment, underscoring the need for careful monitoring [37].
In this study, some patients in the IAABO group experienced pain at the femoral artery puncture site postoperatively, likely mitigated by the widespread use of postoperative analgesia pumps. These symptoms were generally mild and resolved within a few days, thus not included in the postoperative pain incidence statistics. Three patients developed arteriovenous thrombosis in the lower limbs, possibly due to postoperative immobility and maternal blood hypercoagulability, but recovered after follow-up. One patient in each group developed puerperal infection, likely related to repeated vaginal bleeding and prolonged bed rest to protect the fetus. Both patients improved with treatment and were discharged.
Abdominal aortic balloon occlusion can safely cut off the pelvic blood supply during cesarean sections for less than 30 minutes, preventing tissue and organ ischemia and necrosis, and reducing ischemia-reperfusion injury. In this study, the balloon was deflated for 1 minute every 10 minutes to restore blood flow. Follow-up on the duration of postoperative lochia and the time of menstrual rehydration showed that abdominal aortic balloon occlusion did not affect postoperative uterine involution.
The research results indicate that while the incidence of some related diseases after IAABO is relatively low, it does still occur. Therefore, the intraoperative and postoperative care of PPP patients complicated by placental implantation is crucial. It is essential to maintain an unobstructed venous channel and concurrently manage fluid infusion, anemia correction, hemostasis, infection prevention, and anticoagulation. Vital signs must be closely monitored, and observations should include vaginal bleeding volume, consciousness, urine output, uterine contractions, and dorsalis pedis artery pulse. Additionally, it is important to dynamically monitor anemia levels, coagulation function, liver and kidney function, and other biochemical indicators. In the event of any abnormalities, timely symptomatic treatment and preparedness for emergency interventions are necessary.
In this study, the exposure time of the fetus in the IAABO group was (7.2 ± 1.5) seconds, with a dose of (3.4 ± 1.1) mGy. Follow-up results indicated that this dose did not cause fetal tissue damage, dysfunction, or intellectual development disorder, but might increase the incidence of childhood cancer. According to the guidelines of the International Association of Radiology, when the total radiation exposure to the fetus is ≤100 mGy, it does not cause significant fetal damage and is not an indication for termination of pregnancy [41]. In this study, the exposure dose of the included fetuses was far below this threshold. The study showed that abdominal aortic balloon occlusion had no significant adverse effect on the newborn’s condition at birth. Numerous studies have shown that neonatal asphyxia, hospitalization rate, and mortality are related to the interval between emergency cesarean section and fetal delivery [42,43]. Nonetheless, some experts still suggest that ultrasound-guided abdominal aortic balloon occlusion, which has the same effect as angiography-guided occlusion without radiation, can protect the safety of patients, fetuses, doctors, and nurses [44,45].
In this study, 4 out of 118 patients in the IAABO group and 1 out of 22 patients in the routine group had placenta implanted into the bladder during the operation, necessitating bladder repair. The statistical results showed that IAABO did not reduce the risk of bladder injury during cesarean section in patients with implantable PPP. This may be because some patients with dangerous placenta previa and placental implantation had their placenta penetrating the bladder or had severe pelvic adhesions, easily damaging the surrounding organs such as the bladder, ureter, and bowels.
Patients with PPP accompanied by implantation often experience significant and turbulent bleeding during surgery, resulting in an unclear visual field and increased operational difficulty. This can lead to damage to periuterine organs such as the bladder and ureter and complications such as disseminated intravascular coagulation and multiple organ failure, which can be life-threatening. Therefore, patients with dangerous placenta previa and placental implantation often require technical support from urology during cesarean sections. Some scholars advocate for preoperative cystoscopy to assess the bladder shape, the extent and position of placental implantation, and its relationship with the bladder triangle [46-48]. This combined evaluation helps in comprehensively assessing the difficulty of the operation and preparing adequately.
Despite the valuable insights garnered from this study, several limitations warrant consideration. Firstly, the retrospective nature of the study introduces inherent biases and limitations associated with data collection and analysis. Prospective studies leveraging standardized protocols and comprehensive data capture would offer enhanced robustness and reliability, enabling more definitive conclusions. Additionally, the single-center design of the study limits the generalizability of the findings, warranting validation in multicenter cohorts to ensure broader applicability across diverse patient populations and clinical settings.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 82071651) and the Tianfu Jincheng Laboratory Foundation of the Frontier Medical Center (TFJC2023010001).
Disclosure of conflict of interest
None.
References
- 1.Matsuo K, Sangara RN, Matsuzaki S, Ouzounian JG, Hanks SE, Matsushima K, Amaya R, Roman LD, Wright JD. Placenta previa percreta with surrounding organ involvement: a proposal for management. Int J Gynecol Cancer. 2023;33:1633–1644. doi: 10.1136/ijgc-2023-004615. [DOI] [PubMed] [Google Scholar]
- 2.Rowe T. Placenta previa. J Obstet Gynaecol Can. 2014;36:667–668. doi: 10.1016/S1701-2163(15)30503-X. [DOI] [PubMed] [Google Scholar]
- 3.Hou Y, Zhou X, Shi L, Peng J, Wang S. Influence factors and pregnancy outcomes for pernicious placenta previa with placenta accreta. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020;45:1074–1081. doi: 10.11817/j.issn.1672-7347.2020.190656. [DOI] [PubMed] [Google Scholar]
- 4.Jauniaux E, Grønbeck L, Bunce C, Langhoff-Roos J, Collins SL. Epidemiology of placenta previa accreta: a systematic review and meta-analysis. BMJ Open. 2019;9:e031193. doi: 10.1136/bmjopen-2019-031193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu L, Lu J, Huang W, Zhao J, Li M, Zhuang H, Li Y, Liu H, Du L. A modified suture technique for the treatment of patients with pernicious placenta previa and placenta accreta spectrum: a case series. Ann Transl Med. 2021;9:1140. doi: 10.21037/atm-21-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kingdom JC, Hobson SR, Murji A, Allen L, Windrim RC, Lockhart E, Collins SL, Soleymani Majd H, Alazzam M, Naaisa F, Shamshirsaz AA, Belfort MA, Fox KA. Minimizing surgical blood loss at cesarean hysterectomy for placenta previa with evidence of placenta increta or placenta percreta: the state of play in 2020. Am J Obstet Gynecol. 2020;223:322–329. doi: 10.1016/j.ajog.2020.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afshar Y, Dong J, Zhao P, Li L, Wang S, Zhang RY, Zhang C, Yin O, Han CS, Einerson BD, Gonzalez TL, Zhang H, Zhou A, Yang Z, Chou SJ, Sun N, Cheng J, Zhu H, Wang J, Zhang TX, Lee YT, Wang JJ, Teng PC, Yang P, Qi D, Zhao M, Sim MS, Zhe R, Goldstein JD, Williams J 3rd, Wang X, Zhang Q, Platt LD, Zou C, Pisarska MD, Tseng HR, Zhu Y. Circulating trophoblast cell clusters for early detection of placenta accreta spectrum disorders. Nat Commun. 2021;12:4408. doi: 10.1038/s41467-021-24627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, Wang Y, Li M. CXCL12/CXCR4/CXCR7 axis in placenta tissues of patients with placenta previa. Open Life Sci. 2023;18:20220642. doi: 10.1515/biol-2022-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Illsley NP, DaSilva-Arnold SC, Zamudio S, Alvarez M, Al-Khan A. Trophoblast invasion: lessons from abnormally invasive placenta (placenta accreta) Placenta. 2020;102:61–66. doi: 10.1016/j.placenta.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuzaki S, Nagase Y, Ueda Y, Lee M, Matsuzaki S, Maeda M, Takiuchi T, Kakigano A, Mimura K, Endo M, Tomimatsu T, Kimura T. The association of endometriosis with placenta previa and postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2021;3:100417. doi: 10.1016/j.ajogmf.2021.100417. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Song Z, Wang X, Zhang M, Chen X, Zhang D. Ultrasound-based nomogram for postpartum hemorrhage prediction in pernicious placenta previa. Front Physiol. 2022;13:982080. doi: 10.3389/fphys.2022.982080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelwahab M, Cackovic M. Placenta accreta spectrum and postpartum hemorrhage. Clin Obstet Gynecol. 2023;66:399–407. doi: 10.1097/GRF.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 13.Franke D, Zepf J, Burkhardt T, Stein P, Zimmermann R, Haslinger C. Retained placenta and postpartum hemorrhage: time is not everything. Arch Gynecol Obstet. 2021;304:903–911. doi: 10.1007/s00404-021-06027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez MG, Coutinho de Carvalho SF, Martins BA, Santos FDSM, Neto FAFP, Medeiros MOA, Bastos Metzger P. Uterine artery embolization versus hysterectomy in postpartum hemorrhage: a systematic review with meta-analysis. J Endovasc Ther. 2024:15266028241252730. doi: 10.1177/15266028241252730. [DOI] [PubMed] [Google Scholar]
- 15.Pettersen S, Falk RS, Vangen S, Nyfløt LT. Peripartum hysterectomy due to severe postpartum hemorrhage: a hospital-based study. Acta Obstet Gynecol Scand. 2022;101:819–826. doi: 10.1111/aogs.14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu C, Feng S, Liu J, Chen Y. Prediction of hysterectomy in pernicious placenta previa by machine learning. Asian J Surg. 2023;46:1957–1958. doi: 10.1016/j.asjsur.2022.11.068. [DOI] [PubMed] [Google Scholar]
- 17.Jain V, Bos H, Bujold E. Guideline No. 402: diagnosis and management of placenta previa. J Obstet Gynaecol Can. 2020;42:906–917. e901. doi: 10.1016/j.jogc.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Naunheim MR, Dai JB, Rubinstein BJ, Goldberg L, Weinberg A, Courey MS. A visual analog scale for patient-reported voice outcomes: the VAS voice. Laryngoscope Investig Otolaryngol. 2020;5:90–95. doi: 10.1002/lio2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jassim G, AlAnsari A. Reliability and validity of the arabic version of the EORTC QLQ-C30 and QLQ-BR23 questionnaires. Neuropsychiatr Dis Treat. 2020;16:3045–3052. doi: 10.2147/NDT.S263190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H, Wang Q, Han M, Xiao X. Current state of interventional procedures to treat pernicious placenta previa accompanied by placenta accreta spectrum: a review. Medicine (Baltimore) 2023;102:e34770. doi: 10.1097/MD.0000000000034770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo P, Wu Y, Yuan X, Wan Z. Clinical diagnostic value and analysis of MRI combined with ultrasound in prenatal pernicious placenta previa with placenta accreta. Ann Palliat Med. 2021;10:6753–6759. doi: 10.21037/apm-21-1285. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Jiang T, Huang G, Han X, Chen Z, Liu C, Wang X, Zhao X. Long-term follow-up of abdominal aortic balloon occlusion for the treatment of pernicious placenta previa with placenta accreta. J Interv Med. 2020;3:34–36. doi: 10.1016/j.jimed.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng X, Li X, Xu J, Wei Y. Intelligent recognition algorithm-based color doppler ultrasound in the treatment of dangerous placenta previa. J Healthc Eng. 2021;2021:9886521. doi: 10.1155/2021/9886521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pun I, Singh A. Feto-maternal outcomes in placenta previa with and without previous cesarean section. J Nepal Health Res Counc. 2022;20:142–146. doi: 10.33314/jnhrc.v20i01.3640. [DOI] [PubMed] [Google Scholar]
- 25.Takeda S, Takeda J, Makino S. Cesarean section for placenta previa and placenta previa accreta spectrum. Surg J (N Y) 2020;6(Suppl 2):S110–S121. doi: 10.1055/s-0039-3402036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park HS, Cho HS. Management of massive hemorrhage in pregnant women with placenta previa. Anesth Pain Med (Seoul) 2020;15:409–416. doi: 10.17085/apm.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenabi E, Salimi Z, Bashirian S, Khazaei S, Ayubi E. The risk factors associated with placenta previa: an umbrella review. Placenta. 2022;117:21–27. doi: 10.1016/j.placenta.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Huang S, Zuo Q, Wang T, Tang X, Ge Z, Lu H, Zhou X, Jiang Z. Maternal and neonatal outcomes of repeated antepartum bleeding in 493 placenta previa cases: a retrospective study. J Matern Fetal Neonatal Med. 2022;35:5318–5323. doi: 10.1080/14767058.2021.1878495. [DOI] [PubMed] [Google Scholar]
- 29.Smith DD, Adesomo AA, Gonzalez-Brown VM, Russo J, Shellhaas C, Costanstine MM, Frey HA. Sonographic predictors of antepartum bleeding in placenta previa. Am J Perinatol. 2024;41:961–968. doi: 10.1055/a-2257-3106. [DOI] [PubMed] [Google Scholar]
- 30.Li P, Liu X, Li X, Wei X, Liao J. Clinical outcomes and anesthetic management of pregnancies with placenta previa and suspicion for placenta accreta undergoing intraoperative abdominal aortic balloon occlusion during cesarean section. BMC Anesthesiol. 2020;20:133. doi: 10.1186/s12871-020-01040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu Y, Zhou Y, Li L, Li H, Wang S, Wang Y, Zuo C. Cook cervical ripening balloon for placenta accreta spectrum disorders with placenta previa: a novel approach to uterus preserving. Arch Gynecol Obstet. 2022;306:1979–1987. doi: 10.1007/s00404-022-06476-6. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Wu T, Peng Y, Luo R. Grade prediction of bleeding volume in cesarean section of patients with pernicious placenta previa based on deep learning. Front Bioeng Biotechnol. 2020;8:343. doi: 10.3389/fbioe.2020.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang LL, Wang WH, Hou YL. Analysis of the risk factors for massive hemorrhage in pernicious placenta previa and evaluation of the efficacy of internal iliac artery balloon occlusion. Int J Womens Health. 2022;14:1769–1776. doi: 10.2147/IJWH.S379965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang S, Xia A, Jamail G, Long M, Cheng C. Efficacy of temporary ligation of infrarenal abdominal aorta during cesarean section in pernicious placenta previa. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42:313–319. doi: 10.11817/j.issn.1672-7347.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Li J, Shen J, Jin J, Zhang W, Zhong W. Direct puncture embolization of the internal iliac artery during cesarean delivery for pernicious placenta previa coexisting with placenta accreta. Int J Gynaecol Obstet. 2016;135:264–267. doi: 10.1016/j.ijgo.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Chen K, Zhang G, Li F, Liu J, Xie K, Zhu E, Li W, Zhang M, Gen C, Wang A. Application of ultrasound-guided balloon occlusion in cesarean section in 130 cases of sinister placenta previa. J Interv Med. 2020;3:41–44. doi: 10.1016/j.jimed.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang ZX, Zhao YF, Li L. Is prophylactic intraoperative abdominal aortic balloon occlusion beneficial in pregnancies with placenta previa and placenta accreta spectrum during cesarean section? A 5-year retrospective study. Int J Gynaecol Obstet. 2023;163:572–578. doi: 10.1002/ijgo.14852. [DOI] [PubMed] [Google Scholar]
- 38.Gambhir M, Gamanagatti S, Sharma R, Manchanda S, Hemachandran N, Dadhwal V, Sharma JB. Prophylactic bilateral internal iliac balloon occlusion to control hemorrhage in placenta accreta spectrum: a boon for obstetricians. Acta Radiol. 2023;64:2180–2189. doi: 10.1177/02841851231164998. [DOI] [PubMed] [Google Scholar]
- 39.Yang Z, Yang Y, Yin Z, Yao J. The role of internal iliac artery intraoperative vascular clamp temporary occlusion in abnormally invasive placenta. Int J Gynaecol Obstet. 2023;161:175–181. doi: 10.1002/ijgo.14422. [DOI] [PubMed] [Google Scholar]
- 40.Wang YL, Duan XH, Han XW, Wang L, Zhao XL, Chen ZM, Chu QJ, Zhang W. Comparison of temporary abdominal aortic occlusion with internal iliac artery occlusion for patients with placenta accreta - a non-randomised prospective study. Vasa. 2017;46:53–57. doi: 10.1024/0301-1526/a000577. [DOI] [PubMed] [Google Scholar]
- 41.Seven M, Yigin AK, Agirbasli D, Alay MT, Kirbiyik F, Demir M. Radiation exposure in pregnancy: outcomes, perceptions and teratological counseling in Turkish women. Ann Saudi Med. 2022;42:214–221. doi: 10.5144/0256-4947.2022.03.03.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ajibo BD, Wolka E, Aseffa A, Nugusu MA, Adem AO, Mamo M, Temesgen AS, Debalke G, Gobena N, Obsa MS. Determinants of low fifth minute Apgar score among newborns delivered by cesarean section at Wolaita Sodo University Comprehensive Specialized Hospital, Southern Ethiopia: an unmatched case control study. BMC Pregnancy Childbirth. 2022;22:665. doi: 10.1186/s12884-022-04999-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorg AL, Von Kries R, Klemme M, Gerstl L, Beyerlein A, Lack N, Felderhoff-Müser U, Dzietko M. Incidence and risk factors of cerebral sinovenous thrombosis in infants. Dev Med Child Neurol. 2021;63:697–704. doi: 10.1111/dmcn.14816. [DOI] [PubMed] [Google Scholar]
- 44.Grewal M, Magro M, Premnath KPB, Bologa S, Otigbah C. Ultrasound-guided prophylactic abdominal aortic balloon occlusion for placenta accreta spectrum disorder: a case series. J Clin Imaging Sci. 2023;13:9. doi: 10.25259/JCIS_141_2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogura T, Lefor AK, Nakamura M, Fujizuka K, Shiroto K, Nakano M. Ultrasound-guided resuscitative endovascular balloon occlusion of the aorta in the resuscitation area. J Emerg Med. 2017;52:715–722. doi: 10.1016/j.jemermed.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Albukhari SN, Khawaji A, Azhar RA. Non-operative management of iatrogenic intraperitoneal bladder injury following a cesarean section. Cureus. 2021;13:e13150. doi: 10.7759/cureus.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khalil AS, Flora S, Hagglund K, Aslam M. Increased bladder injury rate during emergency and repeat cesarean section. J Turk Ger Gynecol Assoc. 2023;24:97–100. doi: 10.4274/jtgga.galenos.2023.2022-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parisio-Poldiak N, Morel E, Hua C, Gibbs SL, Billue D. Cesarean section complications followed by bladder cystotomy and gross hematuria due to unknown dense scar tissue. Cureus. 2020;12:e11902. doi: 10.7759/cureus.11902. [DOI] [PMC free article] [PubMed] [Google Scholar]