Abstract
Objective: The goal of this study was to find out if polyherbal paste (PHP) with Rosadamascena, Terminalia chebula, and Trachyspermumammi in honey could help rats that were constipated because of loperamide. Methods: Thirty male rats were divided into 6 groups: a control group receiving saline, a model group receiving loperamide at 10 mg/kg and saline, a phenolphthalein group (positive control) receiving loperamide at 10 mg/kg and phenolphthalein at 10 mg/kg, and low (20 mg/kg), medium (40 mg/kg), and high (60 mg/kg) doses of PHP, via intragastric administration for 7 days. Various parameters, including food consumption, water consumption, body weight, fecal characteristics, gastrointestinal transit rate, histological changes, serum biomarkers, and aquaporin-3 (AQP3) and C-kit protein expression levels, were assessed. Results: Administering PHP at a dose of 60 mg/kg resulted in a 16.89% increase in fecal water content, a 12.14% increase in the amount of feces, and a 23.67% increase in gastrointestinal transit rate, while also reducing the time to black stool and restoring appearance by 23.41%. At the 40 mg/kg dose, PHP increased motilin levels in the blood by 31.22%, gastrin by 52.78%, and substance P by 19.45% while decreasing somatostatin by 20.17%. Furthermore, at the 60 mg/kg dose, PHP decreased mucous membrane damage and goblet cell function in the colon, reduced AQP3 protein production by 33.39%, and increased c-kit protein production by 12.14%. Conclusion: The PHP showed promising therapeutic potential for loperamide-induced constipation in rats.
Keywords: Poly herbal paste, Terminalia chebula, loperamide, motilin, gastrin
Introduction
Constipation is a digestive condition marked by infrequent bowel movements and the challenging passage of stools, accompanied by pain and stiffness. In severe cases, acute constipation can lead to intestinal blockage, potentially necessitating surgical intervention. Constipation appears in various forms, including chronic idiopathic constipation, secondary constipation, and functional constipation, each characterized by distinct causes and symptoms [1]. Constipation stands as a prevalent gastrointestinal issue, incurring considerable community expenses. Its estimated prevalence spans from 1% to 80% globally, showcasing notable geographical variation. The diverse definitions associated with constipation contribute to a wide range of prevalence rates [2]. The prevalence of constipation is approximately 16%, with rates ranging from 0.7% to 79.0%, with a higher occurrence in women. The likelihood of constipation increases with age, reaching up to 33% in individuals aged 60-110 [3]. Despite this trend, it is crucial to note that constipation should not be viewed as a natural outcome of the aging process. Between 16% and 40% of individuals with chronic constipation (CC) in various nations rely on laxatives. The onset of the condition is impacted by various factors, including inadequate fiber intake, socioeconomic status, genetic predisposition, insufficient fluid consumption, limited physical activity, hormonal imbalances, medication side effects, and anatomical aspects of the body, among others [4].
Constipation is more prevalent among individuals aged 65 and older, often due to factors such as poorly fitting dentures or tooth loss, which can lead to chewing difficulties and a preference for softer, fiber-rich foods. Elderly individuals also experience a higher incidence of anatomical abnormalities like pelvic floor dyssynergia, rectocele, and prolapse [5]. Some neurological conditions, like tethered cord, spina bifida, Hirschsprung disease, pseudo-obstruction, spinal cord defects, and intestinal neuronal dysplasia, can make it hard to go to the bathroom. So can Parkinson’s disease, stroke, multiple sclerosis, Chagas disease, and familial dysautonomia [6]. Antidepressants, anticholinergics, nonsteroidal anti-inflammatory drugs, psychotropic medications, narcotics, vitamin D, lead, iron, bismuth, and calcium channel blockers can all cause constipation [7]. Metabolic and endocrine disorders that can contribute to constipation include high calcium levels (hypercalcemia), diabetes insipidus, low potassium levels (hypokalemia), underactive thyroid (hypothyroidism), and diabetes mellitus (DM). Other causes of constipation include celiac disease, cystic fibrosis, inflammatory bowel disease (IBD), scleroderma, and cow milk protein allergies. Researchers have found that problems with the rectum’s sensory and motor functions can lead to chronic constipation by impacting feelings, movement, and biomechanics, especially when it comes to problems with evacuation [8]. Functional defecation disorders include aspects such as reduced sensitivity in the rectum, changes in reflex activity, increased capacity of the rectal reservoir, and dysfunction in rectal motor function. Constipated individuals, especially those with dyssynergia defecation, often experience psycho-affective disorders like anxiety, depression, and eating disorders [9]. Constipation imposes significant physical and mental burdens, affecting daily life and well-being and resulting in substantial healthcare costs, underscoring the importance of preventive programs [10]. More than half of patients with constipation do not achieve a cure, despite the significant global expenditure on constipation therapy [11]. Laxatives and prokinetic agents, such as chloride-channel activators, opioid antagonists, 5-hydroxytryptamine modulators, and MTL agonists, are commonly used in clinical settings. Prolonged use of these drugs can lead to issues like melanosis coli, drug dependency, severe diarrhea, low blood pressure, rapid heart rate, and dizziness upon standing [12]. Therefore, there is a pressing necessity to create novel, safe, and efficient therapies for constipation. Due to its perceived safety and efficacy, traditional Chinese medicine frequently serves as an alternate remedy for constipation [13]. Some studies suggest that certain plant extracts can also effectively control constipation with minimal side effects [14].
Rosa damascena, known as Damask rose, is cultivated for its aromatic properties in the perfume, pharmaceutical, and food industries [15]. Originating in the Middle East, it is globally cultivated, with significant regions including Iran, Europe, Bulgaria, Turkey, and India. Iran, particularly Kashan, Fars, and Azerbaijan, has a rich history of cultivation. The plant is a perennial shrub with vibrant flowers and compound leaves containing anthocyanins, flavonoids, terpenes, and glycosides, as well as quercetin, vitamin C, kaempferol, carboxylic acid, and myrcene [16]. The essential oil has phenolic compounds that fight free radicals, cancer, inflammation, mutations, and depression [17]. These compounds include geraniol, nerol, nonadecane, β-citronellol, and kaempferol. Terminalia chebula, known as Black Myrobalan or Harda, is a medicinal plant that originates from the Middle East and tropical areas. It has spreading branches, dark brown fissured bark, thin, elliptic-oblong leaves, inconspicuous white to yellowish flowers, and ovate-drupe-shaped fruits. T. chebula has phenolic compounds, tannins, and flavonoids that can be broken down by water. Its extracts have anti-inflammatory, anti-oxidant, and acetylcholinesterase-inhibiting properties, which could help with Alzheimer’s disease and protect neurons [18]. Trachys permumammi, known as Ajwain or Bishop’s weed, is an annual herbaceous plant indigenous to Egypt, cultivated in countries like Pakistan, India, Iran, and Afghanistan. It grows up to 90 cm tall with aromatic ovoid fruits and pinnate leaves [19]. Ajwain seeds are rich in protein, fat, carbohydrates, moisture, mineral matter, tannins, glycosides, saponins, flavones, and essential oils like thymol and carvacrol [20]. In traditional medicine, ajwain seeds are used to help with digestion and gas. They are also good for killing germs, lowering fever, encouraging coughing, cleaning the intestines, protecting the liver, relaxing muscles, opening up airways, reducing inflammation, getting rid of parasites, and preventing infections. Apis mellifera, commonly known as the honey bee, is a eusocial insect with a sophisticated social organization and substantial economic value as a pollinator. This bee, originating from Africa, Asia, Europe, and the Middle East, produces propolis from plant resins, serving several purposes within the hive.
Traditional medicine uses propolis, which possesses medicinal qualities, to treat vascular ailments, respiratory infections, ulcers, and to strengthen the immune system. It is known for its antimicrobial, antioxidant, immunological, antiparasitic, and cytotoxic properties, which show antiviral effects against the SARS-CoV-2 virus [21]. Commercially, propolis-based products are famous for their health benefits and food preservation.
Loperamide is an over-the-counter antidiarrheal drug used to manage diarrhea symptoms without a prescription [22]. It functions by decreasing intestinal movements and fluid secretion, which extends the duration for food to traverse the digestive system, facilitating enhanced absorption of fluids and electrolytes. Similar in structure to opiate receptor agonists like diphenoxylate and haloperidol, loperamide aims to provide antidiarrheal benefits while minimizing the adverse effects on opiate receptors. Loperamide is metabolized by the cytochrome P450 system, specifically CYP3A4, and its levels in the body may increase when used with CYP3A4 inhibitors [23]. Loperamide is considered adequate for treating painless diarrhea and is generally not associated with the potential for abuse [24].
The study aims to assess the impact of PHP on constipation induced by loperamide in rats.
Material and methods
Preparation of polyherbal paste
Rosa Damascena petals (rose petal jam), Terminalia chebula (Black Myrobalan) seeds, and Trachyspermumammi (Carom Seeds), renowned for their traditional medicinal properties, were procured from the local market in Multan. A taxonomist from the Institute of Botany at Bahauddin Zakariya University, Multan, confirmed the authenticity of the herbal mixture paste, and we submitted a herbarium specimen for documentation (Wfo-0001011190; Wfo-0000406875; Wfo-0000411397). To prepare the paste, Trachyspermumammi seeds were finely ground into a powder using a spice grinder. In a clean bowl, 100 grams of the ground Trachyspermumammi powder were mixed with 100 grams of Rosa Damascena petals. Then, 100 grams of crushed Terminalia chebula seeds were added to the mixture. Next, 100 ml of honey was incorporated to bind the ingredients together and enhance the taste, starting with a tablespoon and adjusting according to sweetness preference. All ingredients were thoroughly mixed until a smooth paste-like consistency was achieved, ensuring even distribution. The prepared paste was then transferred to a clean, airtight container and refrigerated to maintain freshness.
Phytochemicals screening
The herbal mixture paste underwent qualitative phytochemical screening to identify various secondary compounds such as alkaloids, flavonoids, glycosides, phenols, and other compounds. This was done with slight modifications to a standard method [25].
Animals
Male rats weighing 300-350 g were acquired from a market close to Multan. They were fed a standard commercial diet and had unrestricted access to tap water. The Muhammad Institute of Medical and Allied Sciences, Multan’s Department of Life Sciences housed the rats and maintained a temperature of 25°C. All experimental protocols followed the National Research Council’s guidelines [26] and were approved by the Ethical Committee of the Muhammad Institute of Medical and Allied Sciences, Multan, Pakistan (Approval No. MIMAS/03/Biochem/315/24).
Experimental design
Thirty rats were arbitrarily allocated to various groups for the study: a control group receiving normal saline (0.25 mL) twice daily, a constipation model group receiving 10 mg/kg loperamide plus normal saline (0.25 mL), a phenolphthalein group receiving 10 m/kg loperamide plus 10 mg/kg phenolphthalein, a low-dose PHP group (L-PHP) receiving 10 mg/kg loperamide plus 20 mg/kg PHP, a medium-dose PHP group (M-PHP) receiving 10 mg/kg loperamide plus 40 mg/kg PHP, and a high-dose PHP group (H-PHP) receiving 10 mg/kg loperamide plus 60 mg/kg PHP. The experimental protocol followed a previously described methodology. Prior to the initial experiment, all rats underwent a 12-hour overnight fast. Constipation was induced by administering loperamide via a tube at a dose of 10 mg/kg once daily for seven days. Subsequently, the rats received PHP and phenolphthalein at corresponding doses, following the same administration procedure. The control group received only normal saline solution administered at a volume of 0.25 mL using the identical method (Table 1) [27].
Table 1.
Drug groups for the animal experiments
| Groups | Treatment (7 days) | |
|---|---|---|
|
| ||
| Construct Model | Treatment (1 h latter) | |
| Control | 0.25 mL Normal saline | 0.25 mL Normal saline |
| Model | 10 mg/kg Loperamide | 0.25 mL Normal saline |
| Phenolphthalein | 10 mg/kg Loperamide | 10 mg/kg Phenolphthalein |
| L-PHP | 10 mg/kg Loperamide | 20 mg/kg PHP |
| M-PHP | 10 mg/kg Loperamide | 40 mg/kg PHP |
| H-PHP | 10 mg/kg Loperamide | 60 mg/kg PHP |
L-PHP, low dose polyherbal paste; M-PHP, medium dose polyherbal paste; H-PHP, high dose polyherbal paste.
Food consumption, water consumption, and body weight gain
Throughout the experimental phase following the induction of constipation with loperamide, the rats’ daily body weight, food consumption, and water intake were assessed. The treatment regimen was maintained for 7 days after the induction of constipation [28].
The moisture level and frequency of fecal excretion
On the 6th day, the fecal moisture level and fecal count were measured. Following intragastric treatment, the rats were transferred to individual clean cages. Three hours later, fecal specimens were gathered in specialized tubes, cooled on ice, weighed, and enumerated. Subsequently, the specimens were entirely desiccated in an oven to ascertain their dry weight [28]. The formula was then employed to compute the fecal water content.
Assessment of initial black feces
On the 7th day, following a 12-hour overnight fast with access to water, rats in the control group were administered 0.25 mL of normal saline solution. Rats in the remaining groups were administered loperamide intragastrically (10 mg/kg; 0.25 mL). After one hour, all rats were fed an activated charcoal meal using the same method. Subsequently, they were housed in individual clean cages with ad libitum access to food and water. The duration from the consumption of the activated charcoal meal to the initial appearance of black feces was recorded.
Assessment of collection of blood and tissue samples and gastrointestinal transit rate
On the 8th day, following 12 hours without food but with access to water, rats were administered a 0.25 mL meal containing activated charcoal. After 1 hour, blood samples were preserved by freezing them at -80°C for further examination. Animals were euthanized by ketamine injection and the abdominal cavities of the rats were then opened to measure the entire length of their small intestines, from the pyloric sphincter to the cecum. This, along with the distance traveled by the activated charcoal, was used to calculate the rate of gastrointestinal transit. Samples of tissue from the proximal colon were collected and placed in 10% formalin for future histological and immunohistochemistry (IHC) examinations [27]. The intestinal transit rate was determined using the formula:
Assessing histological samples
The colon tissues were preserved in 10% formalin and embedded in paraffin. Sections of 5 mm thickness were cut, rehydrated using various ethanol concentrations, and deparaffinized using xylene. The specimens were placed on glass slides and underwent standard staining methods with Hematoxylin and Eosin (H and E). Changes in colon histology were analyzed under a light microscope at 100× magnification, and images were captured for record-keeping [27].
Evaluation of blood levels of SP, SS, MTL, and gas
ELISA kits from various manufacturers were utilized to quantify the blood levels of substance P (SP), somatostatin (SS), motilin (MTL), and gastrin (Gas).
Analysis using immunohistochemistry
The colon tissues underwent the same pre-treatment as for histological evaluation prior to immunohistochemical examination of protein expression (AQP3 and C-kit). 0.1% hydrogen peroxide (H2O2) was employed to eliminate the inherent peroxidase activity in the colon sections. Following this, primary antibodies were administered and left to incubate overnight at 4°C, after which a secondary antibody was applied and incubated for 1 hour. Subsequently, the colon sections were stained with hematoxylin for counterstaining. Ultimately, the sections were analyzed using a light microscope at a magnification of 400× [27].
Statistical analysis
The results were presented as mean ± standard deviation (SD) for every group. Group mean differences were evaluated using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests. Statistical significance was defined at a p-value below 0.05. The statistical analysis was performed using SPSS 16.0 software (SPSS, Chicago, IL).
Results
The feeding status after administering oral treatment to rats with constipation induced by loperamide
Table 2 illustrates the weight gain, food consumption, and water intake subsequent to oral administration of PHP in six groups of rats with loperamide-induced constipation. Weight gain was 17.35 g, 18.33 g, 22.98 g, 18.71 g, 18 g, and 16.41 g in the control, model, phenolphthalein, L-PHP, M-PHP, and H-PHP groups, respectively. The phenolphthalein group exhibited significantly higher weight gain than the control group, whereas the other four treatment groups did not significantly differ from the control group. Both the M-PHP and H-PHP groups had significantly lower weight gains compared to the phenolphthalein group, while the L-PHP group did not significantly differ. Food intake was 18 g, 19.33 g, 18.95 g, 19.47 g, 18.46 g, and 19.69 g in the six groups, respectively, with no significant differences. Water intake was 24.81 mL, 23.82 mL, 19 mL, 19.36 mL, 19.99 mL, and 21.39 mL in the six groups, respectively. The phenolphthalein group exhibited significantly lower water consumption compared to the control and model groups, while the other groups did not show significant differences. Water consumption did not significantly differ among the treatment groups.
Table 2.
Alterations in body weight, food consumption, and water consumption after administering PHP
| Groups | Body weight gain (g) | Feed Intake (g/day) | Water Intake (ml/day) |
|---|---|---|---|
| Control | 17.35±1.00 | 18±1.00 | 24.81±0.71 |
| Model | 18.33±1.52 | 19.33±0.65 | 23.82±0.55 |
| Phenolphthalein | 22.98±0.97 | 18.95±0.54 | 19±1.00 |
| L-PHP | 18.71±1.32 | 19.47±0.58 | 19.36±0.60 |
| M-PHP | 18±1.00 | 18.46±0.77 | 19.99±0.98 |
| H-PHP | 16.41±0.82 | 19.69±0.61 | 21.39±0.54 |
L-PHP, low dose polyherbal paste; M-PHP, medium dose polyherbal paste; H-PHP, high dose polyherbal paste.
Impact of PHP on the quantity and moisture fecal content in rats with constipation induced by loperamide
The model group experienced a substantial decrease in fecal count and moisture content over a 3-hour period compared to the control group (P < 0.01). However, PHP administration led to an increased fecal count in the L-PHP and M-PHP groups (P < 0.05). Additionally, treatment with L-PHP, M-PHP, H-PHP, and phenolphthalein resulted in higher moisture content levels compared to the model group (P < 0.05).
Impact of PHP on the onset time of black feces in rats with constipation induced by loperamide
The time for the first appearance of black feces was notably longer in the model group than in the control group (P < 0.01). Nevertheless, after treatment with H-PHP and M-PHP, this duration decreased compared to the model group (P < 0.05).
Impact of PHP on the gastrointestinal transit rate in rats with constipation induced by loperamide
Table 3 and Figure 1 illustrate a notable reduction in the intestinal transit rate in the model group compared to the control group (P < 0.01). Following PHP administration, there was an enhancement in the rate of intestinal transit in the H-PHP group compared to the model group (P < 0.05).
Table 3.
Effect of treatment on fecal moisture content, feces count, time to first appearance of black feces, and intestinal transit rate
| Groups | Fecal water content (%) | Number of faces (granule) | Defecating time of black faeces (min) | Intestinal transit rate (%) |
|---|---|---|---|---|
| Control | 71.26±2.75 | 13.40±0.92 | 101.43±0.76 | 76.33±1.35 |
| Model | 57.18±1.20## | 9.94±1.21## | 436.97±1.00## | 47.92±0.88## |
| Phenolphthalein | 65.02±1.31#,** | 20.42±0.56 | 384.11±0.99 | 54.52±0.95## |
| L-PHP | 62.28±0.74#,* | 13.14±0.97# | 349.04±1.16## | 54.65±0.79## |
| M-PHP | 64.10±1.02#,* | 12.54±0.54* | 343.66±0.67##,* | 52.71±1.03## |
| H-PHP | 66.40±0.70** | 10.63±0.77* | 331.41±0.52##,* | 64.25±1.02#,** |
Significance levels were set at #P < 0.05 or ##P < 0.01 for comparisons with the control group, and at *P < 0.05 or **P < 0.01 for comparisons with the model group, with five rats in each group. L-PHP, low dose polyherbal paste; M-PHP, medium dose polyherbal paste; H-PHP, high dose polyherbal paste.
Figure 1.
Polyherbal paste (PHP) increased the speed of carbon transit through the intestines in rats with constipation induced by loperamide. Rats were given carbon and their small intestines were removed 30 minutes later in L-PHP (low dose polyherbal paste), M-PHP (medium dose polyherbal paste), and H-PHP (high dose polyherbal paste) treatment groups.
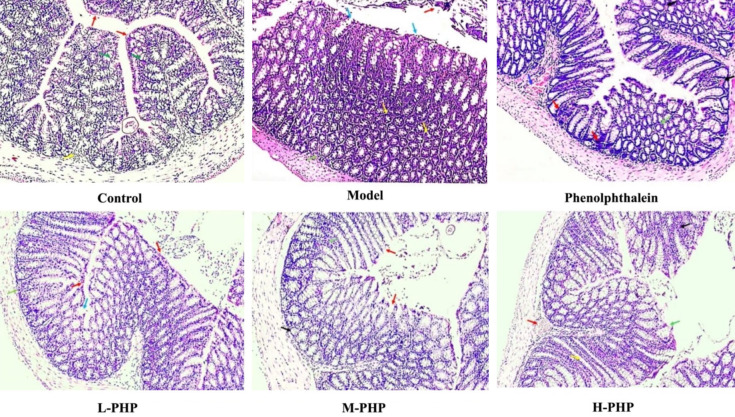
Assessing histological samples
The mucosal folds have normal and intact simple columnar epithelium in most of the areas (red arrows). The morphological features of Crypts of Lieberkühn are normal, with intact goblet cells. The lumen has no necrotic debris. Normal enteroendocrine cells can be seen in the lamina propria of the colon (yellow arrow) (control). The lumen of the colon has necrotic material from dead and dying cells (red arrow). There was moderate disruption of mucosal simple columnar epithelial cells (blue arrow). The lamina propria of the colon also has multiple atrophied Crypts of Lieberkühn (yellow arrows). The paneth cells at the base of Crypts are also subjected to coagulative necrosis (green arrow; model) (Figure 2).
Figure 2.
Polyherbal paste dose dependently decreased histopathological alterations in the colons of rats with constipation induced by loperamide. It shows rat colon sections stained with H and E (magnification ×100) in L-PHP (low dose polyherbal paste), M-PHP (medium dose polyherbal paste), and H-PHP (high dose polyherbal paste) treated groups.
A blood vessel in the submucosa was engorged with red blood cells, resulting in focal submucosal congestion (blue arrow). The presence of red blood cells in interstitium indicates mild interstitial hemorrhage (black arrow). Mononuclear inflammatory cells were aggregated near the basement membrane, which is an indication of mild colitis (red arrow). Various atrophied crypts were found in the mucosa, as shown by the green arrow (green arrow) (Phenolphthalein). The infiltration of red blood cells in the lamina propria indicates mild intestinal interstitial hemorrhage (red arrow). The disruption of the mucosal epithelium also suggests mild coagulative necrosis (blue arrow). The aggregation of mononuclear inflammatory cells near the base of Crypts of Lieberkühn also suggests mild intestinal inflammation (green arrow) (L-PHP). The intestinal simple columnar epithelial cells in most of the areas have been sloughed off, leading to coagulative necrosis in the intestinal mucosa (red arrow). Also, atrophied Crypts of Lieberkühn were present near the basement membrane of the mucosa (black arrow). The infiltration of mononuclear cells in the lamina propria may indicate moderate intestinal inflammation (green arrow) (M-PHP). The blood vessel in the mucosa was congested (red arrow). The mucosal folds showed hyperplasia of goblet cells in Crypts of Lieberkühn (yellow arrow). Simple columnar epithelial cells have been sloughed off in a few areas (green arrow). The presence of eosinophilic mononuclear inflammatory cells in the mucosa may indicate mild to moderate enteritis (black arrow) (H-PHP).
Serum variables
To assess the impact of PHP on blood biochemical constituents in rats with constipation induced by loperamide, changes in various biomarkers associated with gastrointestinal motility were measured using ELISA. As shown in Table 4, the levels of SP, gas, and MTL were significantly lower in the model group than in the control group (P < 0.01), while SS levels were slightly elevated but not significantly. Treatment with PHP or phenolphthalein led to increased levels of SP, gas, and MTL but decreased SS levels compared to the model group. These findings indicate that PHP treatment could potentially modulate gastrointestinal motility-related biomarkers, providing relief from loperamide-induced constipation in male rats (Table 4).
Table 4.
The levels of SS, SP, Gas and MTL rats with constipation induced by loperamide post-treatment
| Groups | MTL (pg/ml) | Gas (pg/ml) | SP (pg/ml) | SS (pg/ml) |
|---|---|---|---|---|
| Control | 511.95±1.31 | 126.11±0.76 | 176.41±0.89 | 24.96±1.75 |
| Model | 312.77±0.81## | 66.17±1.51## | 118.18±0.92## | 27.76±0.81 |
| Phenolphthalein | 431.09±1.21** | 85.25±0.91# | 138.37±1.23## | 25.29±1.09* |
| L-PHP | 421.68±0.74#,* | 94.15±1.08# | 136.54±1.36## | 23.48±1.38* |
| M-PHP | 451.18±1.04** | 143.04±1.13** | 142.93±1.61#,* | 22.27±1.28* |
| H-PHP | 410.66±0.65#,* | 108.22±0.88* | 155.41±1.32** | 22.60±1.42** |
Significance levels were set at #P < 0.05 or ##P < 0.01 for comparisons with the control group, and at *P < 0.05 or **P < 0.01 for comparisons with the model group, with five rats in each group. L-PHP, low dose polyherbal paste; M-PHP, medium dose polyherbal paste; H-PHP, high dose polyherbal paste; MTL, motilin; SP, substance P; SS, somatostatin.
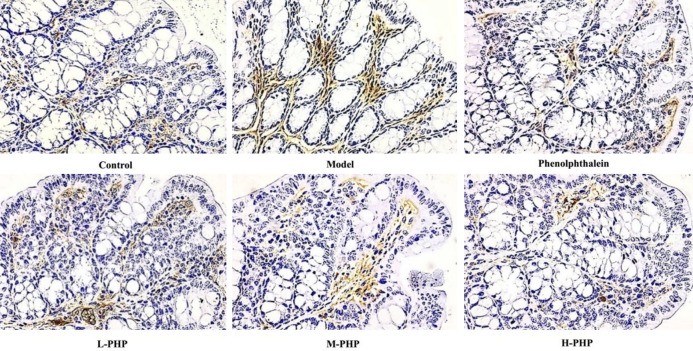
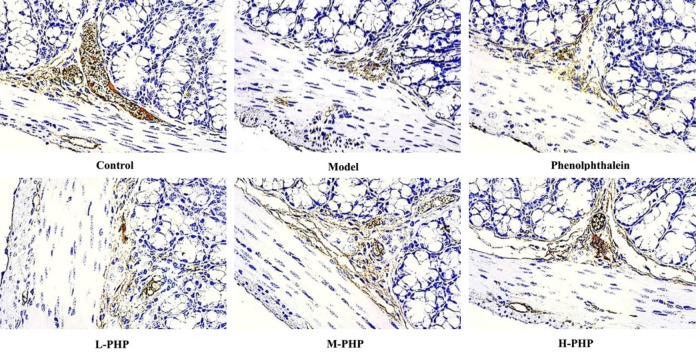
Impact of PHP on the levels of AQP3 and C-kit in rats with constipation induced by loperamide
AQP3 is crucial in controlling water movement in the colon, while the interstitial cells of Cajal (ICC) act as an intestinal contraction pacemaker. Therefore, our research aimed to examine PHP’s effects on AQP3 and C-kit protein levels in the large intestine of male rats with constipation induced by loperamide. Our findings, outlined in Table 5 and Figure 3, reveal that AQP3 levels were higher in the model group than in the control group (P < 0.05), but lower in both the PHP and phenolphthalein groups than in the model group (P < 0.05 or P < 0.01). Conversely, C-kit manifestation was lower in the model group than in the control group (P < 0.05). Significantly, C-kit manifestation was markedly elevated in the H-PHP group (P < 0.01), as depicted in Table 5 and Figure 4.
Table 5.
The levels of AQP3 and C-kit protein in the colons of rats experiencing constipation induced by loperamide
| Groups | AQP3 | C-kit |
|---|---|---|
| Control | 41.22±1.24 | 62.50±0.66 |
| Model | 68.12±1.17## | 49.72±0.98## |
| Phenolphthalein | 53.62±1.37##,** | 51.31±1.37## |
| L-PHP | 62.06±1.24## | 50.24±1.10## |
| M-PHP | 57.29±1.29##,* | 53.07±1.13## |
| H-PHP | 45.48±1.37** | 55.45±0.77#,* |
Significance levels were set at #P < 0.05 or ##P < 0.01 for comparisons with the control group, and at *P < 0.05 or **P < 0.01 for comparisons with the model group, with five rats in each group. L-PHP, low dose polyherbal paste; M-PHP, medium dose polyherbal paste; H-PHP, high dose polyherbal paste; AQP3, aquaporin-3; IOD, Integral optical density.
Figure 3.
Polyherbal paste (PHP) reduced aquaporin-3 (AQP3) levels in the colon, which were assessed through IHC examination at 400× magnification. L-PHP, low dose polyherbal paste; M-PHP, medium dose polyherbal paste; H-PHP, high dose polyherbal paste.
Figure 4.
Polyherbal paste (PHP) increased the presence of C-kit protein in the colon. The levels of C-kit protein in the colon were assessed through immunohistochemistry examination at 400× magnification in L-PHP (low dose polyherbal paste), M-PHP (medium dose polyherbal paste), and H-PHP (high dose polyherbal paste) treated groups.
Discussion
Constipation is defined as rare bowel movements or difficulties passing stools. Symptoms include straining, the sensation of rectal blockage, incomplete emptying, abdominal discomfort, bloating, and firm stools [29]. Certain studies indicate that the use of various plant extracts is gaining attention due to their laxative properties, with minimal side effects observed in the treatment of constipation [30]. According to our research, PHP has a positive effect on reducing the symptoms of loperamide-induced constipation in rats.
Loperamide works by connecting to mu- and delta-opioid receptors to slow down the movement of the stomach and intestines, lower the production of intestinal fluids, making it easier for the body to absorb water and electrolytes from the inside of the intestines. Moreover, it restrains the release of specific inflammatory substances, leading to decreased inflammation and a perception of visceral pain [31]. Several studies have used loperamide to induce experimental constipation, aiding researchers in understanding its mechanism and identifying potential therapeutic compounds [32]. The group that was given loperamide (called the “model group”) in this study had clear signs of constipation, such as a lot less water in their feces, fewer pellets in their feces, and a slower small intestine transit rate. Additionally, these rats took significantly longer to pass their first black feces compared to the control group. Significantly, there were no fatalities among the animals throughout the study, suggesting that the use of loperamide to induce constipation was both safe and effective.
Numerous factors affect the onset of the condition, such as insufficient fiber intake, socioeconomic status, genetic predisposition, insufficient fluid intake, insufficient physical activity, hormonal imbalances, medication side effects, and bodily anatomical features [4]. Constipation is more prevalent among older individuals than younger ones, and its causes in the elderly are associated with various factors. We can evaluate the functionality of intestinal transit by tracking the time it takes for the first appearance of black stool [12], which reflects the total transit time through both the large and small intestines [30]. A shorter duration suggests a better response to treatment and a more favorable prognosis, while a more prolonged duration indicates a less effective treatment. Similarly, lower intestinal transit rates indicate a faster transit time through the small intestine, which is associated with better treatment efficacy and a more favorable prognosis [12]. In rats with loperamide-induced constipation, there were noticeable changes in defecation frequency, intestinal transit rates, fecal moisture content, and the time until the first appearance of black feces, according to the research. Treatment with PHP was found to improve these parameters. However, phenolphthalein only improved the moisture content of feces.
Mucins play a critical role in safeguarding the gastrointestinal epithelium, and changes in their secretion can impact the protective barrier of the gastrointestinal tract (GIT) [33]. Goblet cells, which are abundant in the GIT, primarily secrete mucin. These proteins, recognized for their ability to form gels, aid in safeguarding the GIT and promoting intestinal motility [34]. Our research indicates that PHP could reinstate the secretory role of goblet cells. Nonetheless, further investigation is required to grasp the underlying mechanisms comprehensively.
Prior research has indicated that constipation is associated with abnormal colon function, particularly a disruption in colon motility. The enteric nervous system regulates the regular function of the intestines, influenced by various biomarkers associated with intestinal motility. SP, MTL, and gas function as excitatory neurotransmitters within the intestinal tract, stimulating intestinal motility. The primary cause of constipation might be the irregular release of biomarkers associated with enteric motility [35]. As previously stated, MTL is the intestinal hormone that controls gastrointestinal motility in reaction to food intake and hunger signals. Studies suggest that MTL’s primary role is to enhance pepsin production and stimulate the migrating motor complex (MMC) to regulate gastrointestinal motility [32]. SP, an excitatory neurotransmitter, heightens intestinal sensitivity and strongly stimulates intestinal smooth muscle cells once it binds to its receptors. It induces mucus secretion and enhances vascular permeability in the bowel. Studies suggest that SP directly induces intestinal contractions in rats by targeting neurokinin type 1 receptors [36]. This passage highlights the multifaceted role of gastrin in the GIT. Gastrin plays a critical role in various functions, including stimulating gastric acid secretion, which is vital for the digestive process. It also promotes the growth and repair of the stomach’s protective lining, known as the gastric mucosa.
Moreover, gastrin helps regulate the movement of food through the GIT, ensuring that digestion occurs efficiently. It also regulates the lower esophageal sphincter’s tone, which aids in preventing the reflux of stomach content into the esophagus. Furthermore, gas has been linked to cell proliferation in the gastrointestinal mucosa, which is the digestive tract’s inner lining [37]. On the other hand, the secretion of gastrin is suppressed by SS [38]. SS has a crucial role in the GIT by suppressing the release of several hormones like gastrin, insulin, glucagon, and growth hormone. It also controls gastrointestinal motility and is involved in regulating intestinal absorption and secretion.
Furthermore, SS has been implicated in the development of constipation, potentially due to its impact on gastrointestinal motility and secretion [39]. According to the most recent ELISA results, phenolphthalein treatment increased MTL levels but decreased SS levels compared to the model group (Table 4). Additionally, PHP significantly elevated blood levels of SP, gas, and MTL while reducing SS levels compared to the model group. However, additional research is necessary to comprehend the precise association and mechanism behind these alterations.
AQPs are a group of proteins that act as water channels and are vital for the GIT. In constipation, AQPs play a role in regulating water balance in the intestines, which is essential for regular bowel movements. Studies suggest that alterations in AQPs expression or function may contribute to the pathophysiology of constipation by affecting water transport across the intestinal epithelium. Additionally, AQPs have been implicated in other gastrointestinal functions, such as mucosal protection, ion transport, and cell proliferation [40]. AQP3 is a protein that acts as a water channel, playing a crucial role in the GIT by aiding in the movement of water through cell membranes. In the context of constipation, AQP3 is involved in maintaining proper hydration of the intestinal epithelium, which is essential for normal bowel function. Research has indicated that changes in the expression or function of AQP3 may have a role in the onset of constipation by influencing water transport in the intestines [41]. In this research, we measured the manifestation of AQP3 and found that phenolphthalein and PHP alleviate loperamide-induced constipation by reducing AQP3 levels in rat colons (Table 5). However, the exact mechanisms behind this effect require further investigation.
The ICC is crucial for regulating gastrointestinal movements [32]. They act as pacemaker cells, controlling the release of neurotransmitters and generating the electrical slow wave that coordinates muscle contractions in the intestines [42]. In the colon, submucosal ICCs are responsible for producing this slow wave, which is essential for smooth muscle function [43]. A decrease in ICCs can lead to irregular slow waves, which disrupt regular muscle contractions and can result in constipation [44]. C-kit, a receptor found on ICCs, is crucial for maintaining the ICC network. Reduced C-kit expression may indicate a disruption in the ICC network in people with constipation [45]. IHC techniques can reliably detect C-kit [44], providing an indirect way to assess the quantity and density of ICCs in the gut [32]. In our study, we found that PHP could increase the expression of the C-kit protein, which is crucial for maintaining ICC function. Nevertheless, additional investigation is required to understand its mechanism comprehensively.
Currently, researchers have identified and characterized several monomeric compounds from the PHP. Rosa damascena contains compounds such as anthocyanins, flavonoids, terpenes, and glycosides. It also contains quercetin, vitamin C, kaempferol, carboxylic acid, myrcene, geraniol, nerol, nonadecane, β-citronellol, and kaempferol [17]. Terminalia chebula possesses a broad array of medicinal properties due to its composition of various biochemicals like hydrolysable phenolic compounds, tannins, and flavonoids, making it a potentially effective therapeutic choice. It also contains chebulagic acid, chebulinic acid, and gallic acid [18]. Trachyspermumammi seeds contain a diverse range of compounds, including protein, fat, carbohydrates, fiber, moisture, mineral matter such as calcium, phosphorus, iron, and nicotinic acid, along with tannins, glycosides, saponins, and flavones [20]. The primary phytochemicals identified in the honey samples included sapogenin, sparteine, lunamarin, flavanone, phenols, and proanthocyanin [20]. Among these components, chebulagic acid, chebulinic acid, tannins, anthraquinone glycosides, quercetin, kaempferol, and gallic acid demonstrated notable pharmacological effects in alleviating constipation, relaxing smooth muscles, and diminishing inflammation [46].
Interestingly, apart from its use in treating constipation, this PHP is also traditionally employed for managing diarrhea, although no scientific evidence or research supports these effects. Some well-known herbs have been reported to have similar effectiveness for both diarrhea and constipation. Ginger and Psyllium husk are popular herbs known to treat both diarrhea and constipation effectively. This dual effect is believed to balance out the potential side effects of using antidiarrheal or laxative drugs individually at high doses [47]. This principle supports a holistic approach to natural products, recognizing their ability to address various disease aspects and potentially mitigate side effects [48]. More research is needed to fully grasp the antidiarrheal efficacy of PHP.
Conclusion
The study looked into the effectiveness of a polyherbal paste (PHP) in treating loperamide-induced constipation in rats. Results showed that PHP treatment led to improvements in the fecal count and moisture content, reduced the onset time of black feces, and increased the gastrointestinal transit rate compared to the model group. Histological analysis revealed mitigation of mucosal damage and restoration of goblet cell secretary activity in the colon. The PHP treatment also changed biomarkers related to gastrointestinal motility. Compared to the model group, it increased gas and MTL SP levels while decreasing SS levels. Additionally, PHP treatment decreased AQP3 levels and increased C-kit expression in the colon. These results indicate that PHP could be a viable treatment choice for constipation management.
Acknowledgements
The authors extend their appreciation to King Saud University for funding this work through research supporting project (RSP2024R376), Riyadh, Saudi Arabia.
Disclosure of conflict of interest
None.
Abbreviations
- PHP
Polyherbal paste
- AQP3
Aquaporin-3
- CC
Chronic constipation
- DM
Diabetes mellitus
- IBD
Inflammatory bowel disease
- L-PHP
Low-dose PHP group
- M-PHP
Medium-dose PHP group
- H-PHP
High-dose PHP group
- IHC
Immunohistochemistry
- H and E
Hematoxylin and Eosin
- SP
Substance P
- SS
Somatostatin
- MTL
Motilin
- Gas
Gastrin
- H2O2
Hydrogen peroxide
- SD
Standard deviation
References
- 1.Shaito A, Thuan DTB, Phu HT, Nguyen THD, Hasan H, Halabi S, Abdelhady S, Nasrallah GK, Eid AH, Pintus G. Herbal medicine for cardiovascular diseases: efficacy, mechanisms, and safety. Front Pharmacol. 2020;11:422. doi: 10.3389/fphar.2020.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan IA, Aziz A, Manzoor Z, Munawar SH, Sarwar HS, Afzal A, Raza MA. Study on antipyretic activity of Rumex vesicarius leaves extract in albino rabbits. Vet World. 2014;7:44–48. [Google Scholar]
- 3.Chattopadhyay D, Arunachalam G, Ghosh L, Rajendran K, Mandal AB, Bhattacharya SK. Antipyretic activity of Alstonia macrophylla wall ex A. DC: an ethnomedicine of Andaman Islands. J Pharm Pharm Sci. 2005;8:558–64. [PubMed] [Google Scholar]
- 4.Akinpelu DA, Onakoya TM. Antimicrobial activities of medicinal plants used in folklore remedies in South-Western. Afr J Biotechnol. 2006;5:1078–1081. [Google Scholar]
- 5.Zhang H, Hou Y, Liu Y, Yu X, Li B, Cui H. Determination of mangiferin in rat eyes and pharmacokinetic study in plasma after oral administration of mangiferin-hydroxypropyl-beta-cyclodextrin inclusion. J Ocul Pharmacol Ther. 2010;26:319–24. doi: 10.1089/jop.2010.0024. [DOI] [PubMed] [Google Scholar]
- 6.Khan IA, Janbaz KH, Saqib F. Antidiarrheal activity of methanolic leaf extract of Rumex vesicarius. Bangladesh J Pharmacol. 2016;11:175–180. [Google Scholar]
- 7.Ain QU, Abid MUH, Hashim M, Ishaq S, Manzoor A, Perwasha P, Mizgan GE, Iqbal MO, Munawar SH, Manzoor Z, Khan IA. Anticoagulant and thrombolytic activities of leaf extract of mangifera indica in smokers. Tob Regul Sci. 2022;8:1189–1201. [Google Scholar]
- 8.Ain QU, Iqbal MO, Khan IA, Bano N, Naeem M, Jamaludin MI, Devaraj S. Phytochemical, antioxidant, antipyretic and anti-inflammatory activities of aqueous-methanolic leaf extract of Mangifera indica. Am J Transl Res. 2023;15:4533–4543. [PMC free article] [PubMed] [Google Scholar]
- 9.Mizgan GE, Kousar S, Khan IA, Perwasha P, Gul H, Aslam I, Abbas A, Ul-Ain Q. Evaluation of anti-clotting and thrombolytic potential of the aqueous-methanolic extract of Jasminum sambac. Pak J Pharm Sci. 2022;35:1747–1754. [PubMed] [Google Scholar]
- 10.Iqbal MO, Naeem M, Mumtaz A, Ahmed MM, Ahmad A, Riaz R, Mesbah Z, Munawar N. Biochemical evaluation and medicinal ability of Jatropha mollissima in hepatic disorders. Am J Transl Res. 2022;14:7178–7188. [PMC free article] [PubMed] [Google Scholar]
- 11.Khan IA, Hussain M, Munawar SH, Iqbal MO, Arshad S, Manzoor A, Shah MA, Abbas K, Shakeel W, Syed SK. Jasminum sambac: a potential candidate for drug development to cure cardiovascular ailments. Molecules. 2021;26:5664. doi: 10.3390/molecules26185664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan IA, Lodhi AH, Munawar SH, Manzoor A, Manzoor Z, Raza MA. Formulation and evaluation of rutin-allicin gel against diabetic foot ulcer. Lat Am J Pharm. 2020;39:725–729. [Google Scholar]
- 13.Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacol. 6th edition. Churchill Livingstone Edinb; 2007. [Google Scholar]
- 14.Alvarez AM, DeOcesano-Pereira C, Teixeira C, Moreira V. IL-1β and TNF-α modulation of proliferated and committed myoblasts: IL-6 and COX-2-derived prostaglandins as key actors in the mechanisms involved. Cells. 2020;9:2005. doi: 10.3390/cells9092005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jabbarzadeh Z, Khosh-Khui M. Factors affecting tissue culture of Damask rose (Rosa damascena Mill.) Sci Hortic. 2005;105:475–482. [Google Scholar]
- 16.Khan IA, Lodhi AH, Munawar SH, Manzoor A, Manzoor Z, Raza MA. Formulation and evaluation of rutin-allicin gel against diabetic foot ulcer. Lat Am J Pharm. 2020;39:725–729. [Google Scholar]
- 17.Wang H. Beneficial medicinal effects and material applications of rose. Heliyon. 2023;10:e23530. doi: 10.1016/j.heliyon.2023.e23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceccacci S, Lucia A, Tortora A, Colantuono A, Carotenuto G, Tito A, Monti MC. Jasminum sambac cell extract as antioxidant booster against skin aging. Antioxidants (Basel) 2022;11:2409. doi: 10.3390/antiox11122409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan IA, Hussain M, Syed SK, Saadullah M, Alqahtani AM, Alqahtani T, Aldahish AA, Asiri S, Zeng LH. Pharmacological justification for the medicinal use of Plumeria rubra Linn. in cardiovascular disorders. Molecules. 2021;27:251. doi: 10.3390/molecules27010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaki Z, Ahmad G. A review on Trachyspermum ammi (Ajwain) J of Int Community Health. 2021;10:22–26. [Google Scholar]
- 21.Zhang L, Liang X, Wang B, Lin Z, Ye M, Ma R, Zheng M, Xiang H, Xu P. Six herbs essential oils suppressing inflammatory responses via inhibiting COX-2/TNF-α/IL-6/NF-κB activation. Microchem J. 2020;156:104769. [Google Scholar]
- 22.Iqbal MO, Khan IA, Manzoor A, Arshad S, Sial AS, Dar E, Shaikh AR. Cardioprotective effect of hydroalcoholic leaf extract of Jatropha mollissima on isoproterenol-induced myocardial infarction in rats. Pharmacogn Mag. 2021;17:251–256. [Google Scholar]
- 23.Bukhari KA, Khan IA, Ishaq S, Iqbal MO, Alqahtani AM, Alqahtani T, Menaa F. Formulation and evaluation of diclofenac potassium gel in sports injuries with and without phonophoresis. Gels. 2022;8:612. doi: 10.3390/gels8100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faizi S, Zikr-Ur-Rehman S, Ali M, Naz A. Temperature and solvent dependent NMR studies on mangiferin and complete NMR spectral assignments of its acyl and methyl derivatives. Magn Reson Chem. 2006;44:838–44. doi: 10.1002/mrc.1854. [DOI] [PubMed] [Google Scholar]
- 25.Jahng J, Jung IS, Choi EJ, Conklin JL, Park H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Neurogastroenterol Motil. 2012;24:185–190. e92. doi: 10.1111/j.1365-2982.2011.01819.x. [DOI] [PubMed] [Google Scholar]
- 26.Lawson PA. Bergey’s manual of systematics of archaea and bacteria. Chichester: John Wiley & Sons; 2020. pp. 1–8. [Google Scholar]
- 27.Akinpelu DA, Onakoya TM. Antimicrobial activities of medicinal plants used in folklore remedies in South-Western. Afr J Biotechnol. 2006;5:1078–1081. [Google Scholar]
- 28.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Jani B, Marsicano E. Constipation: evaluation and management. Mo Med. 2018;115:236–240. [PMC free article] [PubMed] [Google Scholar]
- 30.Makizaki Y, Maeda A, Oikawa Y, Tamura S, Tanaka Y, Nakajima S, Yamamura H. Alleviation of low-fiber diet-induced constipation by probiotic Bifidobacterium bifidum G9-1 is based on correction of gut microbiota dysbiosis. Biosci Microbiota Food Health. 2019;38:49–53. doi: 10.12938/bmfh.18-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulke A, Eckert GP, Schubert-Zsilavecz M, Wurglics M. Isoquercitrin provides better bioavailability than quercetin: comparison of quercetin metabolites in body tissue and brain sections after six days administration of isoquercitrin and quercetin. Pharmazie. 2012;67:991–6. [PubMed] [Google Scholar]
- 32.Liu LW. Chronic constipation: current treatment options. Can J Gastroenterol. 2011;25(Suppl B):22B–28B. [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Zheng T, Ding H, Chen J, Li B, Zhang Q, Yang S, Zhang S, Guan W. Exploring the benefits of probiotics in gut inflammation and diarrhea-from an antioxidant perspective. Antioxidants (Basel) 2023;12:1342. doi: 10.3390/antiox12071342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Nie SP, Zhu KX, Xiong T, Li C, Gong J, Xie MY. Effect of Lactobacillus plantarum NCU116 on loperamide-induced constipation in mice. Int J Food Sci Nutr. 2015;66:533–538. doi: 10.3109/09637486.2015.1024204. [DOI] [PubMed] [Google Scholar]
- 35.Kwon JI, Park Y, Noh DO, Suh HJ, Han SH. Complex-oligosaccharide composed of galacto-oligosaccharide and lactulose ameliorates loperamide-induced constipation in rats. Food Sci Biotechnol. 2018;27:781–788. doi: 10.1007/s10068-017-0300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appleton J. Evaluating the bioavailability of isoquercetin. Nat Med J. 2010;2:1–6. [Google Scholar]
- 37.Karakan T, Tuohy KM, Janssen-van, Solingen G. Low-dose lactulose as a prebiotic for improved gut health and enhanced mineral absorption. Front Nutr. 2021;8:672925. doi: 10.3389/fnut.2021.672925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan IA, Hussain M, Hussain N, Alqahtani AM, Alqahtani T. Cardioprotective effect of Rumex vesicarius Linn. Leaf extract against catecholamine-induced cardiotoxicity. Molecules. 2022;27:3383. doi: 10.3390/molecules27113383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim MG, Jo K, Chang YB, Suh HJ, Hong KB. Changes in the gut microbiome after galacto-oligosaccharide administration in loperamide-induced constipation. J Pers Med. 2020;10:161. doi: 10.3390/jpm10040161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krammer HJ, von Seggern H, Schaumburg J, Neumer F. Effect of Lactobacillus casei Shirota on colonic transit time in patients with chronic constipation. Coloproctology. 2011;33:109–113. [Google Scholar]
- 41.Dimidi E, Mark Scott S, Whelan K. Probiotics and constipation: mechanisms of action, evidence for effectiveness and utilisation by patients and healthcare professionals. Proc Nutr Soc. 2020;79:147–157. doi: 10.1017/S0029665119000934. [DOI] [PubMed] [Google Scholar]
- 42.Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008;373:545–9. doi: 10.1016/j.bbrc.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 43.Khan IA, Janbaz KH, Saqib F. Antidiarrheal activity of methanolic leaf extract of Rumex vesicarius. Bangladesh J Pharmacol. 2016;11:175–180. [Google Scholar]
- 44.Saper CB, Breder CD. The neurologic basis of fever. N Engl J Med. 1994;330:1880–6. doi: 10.1056/NEJM199406303302609. [DOI] [PubMed] [Google Scholar]
- 45.He Y, Liu G, Xia C, Chen J, Zhao J, Li X. Laxative effect of mulberry ferment on two models of constipated mice. J Funct Foods. 2022;90:104971. [Google Scholar]
- 46.Luo C, He ML, Bohlin L. Is COX-2 a perpetrator or a protector? Selective COX-2 inhibitors remain controversial. Acta Pharmacol Sin. 2005;26:926–33. doi: 10.1111/j.1745-7254.2005.00150.x. [DOI] [PubMed] [Google Scholar]
- 47.Quyang H, Chen JD. Review article: therapeutic roles of acupuncture in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20:831–841. doi: 10.1111/j.1365-2036.2004.02196.x. [DOI] [PubMed] [Google Scholar]
- 48.Giliani AU, Ghayur MN, Khalid A, Zaheer-ul-Haq, Choudhary MI, Atta-ur-Rahman Presence of antispasmodic, antidiarrheal, antisecretory, calcium antagonist and acetylcholinesterase inhibitory steroidal alkaloids in Sarcococca saligna. Planta Med. 2005;71:120–125. doi: 10.1055/s-2005-837777. [DOI] [PubMed] [Google Scholar]