Abstract
Objective: To detect the expression of Spectrin Repeat Containing Nuclear Envelope Family Member 3 (SYNE3) and Cluster of Differentiation 34 (CD34) in non-small cell lung cancer (NSCLC). It also aimed to explore the relationship between SYNE3 and NSCLC angiogenesis and clinicopathologic features to identify new biomarkers for NSCLC. Methods: Forty-five NSCLC stage IA-IVB tissue specimens from patients diagnosed at Bazhong Central Hospital were collected from January to September 2022, along with 45 para-cancerous normal lung tissues as controls. None of the NSCLC patients had received anti-tumor therapies, including radiotherapy, chemotherapy, targeted therapy, immunotherapy, or traditional Chinese medicine. All specimens were stained for SYNE3 and CD34 using the Streptavidin-Peroxidase (SP) method. The expression levels of SYNE3 and CD34 in NSCLC tissues and para-cancerous tissues were detected, and a correlation analysis between SYNE3 and clinicopathological features was performed. The number of CD34-labeled microvessels was counted using the Microvessel density (MVD) method. The relationship between SYNE3 and NSCLC angiogenesis was examined through the correlation between CD34-MVD and NSCLC clinicopathologic features. Results: The expression of SYNE3 in NSCLC was significantly lower than that in para-cancerous normal lung tissues, while the expression of CD34 in NSCLC was significantly higher than in para-cancerous normal lung tissues (P=0.037). SYNE3 expression in NSCLC was negatively correlated with tumor diameter and was lower in male patients with a smoking history compared to female patients without a smoking history. CD34 expression was positively correlated with Tumor, Node, Metastasis staging and lymph node metastasis. There was a significant correlation between the expression of SYNE3 and CD34 in NSCLC (r=0.450, P=0.000). Conclusion: SYNE3 was lowly expressed and negatively correlated with tumor size in NSCLC, whereas CD34 was highly expressed and positively correlated with TNM stage and lymph node metastasis. The significant correlation between the expressions of SYNE3 and CD34 suggests that SYNE3 may play a key role in NSCLC angiogenesis and progression.
Keywords: SYNE3, CD34, non-small cell lung cancer, tumor diameter, angiogenesis
Introduction
The onset of lung cancer is insidious, and over the past 30 years, its incidence has increased the fastest among all cancers, with a persistently high mortality rate [1]. This trend poses a serious threat to human life and health, and negatively impacts social development. In 2020, an estimated 2.2 million new cases of lung cancer were reported worldwide, accounting for 11.4% of the total cancer incidence. Additionally, lung cancer caused an estimated 1.8 million deaths, representing 18.0% of cancer-related deaths, making it the leading cause of cancer death globally [1]. Factors such as population aging, increasing air pollution, poor dietary and lifestyle habits, smoking, and inadequate cancer screening have contributed to the rising incidence and mortality rates of lung cancer, which increasingly affects younger populations [2]. Therefore, the prevention and treatment of lung cancer are urgent priorities.
Due to the lack of early screening and effective diagnostic methods, most lung cancer patients are diagnosed at advanced stages, resulting in poor overall prognosis and low clinical cure rates [3]. Improved lung cancer screening methods are critically needed. Lung cancer development is multifactorial, and its etiology and pathogenesis remain to be fully understood. Histologically, lung cancer is classified into two categories: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), with NSCLC accounting for about 85% of all lung cancers [4]. Despite recent advancements in treatments, including targeted therapy, immunotherapy, and anti-angiogenic therapy, along with traditional methods like surgery, chemotherapy, and radiotherapy [5,6], lung cancer patients continue to face challenges such as early systemic metastasis, poor quality of life, treatment difficulties, poor prognosis, and high mortality rates due to non-specific clinical manifestations, late diagnosis, and the complex biological behavior of the tumor [7]. Consequently, early detection and diagnosis are crucial for improving treatment outcomes. Exploring effective molecular biomarkers and therapeutic targets with higher diagnostic value is essential for developing individualized and precise treatment plans for lung cancer patients. With the advancement of sequencing technology, genomes are widely used in tumor research. Genetic markers help determine tumor progression, predict treatment outcomes, reduce recurrence and mortality, and prolong survival [8]. The role of Spectrin Repeat Containing Nuclear Envelope Family Member 3 (SYNE3) in different tumors has been extensively studied. SYNE3, also known as C14orf139, C14orf49, and LINC00341, is a gene located at 14q32.13, spanning 58.2 kb and containing 18 exons and 17 introns.
Liao et al. [9] compared the protein sequences encoded by SYNE3 among different species and found that human SYNE3 shares varying degrees of homology with species such as mice, sheep, and others, indicating its conservation among species. However, significant differences were noted with zebrafish, commonly used in neuronal and stem cell research. Sur et al. [10] demonstrated that Nuclear Envelope Spectrin Repeat Protein 1 (Nesprin-1) encoded by SYNE1 plays a crucial role in DNA repair. They later reported that knockdown of SYNE3 led to DNA damage, loss of genome organization, and transcriptional changes, which are closely associated with tumorigenesis [11].
SYNE3 is positively expressed in the intestine, lungs, trachea, esophagus, stomach, and heart. It is also expressed to varying degrees in the liver, thyroid, and brain, but minimally or not at all in the spleen, kidney, and pancreas. The copy number of SYNE3 varies significantly in 12 different types of epithelial tumors, including ovarian cancer, hepatocellular carcinoma, bladder cancer, cutaneous melanoma, and lung cancer [12]. Liao et al. [9] found that the expression of SYNE3 was significantly decreased in malignant tumors such as breast and lung cancers, but up-regulated in acute myeloid leukemia (AML). Patients with high SYNE3 expression in renal clear cell carcinoma, cervical cancer, and squamous carcinoma of the head and neck had better prognoses, while the opposite was true for those with low-grade gliomas.
The NEAT1/SATB1-miR-330-3p/miR-149-5p-SYNE3 signaling pathway is involved in the regulation of the nucleus and transcription. SYNE3 can promote apoptosis of tumor cells by regulating the cell cycle, recruiting immune cells, and altering the nuclear skeleton and ribosomes. High expression of SYNE3 can recruit dendritic cells and B-lymphocytes, thereby regulating the tumor microenvironment, suggesting that SYNE3 may be a potential tumor biomarker.
Nuclear Envelope Spectrin Repeat Protein 3 (nesprin-3), also known as Klarsicht, Actinin-associated LIM protein 1 (ANC-1), is encoded by SYNE3 and was first discovered in 2005 [13]. It is a protein consisting of 975 amino acids with a molecular weight of about 112 kDa. Nesprin-3 consists of a Klarsicht, ANC-1, Syne Homology (KASH) structural domain and a series of Spectrin repeat regions (SRs). It has two isoforms, Nesprin-3α and Nesprin-3β, which differ structurally in that Nesprin-3α contains 8 SRs, whereas Nesprin-3β contains only 7 SRs (Figure 1). Nesprin-3 is highly conserved, located in the outer nuclear membrane, and widely distributed in various vertebrate tissues. It is prominently expressed in non-neuronal nuclei, adipose tissue, and bone marrow [14,15], while exhibiting lower expression levels in the pancreas and salivary glands. Nesprin-3, the third member of the Nesprin family, is an essential component of the LINC complex (link of the nucleoskeleton and cytoskeleton), which is involved in the localization and migration of the nucleus, the maintenance of cellular morphology and tone, and the transmission of mechanical forces [16,17].
Figure 1.
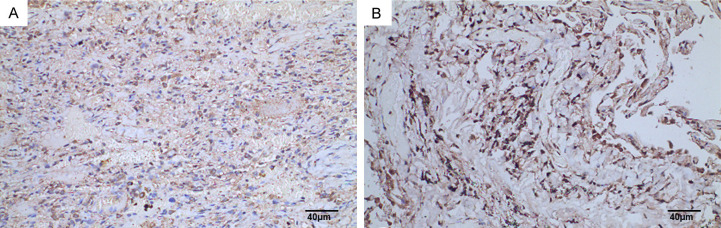
SYNE3 expression in NSCLC paraneoplastic lung tissues. Note: A: Lung tissue adjacent to squamous lung cancer (SP method, ×200), Scale bar, 40 μm; B: Lung tissue adjacent to lung adenocarcinoma (SP method, ×200), Scale bar, 40 μm. SYNE3: Spectrin Repeat Containing Nuclear Envelope Family Member 3; NSCLC: non-small cell lung cancer.
Malignant tumors are characterized by abnormal cell differentiation and proliferation, uncontrolled growth, invasion, and metastasis. Dysregulation of the LINC complex’s maintenance and regulation of the cytoskeleton and nucleoskeleton is closely related to tumorigenesis and development [18-20]. Studies have reported that Nesprin-3 mediates tumor cell migration in lung cancer and fibrosarcoma [21,22]. However, the expression of the SYNE3/Nesprin-3 gene in lung cancer has rarely been reported, and its relationship with clinicopathologic features has not been elucidated.
Tumor growth, migration, and invasion are closely linked to microvessel formation [23]. Microvessel density (MVD) is an important indicator of angiogenesis [24]. Cluster of Differentiation 34 (CD34) is a highly glycosylated type I transmembrane protein, a member of the calreticulin family, expressed on the surface of all small vascular endothelial cells and human and mammalian hematopoietic stem/progenitor cells. Its expression gradually weakens and disappears with cell maturation, quantitatively reflecting the growth of new capillaries. In 1984, Civin first discovered that CD34 is a heavily glycosylated monomeric surface protein with a molecular weight of about 115-120 kDa, sharing no homologous sequence with other known proteins [25]. CD34 consists of three structural regions: the intracellular region, the transmembrane region, and the extracellular region, with a total of 373 amino acid residues. The cytoplasmic region contains 73 hydrophobic amino acid residues, the transmembrane region contains 22 amino acid residues, and the extracellular region contains 278 amino acid residues [26].
Studies have shown that CD34 is essential for the diagnosis and treatment of lymphohematopoietic system tumors, identification of solid tumors, and modulation of inflammatory responses [27,28]. CD34 serves as an antibody that labels microvascular endothelial cells, identifiable microscopically by staining tissue sections with an anti-CD34 antibody [29]. MVD assesses tumor angiogenesis by counting these labeled microvessels [30]. Malignant tumors are rich in blood vessels, highly malignant, and prone to malignant behaviors such as infiltration and metastasis. Guo et al. [31,32] demonstrated that CD34-MVD in lung cancer tissues was significantly higher than in adjacent normal lung tissues and significantly correlated with the clinical stage. In recent years, the therapeutic landscape of NSCLC has undergone remarkable changes. Targeted therapies and immunotherapy have become pivotal in the treatment of NSCLC. The proteins encoded by the SYNE3 gene may be involved in biological processes such as cell structure, differentiation, and growth, playing key roles in tumorigenesis and progression. Therefore, studying SYNE3 expression may provide new insights into the biological mechanisms of lung cancer. Although there are no direct studies on SYNE3 in NSCLC, considering the rapid advancements in NSCLC treatment, future research may focus on the role and potential therapeutic value of SYNE3 in this disease.
In this study, we examined the expression of SYNE3 and CD34 in NSCLC tissues and adjacent normal lung tissues using immunohistochemistry (IHC) to investigate the relationship between SYNE3, CD34, and the clinicopathological features of NSCLC, aiming to identify new biomarkers for NSCLC.
Materials and methods
Materials
Experimental subjects
Forty-five tissue specimens diagnosed as NSCLC in the Department of Pathology at Bazhong Central Hospital from January to September 2022 were collected, along with 37 cases of adjacent normal lung tissues as controls. All NSCLC patients included in the study had not received antitumor therapy, such as radiotherapy, targeted therapy, immunotherapy, or traditional Chinese medicine, before surgery. Clinical data, including sex, age, smoking history, histologic type, and clinical stage, were collected from the hospital’s electronic medical record system based on the patients’ names, hospitalization numbers, and pathology numbers. The study protocol and ethics were approved by the Ethics Committee of Bazhong Central Hospital (No. [2022] 61), and informed consent was waived due to the use of electronic medical record information.
General information
Among the 45 NSCLC patients, there were 26 cases of adenocarcinoma and 19 cases of squamous carcinoma; 25 cases were male, and 20 cases were female, aged 39-79 years, with a mean age of 62.87 years. There were 23 cases with a smoking history and 22 cases without. Lymph node metastasis was present in 27 cases and absent in 18 cases. The Tumor Node Metastasis (TNM) staging of lung cancer was based on the 8th edition of the Revised International Guidelines for TNM Staging of Lung Cancer [33], with 27 cases in stage I+II and 18 cases in stage III+IV. The tumors were divided into three groups according to their maximum diameters: 32 cases with diameters ≤ 3 cm, 6 cases with diameters 3-5 cm, and 7 cases with diameters > 5 cm. Tumor location distribution included 27 cases in the right lung, 18 cases in the left lung, 23 cases in the upper lobe, 5 cases in the middle lobe, and 17 cases in the lower lobe.
Methods
Paraffin specimen production
Successive slices of the paraffin specimens were prepared by professional staff in the Department of Pathology at Bazhong Central Hospital. Each wax block was cut into five slices, each approximately 4 μm thick. These slices were used for the immunohistochemical staining of SYNE3 and CD34, as well as for negative controls. One additional slice was preserved for future use.
Kidney tissues were used as positive control tissues for SYNE3 and CD34 antibodies. Immunohistochemical pre-tests of SYNE3 and CD34 antibodies were performed with different concentration gradients according to the manufacturer’s recommended ranges. The optimal dilution ratios were determined to be 1:200 for both the SYNE3 and CD34 antibodies. Phosphate-buffered saline (PBS) was used instead of the primary antibody as a blank control to exclude false positive results.
Immunohistochemical staining steps
(1) Baking the slices: the paraffin sections were placed in a convection drying oven at 60°C for at least 2 H; (2) Dewaxing the sections: paraffin sections were dewaxed to water: sequentially, the sections were put into xylene I for 15 min, xylene II for 15 min, xylene III for 15 min, anhydrous ethanol I for 5 min, anhydrous ethanol II for 5 min, 85% alcohol for 5 min, 75% alcohol for 5 min, and washed with distilled water; (3) Antigen repair (CD34): the sections were immersed in citrate buffer (pH 6.0), microwaved on high for 10 min, cooled for 8 min, and heated again on medium-high for 10 min; after cooling, the sections were washed with PBS for three times, 5 min each; (4) Blocking endogenous peroxidase: the sections were placed in 3% hydrogen peroxide for 10 min at room temperature and washed three times with PBS for 5 min each; (5) Serum blocking: goat serum closure solution dropwise was added for 20 min at room temperature; (6) Primary antibody incubation: primary antibody dropwise was added and then the sections were incubated overnight at 4°C; (7) Washing: the sections were washed with PBS for 3 times, 5 min each; secondary antibody was added dropwise, and then the sections were incubated at 37°C for 30 min; sections were washed with PBS for 3 times, 5 min each; (8) DAB color development: fresh DAB color development solution was prepared, and drops were added to the tissue; color development was monitored at room temperature under a microscope. Positive staining appeared brown-yellow. Sections were washed with distilled water to terminate the color development; (9) Re-staining of cell nuclei: the nuclei was re-staining with hematoxylin for 3 min, washed in tap water, returned to blue in water, and then rinsed in running water; (10) Dehydration sealing: the sections were sequentially immersed in 75%, 85%, and 95% anhydrous ethanol and dimethylbenzene for 10 minutes, and neutral gum was used to seal the slices.
Judgment of results
The immunohistochemical results for all sections were interpreted by a researcher who only had access to the patient’s pathology number. Two experienced pathologists independently reviewed the sections under randomized and double-blind conditions. In cases of discrepancy between the pathologists’ results, the average of their findings was used.
Interpretation of SYNE3 immunohistochemistry results
Images were captured from the sections using a microcamera system. Each section was first observed at 100× for all tissues, followed by capturing three images at 200× magnification for detailed analysis [34]. The percentage of positive cells (% DAB Positive Nuclei) was calculated for each image using the Halo data analysis system. Hematoxylin-stained nuclei appeared blue, while DAB-stained nuclei showed positive expression in brownish yellow [35].
Scoring criteria: ① three 400× high magnification fields of view were observed, the number of positive cells were counted, and scored 0, 1, 2, 3 and 4 according to the percentage of positive cells < 5%, 5%-25%, 26%-50%, 51%-75% and > 75%, respectively. ② According to the intensity of color development, uncolored, light yellow, tan, and brown were scored 0, 1, 2, and 3, respectively. The final score was determined by multiplying the two criteria ①×②: 0-1 point (-), 2-4 points (+), 5-8 points (+++), 9 points or more (++++). With “(-)” as negative expression and “(+), (++), (+++), (++++)” as positive expression.
MVD counting
Following the methods described by Weidner et al. [36], single brown vascular endothelial cells or clusters of CD34-labeled endothelial cells, clearly demarcated from neighboring blood vessels, tumor cells, or other tissues, were counted as microvessels. The “hotspots” were identified by locating the five most stained areas of vascular endothelial cells in the tumor stroma at low magnification (100×). The number of microvessels in five fields of view was then counted at high magnification (200×) and averaged to obtain the CD34-MVD.
Statistical methods
Data were analyzed using SPSS 26.0 and GraphPad Prism 7.0. Measures conforming to a normal distribution were expressed as mean ± standard deviation (x̅±s), while those not conforming to a normal distribution were expressed as median (interquartile range) [M50 (P25, P75)].
For normally distributed data, comparisons between two or more groups were performed using the independent samples t-test and one-way analysis of variance (ANOVA), respectively. For data not conforming to a normal distribution, the Mann-Whitney U test or Kruskal-Wallis H test was used. Spearman correlation analysis was employed to examine the interrelationships between SYNE3, CD34, and NSCLC. Differences were considered statistically significant at P < 0.05.
Results
SYNE3 expression in NSCLC and para-cancerous lung tissues
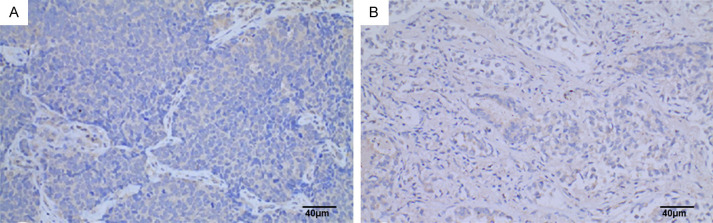
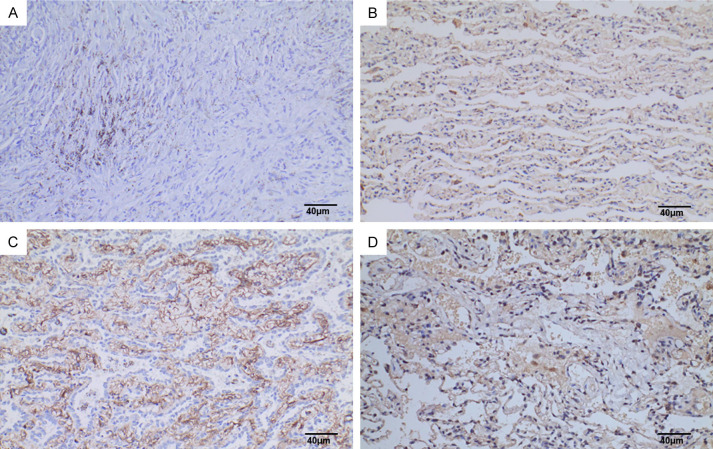
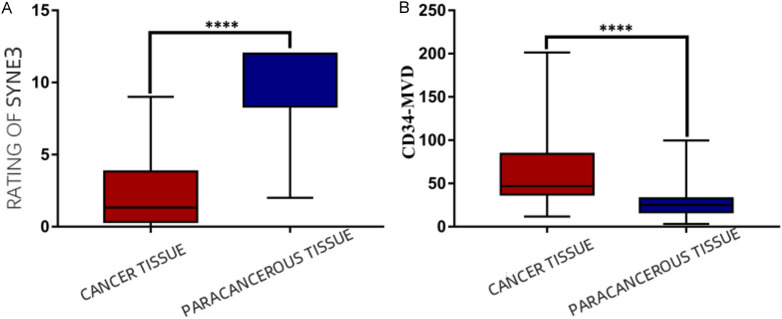
In this study, the immunohistochemical SP method was used to detect SYNE3 expression in NSCLC tissues and adjacent non-cancerous lung tissues. SYNE3 was positively expressed in all adjacent lung tissues, with 6 cases of weak positivity, 4 cases of moderate intensity positivity, and 27 cases of strong positivity (Figure 1). In NSCLC tissues, SYNE3 exhibited varying degrees of positive expression, with an overall positive expression rate of 44.4%. This included 25 cases of negative expression (Figure 2), 12 cases of weak positivity, 5 cases of moderate intensity positivity, and 3 cases of strong positivity. Positive SYNE3 expression in NSCLC cells was indicated by light yellow, brownish-yellow, or tan staining in the cytoplasmic membrane and cytoplasm (Figures 3 and 4). The positive expression score of SYNE3 in NSCLC tissues [1.333 (0.333, 3.833)] was significantly lower than in adjacent normal lung tissues [12.000 (8.333, 12.000)] (Figure 5), with a statistically significant difference (P=0.037).
Figure 2.
SYNE3 expression in NSCLC tissues. Note: A: Lung squamous carcinoma tissue (SP method, ×200), Scale bar, 40 μm; B: Lung adenocarcinoma tissue (SP method, ×200), Scale bar, 40 μm. SYNE3: Spectrin Repeat Containing Nuclear Envelope Family Member 3; NSCLC: non-small cell lung cancer.
Figure 3.
Expression of SYNE3 in lung adenocarcinoma. Note: A: PBS was used as negative control instead of primary antibody (SP method, ×200), Scale bar, 40 μm; B: SYNE3 was weakly positively expressed in lung adenocarcinoma (SP method, ×200), Scale bar, 40 μm; C: SYNE3 was moderately strongly positively expressed in lung adenocarcinoma (SP method, ×200), Scale bar, 40 μm; D: SYNE3 was strongly positively expressed in lung adenocarcinoma (SP method, ×200), Scale bar, 40 μm. SYNE3: Spectrin Repeat Containing Nuclear Envelope Family Member 3; PBS: phosphate buffer saline; SP: Streptavidin-Peroxidase.
Figure 4.
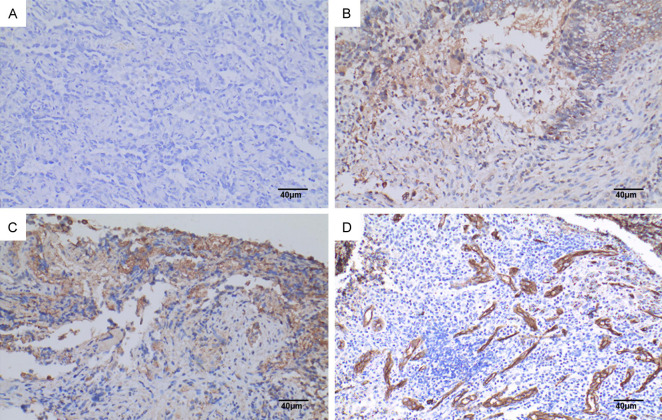
SYNE3 expression in lung squamous carcinoma. Note: A: PBS instead of primary antibody was used as negative control (SP×200), Scale bar, 40 μm; B: SYNE3 was weakly positively expressed in lung squamous carcinoma (SP×200), Scale bar, 40 μm; C: SYNE3 was moderately strongly positively expressed in lung squamous carcinoma (SP×200), Scale bar, 40 μm; D: SYNE3 was strongly positively expressed in lung squamous carcinoma (SP×200), Scale bar, 40 μm. SYNE3: Spectrin Repeat Containing Nuclear Envelope Family Member 3; PBS: phosphate buffer saline; SP: Streptavidin-Peroxidase.
Figure 5.
SYNE3 and CD34 expression in NSCLC cancer tissues and para-cancerous tissues. A: SYNE3 in NSCLC cancer tissues and para-cancerous tissues; B: CD34 expression in NSCLC cancer tissues and para-cancerous tissues. Note: “****” indicates P < 0.0001 compared with para-cancerous lung tissues. SYNE3: Spectrin Repeat Containing Nuclear Envelope Family Member 3; NSCLC: non-small cell lung cancer; CD34: Cluster of Differentiation 34; MVD: Microvessel density.
Relationship between SYNE3 expression and clinicopathologic parameters of NSCLC
The SYNE3 expression scores did not follow a normal distribution. The Mann-Whitney U test or Kruskal-Wallis H test was used to compare SYNE3 expression in NSCLC tissues across different groups based on sex, age, smoking history, histological type, tumor diameter, tumor location, TNM stage, and lymph node metastasis. Statistical analysis showed that SYNE3 expression was lower in male patients and was correlated with smoking history and tumor diameter, with statistically significant differences between groups (P=0.026). However, there was no significant correlation with age, histological type, tumor location, TNM stage, or lymph node metastasis (P=0.053), as shown in Table 1.
Table 1.
Relationship between SYNE3 and CD34 expression levels and clinicopathologic parameters in NSCLC
| Subgroups | SYNE3 score | n | Z-value | P-value | CD34-MVD | n | Z-value | P-value |
|---|---|---|---|---|---|---|---|---|
| Sex | -2.411 | 0.016 | -0.466 | 0.641 | ||||
| Male | 1.000 (0.333333, 2.000) | 25 | 44.833 (35.583, 79.917) | 16 | ||||
| Female | 2.833 (0.750, 7.500) | 20 | 56.167 (37.417, 88.583) | 18 | ||||
| Age | -0.566 | 0.571 | -0.723 | 0.469 | ||||
| ≤ 60 years old | 1.000 (0.000, 4.000) | 19 | 45.333 (34.000, 75.833) | 17 | ||||
| > 60 years old | 1.333 (0.917, 3.750) | 26 | 66.333 (37.500, 105.167) | 17 | ||||
| Histologic type | -1.051 | 0.293 | -0.053 | 0.958 | ||||
| Adenocarcinoma | 2.000 (0.583, 4.000) | 26 | 50.667 (35.667, 84.333) | 21 | ||||
| squamous carcinoma | 1.000 (0.000, 2.333) | 19 | 44.333 (39.333, 96.167) | 13 | ||||
| Stage | -1.467 | 0.142 | -2.338 | 0.019 | ||||
| Stage I+II | 1.333 (0.667, 5.000) | 27 | 44.333 (35.000, 66.667) | 23 | ||||
| Stage III+IV | 1.000 (0.250, 2.083) | 18 | 71.667 (44.667, 134.000) | 11 | ||||
| Mass Location | -0.838 | 0.402 | -0.33 | 0.742 | ||||
| Left Lung | 1.000 (0.500, 3.167) | 18 | 44.667 (34.667, 101.333) | 15 | ||||
| Right Lung | 1.333 (0.333, 5.000) | 27 | 48.667 (37.667, 80.000) | 19 | ||||
| Upper Lobe | 1.000 (0.000, 2.333) | 23 | * | 0.276 | 43.500 (37.167, 94.750) | 18 | * | 0.923 |
| Middle Lobe | 2.000 (1.167, 6.500) | 5 | 3 | |||||
| Lower lobe | 2.000 (0.500, 4.000) | 17 | 66.333 (32.167, 92.833) | 13 | ||||
| Smoking history | -2.408 | 0.016 | -0.173 | 0.862 | ||||
| No | 2.833 (0.667, 6.500) | 22 | 50.667 (37.333, 84.333) | 19 | ||||
| Yes | 1.000 (0.333, 2.000) | 23 | 45.333 (35.000, 84.333) | 15 | ||||
| Lump size | * | 0.015 | * | 0.311 | ||||
| ≤ 3 cm | 1.667 (0.333, 3.500) | 32 | 44.667 (35.000, 84.333) | 27 | ||||
| 3-5 cm | 7.000 (1.250, 9.000) | 6 | 70.833 (49.083, 107.750) | 6 | ||||
| > 5 cm | 0.667 (0.000, 1.333) | 7 | 1 | |||||
| Lymph node metastasis | -0.466 | 0.641 | -4.683 | 0.000 | ||||
| No | 1.167 (0.250, 3.500) | 18 | 36.667 (31.333, 40.000) | 15 | ||||
| Yes | 1.333 (0.667, 4.000) | 27 | 80.000 (61.667, 126.000) | 19 |
Note: “*” indicates that the Kruskal-Wallis H test was used to compare the differences between groups. SYNE3: Spectrin Repeat Containing Nuclear Envelope Family Member 3; CD34: Cluster of Differentiation 34; NSCLC: non-small cell lung cancer; MVD: Microvessel density.
CD34 expression in NSCLC and paracancerous lung tissues
The vascular endothelial cell marker CD34 was positively expressed in NSCLC, normal lung tissues adjacent to the cancer, and lymph node metastases. CD34-positive expression was characterized by a brownish-yellow or brown color in the cytoplasm of vascular endothelial cells. CD34 was expressed in the parenchyma and interstitium of the cancer foci (Figure 6), and some were expressed in foci or tubes. Statistical analysis using the Mann-Whitney U test showed that the expression level of CD34-MVD in NSCLC tissues [47.000 (37.167, 84.333)] was significantly higher than in normal tissues adjacent to the cancer [25.000 (16.833, 32.750)] (Figure 5), with a statistically significant difference (P=0.037).
Figure 6.
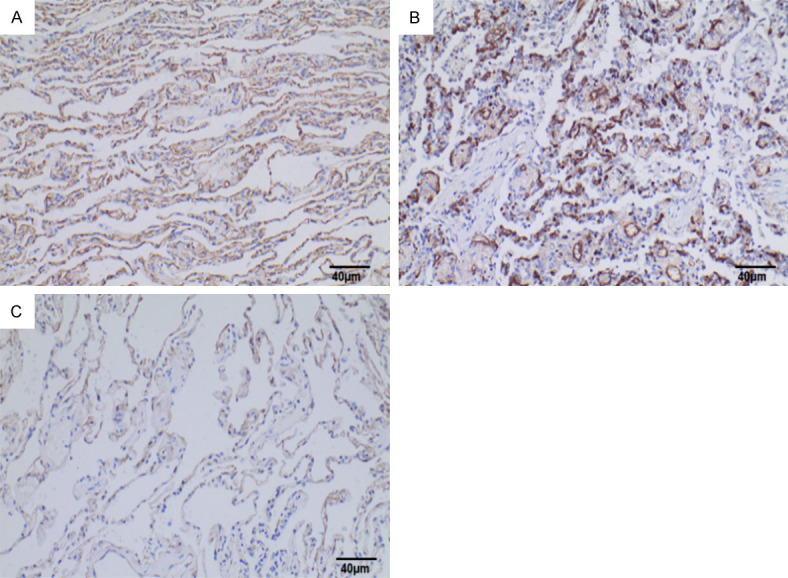
CD34 expression in NSCLC tissues and para-cancerous lung tissues. Note: A: CD34 was positively expressed in lung adenocarcinoma tissues (SP method, ×200), Scale bar, 40 μm; B: CD34 was positively expressed in lung squamous carcinoma tissues (SP method, ×200), Scale bar, 40 μm; C: CD34 was positively expressed in normal lung tissues adjacent to the cancer (SP method, ×200), Scale bar, 40 μm. CD34: Cluster of Differentiation 34; NSCLC: non-small cell lung cancer; SP: Streptavidin-Peroxidase.
Relationship between CD34-MVD and clinicopathologic parameters of NSCLC
The CD34-MVD levels did not follow a normal distribution. The Mann-Whitney U test or Kruskal-Wallis H test was used to compare CD34-MVD expression in NSCLC tissues across different groups based on sex, age, smoking history, histological type, tumor size, tumor location, TNM stage, and lymph node metastasis. Statistical analysis showed that CD34-MVD expression in NSCLC was correlated with TNM stage and lymph node metastasis, with statistically significant differences between groups (P=0.027). However, there was no significant correlation with the patient’s sex, age, smoking history, histological type, tumor location, or maximum tumor diameter (P=0.061), as shown in Table 1.
Correlation analysis between SYNE3 and clinicopathologic parameters
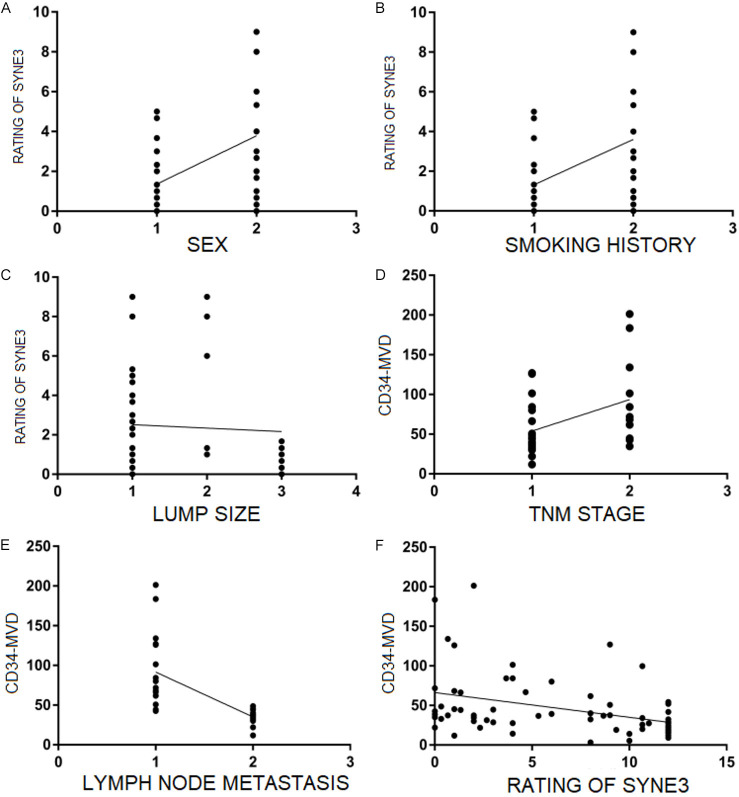
Spearman correlation analysis showed that the SYNE3 score was negatively correlated with the maximum tumor diameter in NSCLC and had a higher correlation with CD34-MVD (r=0.450), which was statistically significant (P=0.036). CD34-MVD was positively correlated with TNM staging and lymph node metastasis (P=0.047), as shown in Table 2 and Figure 7.
Table 2.
Correlation analysis between SYNE3 and CD34-MVD and clinicopathological parameters
| Grouping | Correlation coefficient r | P-value |
|---|---|---|
| SYNE3 and Sex | 0.363 | 0.014 |
| SYNE3 and smoking history | 0.363 | 0.014 |
| SYNE3 and tumor maximum diameter | 0.028 | 0.855 |
| SYNE3 and CD34-MVD | 0.450 | 0.000 |
| CD34-MVD and TNM staging | 0..407 | 0.017 |
| CD34-MVD and lymph node metastasis | 0.815 | 0.000 |
Note: SYNE3: Spectrin Repeat Containing Nuclear Envelope Family Member 3; CD34: Cluster of Differentiation 34; MVD: Microvessel density.
Figure 7.
Correlation analysis between SYNE3 and CD34-MVD and clinicopathological parameters. Note: A: Represents male (1) and female (2); B: Represents smoking history (1) and no smoking history (2); C: Represents tumor size: ≤ 3 cm (1), 3-5 cm (2), > 5 cm (3); D: Represents stage: I+II (1) and III+IV (2); E: Represents lymph node metastasis (1) and no lymph node metastasis (2). F: The expression of SYNE3 in NSCLC was correlated with CD34-MVD. SYNE3: Spectrin Repeat Containing Nuclear Envelope Family Member 3; CD34: Cluster of Differentiation 34; MVD: Microvessel density.
Discussion
The occurrence and development of lung cancer involves a multifactorial, multigene, and multistep complex process. Despite numerous studies on lung cancer, early diagnosis and treatment remain insufficient, and the prognosis of lung cancer is unsatisfactory. Therefore, there is an urgent need to find minimally invasive, rapid, and economical biomarkers. Previous literature has reported that the CD44v/SYNE1/miR-34a axis can be used as a non-invasive biomarker for early diagnosis and monitoring prognosis of oral squamous carcinoma [37]. Doherty et al. [38] found that polymorphisms in the SYNE1 gene were associated with an increased risk of invasive ovarian cancer, suggesting that SYNE is differentially expressed in tumors and is involved in the regulation of the biological processes of tumor cells.
Human SYNE3 protein is a recently discovered membrane protein that is expressed at low levels in lung, breast, kidney, cervical, and squamous head and neck cancers, but is highly expressed in acute myeloid leukemia. It plays roles in tumor pathogenesis such as apoptosis, maintenance of nuclear stability, and regulation of the tumor microenvironment, indicating its potential as a tumor biomarker [9]. Currently, there is no research on SYNE3 in NSCLC angiogenesis or its relationship with clinicopathological features. Based on this, this study explores the relationship between SYNE3 and NSCLC angiogenesis through immunohistochemical analysis of SYNE3 and CD34, aiming to identify a new early biomarker for NSCLC.
This study revealed that the expression level of SYNE3 in primary NSCLC lesions was significantly lower compared to normal lung tissues adjacent to the cancer. Moreover, SYNE3 expression was negatively correlated with tumor size and smoking history. Specifically, male patients with a history of smoking exhibited lower SYNE3 expression compared to female patients without smoking history, suggesting a potential role of SYNE3 in the pathogenesis of NSCLC. Our findings align with previous research by Tessema et al. [39] on SYNE1 methylation in gastric cancer, indicating a tumor-inhibitory role for SYNE proteins. Additionally, Li et al. [40] demonstrated that SYNE1 mutations in renal clear cell carcinoma were associated with poor survival and influenced responses to immune checkpoint inhibitors. Wang et al. [41] suggested that SYNE3 may confer radiation resistance in colorectal cancer cells following radiotherapy, possibly contributing to tumor cell resistance. Previous studies [42] have reported reduced SYNE3 protein expression in epidermal keratinocytes of mice lacking the p63 gene, implicating SYNE3 as a target of p63 in mice. Future studies should investigate the relationship between SYNE3 expression levels and survival rates, tumor cell resistance, and different subtypes of lung cancer to elucidate its mechanisms of action further.
Statistical analysis in this study did not reveal significant differences in SYNE3 expression between lung adenocarcinoma and lung squamous carcinoma. Subgroup analysis indicated that SYNE3 expression levels were slightly lower in lung squamous carcinoma compared to lung adenocarcinoma in both early and advanced-stage NSCLC tissues, although these differences were not statistically significant. Given the varied distribution of TNM stages and the limited sample size, further research is needed to clarify the relationship between SYNE3 expression and histologic types of lung cancer.
Literature has indicated [43] a significant correlation of SYNE3 expression with male patients. Our study supports this correlation, demonstrating lower SYNE3 expression levels in male patients with lung cancer. This observation suggests that SYNE3, possibly influenced by hormonal and chromosomal factors, may play a role in tumorigenesis and development in a sex-specific manner.
Our data also highlight a significant difference in SYNE3 expression between smoking and non-smoking NSCLC patients, although there is currently no literature describing the relationship between SYNE3 and smoking. Future studies should explore how cigarette smoke mediates SYNE3 expression and its involvement in lung cancer pathogenesis.
Neovascularization plays a crucial role in facilitating cancer cell proliferation, metastasis, and infiltration, linking closely with tumorigenesis and progression [23,44]. CD34, a sensitive marker for vascular endothelial cells, is highly expressed in neovasculature [45]. Spencer et al. [46] demonstrated that a small regulatory polypeptide of amino acid response (SPAAR) binds to SYNE1 to promote angiogenesis. This polypeptide is encoded by LINC00961 and co-regulates vascular endothelial cell function. However, the study of SYNE3 in lung cancer angiogenesis has not been reported yet. The results of this study found that the elevated expression of CD34 in NSCLC was closely associated with TNM stage and lymph node metastasis, which is consistent with previous studies [32,47,48]. We also found a high correlation between SYNE3 expression in NSCLC and CD34-MVD (r=0.450, P=0.000), suggesting that SYNE3 may have a role in NSCLC angiogenesis. SYNE3 has an inhibitory role in NSCLC and is a potential biomarker and therapeutic target for NSCLC, which needs to be further investigated in the future. The role and possible mechanisms of SYNE3 in NSCLC need to be further investigated in the future.
In summary, SYNE3 is lowly expressed in NSCLC, negatively correlated with tumor size, and lower in men and patients with smoking history, while CD34 is highly expressed in NSCLC, positively correlated with TNM stage and lymph node metastasis, and it is hypothesized that the sex-related gene SYNE3 may be involved in the process of NSCLC development and progression. The correlation between SYNE3 and angiogenesis in NSCLC is high. SYNE3 may have a role in lung cancer angiogenesis and is expected to be a new target for anti-angiogenic therapy.
Despite revealing associations between SYNE3 expression and NSCLC characteristics, this study is limited by its small sample size, retrospective design, and lack of exploration into molecular mechanisms underlying SYNE3’s influence on CD34 expression. Future research should expand sample sizes, adopt prospective study designs, conduct experimental studies to elucidate SYNE3-CD34 interactions, and consider additional influencing factors. These efforts will provide a more comprehensive scientific basis for precision medicine in NSCLC.
In conclusion, SYNE3 expression was significantly lower in NSCLC tissues compared to adjacent normal lung tissues, while CD34 expression was significantly higher in NSCLC tissues. SYNE3 expression correlated negatively with tumor size and smoking history, and was lower in male patients with smoking history. CD34 expression in NSCLC correlated positively with TNM stage and lymph node metastasis, serving as a valuable indicator for NSCLC prognosis. The strong correlation between SYNE3 expression and CD34-MVD suggests SYNE3’s potential involvement in NSCLC angiogenesis.
Acknowledgements
This work was supported by the Project of Sichuan Medical Association (2021HR24) and Project of Open Fund of Sichuan Key Laboratory of Precision Medicine (2022KF-03).
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Moghbeli M. PI3K/AKT pathway as a pivotal regulator of epithelial-mesenchymal transition in lung tumor cells. Cancer Cell Int. 2024;24:165. doi: 10.1186/s12935-024-03357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Yan B, He S. Advances and challenges in the treatment of lung cancer. Biomed Pharmacother. 2023;169:115891. doi: 10.1016/j.biopha.2023.115891. [DOI] [PubMed] [Google Scholar]
- 6.Hu M, Zhong C, Wang J, Chen J, Zhou T. Current status and breakthroughs in treating advanced non-small cell lung cancer with EGFR exon 20 insertion mutations. Front Immunol. 2024;15:1399975. doi: 10.3389/fimmu.2024.1399975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khasraw M, Yalamanchili P, Santhanagopal A, Wu C, Salas M, Meng J, Karnoub M, Esker S, Felip E. Clinical management of patients with non-small cell lung cancer, brain metastases, and actionable genomic alterations: a systematic literature review. Adv Ther. 2024;41:1815–1842. doi: 10.1007/s12325-024-02799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger MF, Mardis ER. The emerging clinical relevance of genomics in cancer medicine. Nat Rev Clin Oncol. 2018;15:353–365. doi: 10.1038/s41571-018-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao L, Zhang L, Yang M, Wang X, Huang W, Wu X, Pan H, Yuan L, Huang W, Wu Y, Guan J. Expression profile of SYNE3 and bioinformatic analysis of its prognostic value and functions in tumors. J Transl Med. 2020;18:355. doi: 10.1186/s12967-020-02521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sur I, Neumann S, Noegel AA. Nesprin-1 role in DNA damage response. Nucleus. 2014;5:173–91. doi: 10.4161/nucl.29023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heffler J, Shah PP, Robison P, Phyo S, Veliz K, Uchida K, Bogush A, Rhoades J, Jain R, Prosser BL. A balance between intermediate filaments and microtubules maintains nuclear architecture in the cardiomyocyte. Circ Res. 2020;126:e10–e26. doi: 10.1161/CIRCRESAHA.119.315582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harryman WL, Pond E, Singh P, Little AS, Eschbacher JM, Nagle RB, Cress AE. Laminin-binding integrin gene copy number alterations in distinct epithelial-type cancers. Am J Transl Res. 2016;8:940–54. [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dammer EB, Duong DM, Diner I, Gearing M, Feng Y, Lah JJ, Levey AI, Seyfried NT. Neuron enriched nuclear proteome isolated from human brain. J Proteome Res. 2013;12:3193–206. doi: 10.1021/pr400246t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao L, Qu R, Ouang J, Dai J. A glance at the nuclear envelope spectrin repeat protein 3. Biomed Res Int. 2019;2019:1651805. doi: 10.1155/2019/1651805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart-Hutchinson PJ, Hale CM, Wirtz D, Hodzic D. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp Cell Res. 2008;314:1892–905. doi: 10.1016/j.yexcr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balikov DA, Brady SK, Ko UH, Shin JH, de Pereda JM, Sonnenberg A, Sung HJ, Lang MJ. The nesprin-cytoskeleton interface probed directly on single nuclei is a mechanically rich system. Nucleus. 2017;8:534–547. doi: 10.1080/19491034.2017.1322237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tusamda Wakhloo N, Anders S, Badique F, Eichhorn M, Brigaud I, Petithory T, Vassaux M, Milan JL, Freund JN, Rühe J, Davidson PM, Pieuchot L, Anselme K. Actomyosin, vimentin and LINC complex pull on osteosarcoma nuclei to deform on micropillar topography. Biomaterials. 2020;234:119746. doi: 10.1016/j.biomaterials.2019.119746. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Ning X, Chen A, Huang H, Ni C, Zhou C, Yu K, Lan S, Wang Q, Li S, Liu H, Wang X, Chen Z, Ma L, Sun Q. Impaired formation of homotypic cell-in-cell structures in human tumor cells lacking alpha-catenin expression. Sci Rep. 2015;5:12223. doi: 10.1038/srep12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao SY, Sun Y, Lai ZS, Nan QZ, Li K, Zhang ZS. Inhibition of migration and invasion of colorectal cancer cells via deletion of Rac1 with RNA interference. Mol Cell Biochem. 2009;322:179–84. doi: 10.1007/s11010-008-9955-6. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, Jiang J, Huang L, Feng W, Zhang Z, Jin L, Xing X. Sperm‑associated antigen 4 (SPAG4) as a new cancer marker interacts with Nesprin3 to regulate cell migration in lung carcinoma. Oncol Rep. 2018;40:783–792. doi: 10.3892/or.2018.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrie RJ, Harlin HM, Korsak LI, Yamada KM. Activating the nuclear piston mechanism of 3D migration in tumor cells. J Cell Biol. 2017;216:93–100. doi: 10.1083/jcb.201605097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronca R, Benkheil M, Mitola S, Struyf S, Liekens S. Tumor angiogenesis revisited: regulators and clinical implications. Med Res Rev. 2017;37:1231–1274. doi: 10.1002/med.21452. [DOI] [PubMed] [Google Scholar]
- 24.Cox G, Walker RA, Andi A, Steward WP, O’Byrne KJ. Prognostic significance of platelet and microvessel counts in operable non-small cell lung cancer. Lung Cancer. 2000;29:169–77. doi: 10.1016/s0169-5002(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 25.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 26.Gangenahalli GU, Singh VK, Verma YK, Gupta P, Sharma RK, Chandra R, Luthra PM. Hematopoietic stem cell antigen CD34: role in adhesion or homing. Stem Cells Dev. 2006;15:305–13. doi: 10.1089/scd.2006.15.305. [DOI] [PubMed] [Google Scholar]
- 27.Yano T, Tanikawa S, Fujie T, Masutani M, Horie T. Vascular endothelial growth factor expression and neovascularisation in non-small cell lung cancer. Eur J Cancer. 2000;36:601–9. doi: 10.1016/s0959-8049(99)00327-5. [DOI] [PubMed] [Google Scholar]
- 28.Shi H, Zhu F, Xiao AQ, Zhang ZR, Zhang R. Clinical significance of CD117/CD34 co-expression in adult patients with acute leukemia. Ai Zheng. 2006;25:762–4. [PubMed] [Google Scholar]
- 29.Siemerink MJ, Klaassen I, Vogels IM, Griffioen AW, Van Noorden CJ, Schlingemann RO. CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis. 2012;15:151–63. doi: 10.1007/s10456-011-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer. 2002;86:1566–77. doi: 10.1038/sj.bjc.6600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo CR, Han R, Xue F, Xu L, Ren WG, Li M, Feng Z, Hu BC, Peng ZM. Expression and clinical significance of CD31, CD34, and CD105 in pulmonary ground glass nodules with different vascular manifestations on CT. Front Oncol. 2022;12:956451. doi: 10.3389/fonc.2022.956451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bing Z, Jian-Ru Y, Yao-Quan J, Shi-feng C. Evaluation of angiogenesis in non-small cell lung carcinoma by CD34 immunohistochemistry. Cell Biochem Biophys. 2014;70:327–31. doi: 10.1007/s12013-014-9916-5. [DOI] [PubMed] [Google Scholar]
- 33.Ruffini E, Fang W, Guerrera F, Huang J, Okumura M, Kim DK, Girard N, Billè A, Boubia S, Cangir AK, Detterbeck F, Falkson C, Filosso PL, Giaccone G, Kondo K, Infante M, Lucchi M, Marino M, Marom EM, Nicholson AG, Rimner A, Rami-Porta R, Asamura H Staging and Prognostic Factors Committee; Staging and Prognostic Factors-Thymic Domain Subcommittee; Staging and Prognostic Factors Subcommittees; Members of the Advisory Boards. The International Association for the Study of Lung Cancer Thymic Tumors Staging Project: The Impact of the Eighth Edition of the Union for International Cancer Control and American Joint Committee on Cancer TNM Stage Classification of Thymic Tumors. J Thorac Oncol. 2020;15:436–447. doi: 10.1016/j.jtho.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Rashmi R, Prasad K, Udupa CBK. Breast histopathological image analysis using image processing techniques for diagnostic puposes: a methodological review. J Med Syst. 2021;46:7. doi: 10.1007/s10916-021-01786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crowe AR, Yue W. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio Protoc. 2019;9:e3465. doi: 10.21769/BioProtoc.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–87. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 37.Shah K, Patel S, Modi B, Shah F, Rawal R. Uncovering the potential of CD44v/SYNE1/miR34a axis in salivary fluids of oral cancer patients. J Oral Pathol Med. 2018;47:345–352. doi: 10.1111/jop.12678. [DOI] [PubMed] [Google Scholar]
- 38.Doherty JA, Rossing MA, Cushing-Haugen KL, Chen C, Van Den Berg DJ, Wu AH, Pike MC, Ness RB, Moysich K, Chenevix-Trench G, Beesley J, Webb PM, Chang-Claude J, Wang-Gohrke S, Goodman MT, Lurie G, Thompson PJ, Carney ME, Hogdall E, Kjaer SK, Hogdall C, Goode EL, Cunningham JM, Fridley BL, Vierkant RA, Berchuck A, Moorman PG, Schildkraut JM, Palmieri RT, Cramer DW, Terry KL, Yang HP, Garcia-Closas M, Chanock S, Lissowska J, Song H, Pharoah PD, Shah M, Perkins B, McGuire V, Whittemore AS, Di Cioccio RA, Gentry-Maharaj A, Menon U, Gayther SA, Ramus SJ, Ziogas A, Brewster W, Anton-Culver H Australian Ovarian Cancer Study Management Group; Australian Cancer Study (Ovarian Cancer); Pearce CL Ovarian Cancer Association Consortium (OCAC) ESR1/SYNE1 polymorphism and invasive epithelial ovarian cancer risk: an Ovarian Cancer Association Consortium study. Cancer Epidemiol Biomarkers Prev. 2010;19:245–50. doi: 10.1158/1055-9965.EPI-09-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tessema M, Willink R, Do K, Yu YY, Yu W, Machida EO, Brock M, Van Neste L, Stidley CA, Baylin SB, Belinsky SA. Promoter methylation of genes in and around the candidate lung cancer susceptibility locus 6q23-25. Cancer Res. 2008;68:1707–14. doi: 10.1158/0008-5472.CAN-07-6325. [DOI] [PubMed] [Google Scholar]
- 40.Li P, Xiao J, Zhou B, Wei J, Luo J, Chen W. SYNE1 mutation may enhance the response to immune checkpoint blockade therapy in clear cell renal cell carcinoma patients. Aging (Albany NY) 2020;12:19316–19324. doi: 10.18632/aging.103781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang XC, Yue X, Zhang RX, Liu TY, Pan ZZ, Yang MJ, Lu ZH, Wang ZY, Peng JH, Le LY, Wang GY, Peng QH, Meng Y, Huang W, Liu RY. Genome-wide RNAi screening identifies RFC4 as a factor that mediates radioresistance in colorectal cancer by facilitating nonhomologous end joining repair. Clin Cancer Res. 2019;25:4567–4579. doi: 10.1158/1078-0432.CCR-18-3735. [DOI] [PubMed] [Google Scholar]
- 42.Rapisarda V, Malashchuk I, Asamaowei IE, Poterlowicz K, Fessing MY, Sharov AA, Karakesisoglou I, Botchkarev VA, Mardaryev A. p63 transcription factor regulates nuclear shape and expression of nuclear envelope-associated genes in epidermal keratinocytes. J Invest Dermatol. 2017;137:2157–2167. doi: 10.1016/j.jid.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeung MW, Wang S, van de Vegte YJ, Borisov O, van Setten J, Snieder H, Verweij N, Said MA, van der Harst P. Twenty-five novel loci for carotid intima-media thickness: a genome-wide association study in > 45 000 individuals and meta-analysis of > 100 000 individuals. Arterioscler Thromb Vasc Biol. 2022;42:484–501. doi: 10.1161/ATVBAHA.121.317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harlozinska A. Progress in molecular mechanisms of tumor metastasis and angiogenesis. Anticancer Res. 2005;25:3327–3333. [PubMed] [Google Scholar]
- 45.Hassanpour M, Salybekov AA, Kobayashi S, Asahara T. CD34 positive cells as endothelial progenitor cells in biology and medicine. Front Cell Dev Biol. 2023;11:1128134. doi: 10.3389/fcell.2023.1128134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spencer HL, Sanders R, Boulberdaa M, Meloni M, Cochrane A, Spiroski AM, Mountford J, Emanueli C, Caporali A, Brittan M, Rodor J, Baker AH. The LINC00961 transcript and its encoded micropeptide, small regulatory polypeptide of amino acid response, regulate endothelial cell function. Cardiovasc Res. 2020;116:1981–1994. doi: 10.1093/cvr/cvaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadota K, Huang CL, Liu D, Ueno M, Kushida Y, Haba R, Yokomise H. The clinical significance of lymphangiogenesis and angiogenesis in non-small cell lung cancer patients. Eur J Cancer. 2008;44:1057–67. doi: 10.1016/j.ejca.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Chuanliang P, Yunpeng Z, Yingtao H, Qifeng S, Xiaogang Z, Bo C. Syk expression in non-small-cell lung cancer and its relation with angiogenesis. J Cancer Res Ther. 2016;12:663–6. doi: 10.4103/0973-1482.154082. [DOI] [PubMed] [Google Scholar]