Abstract
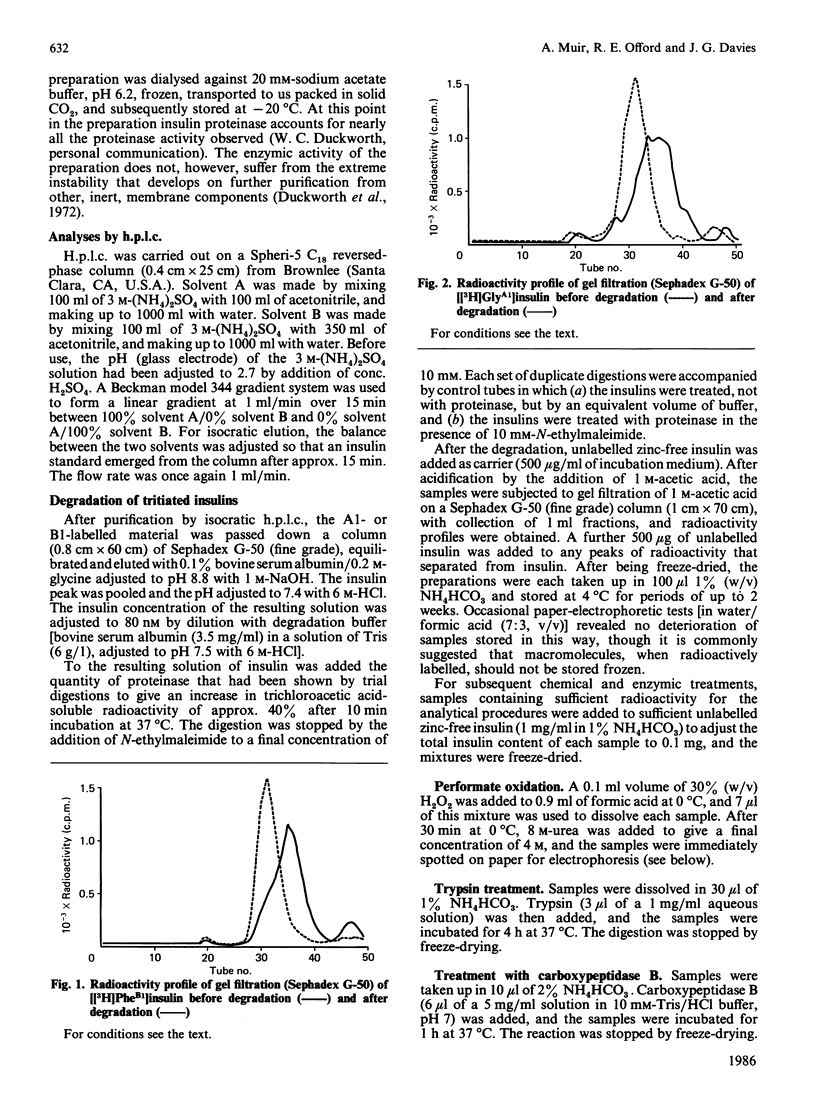
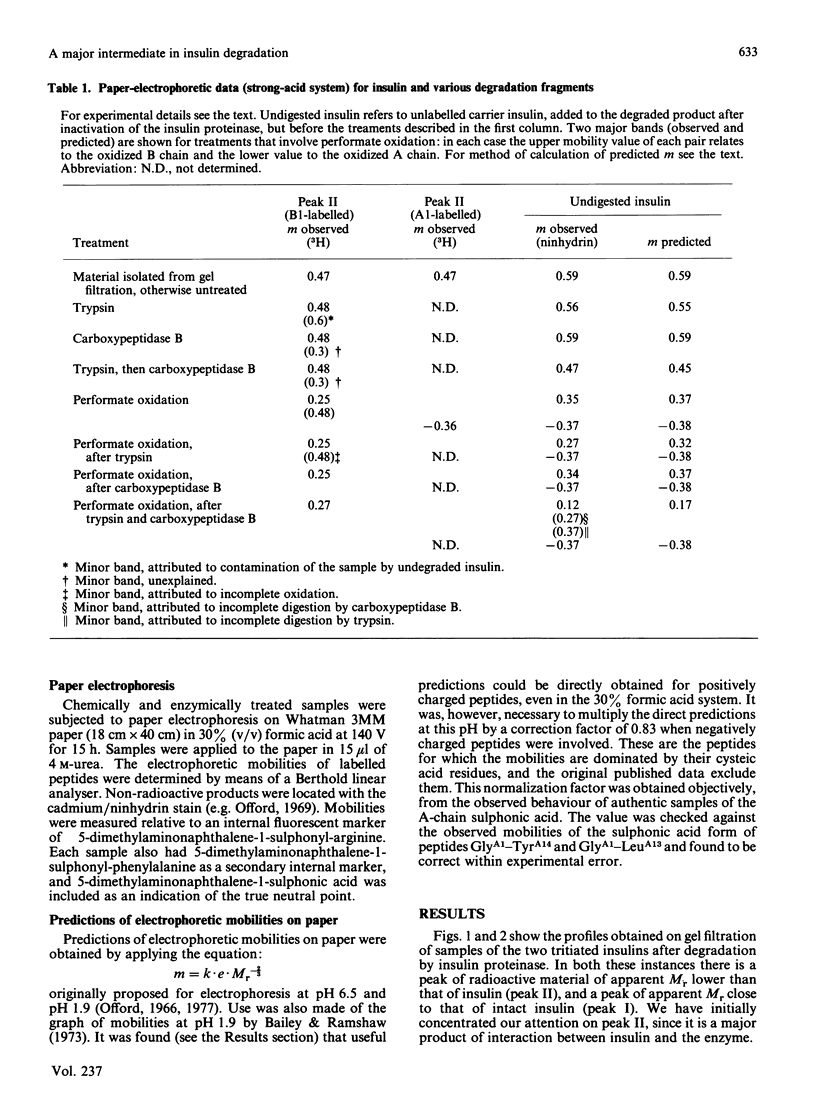
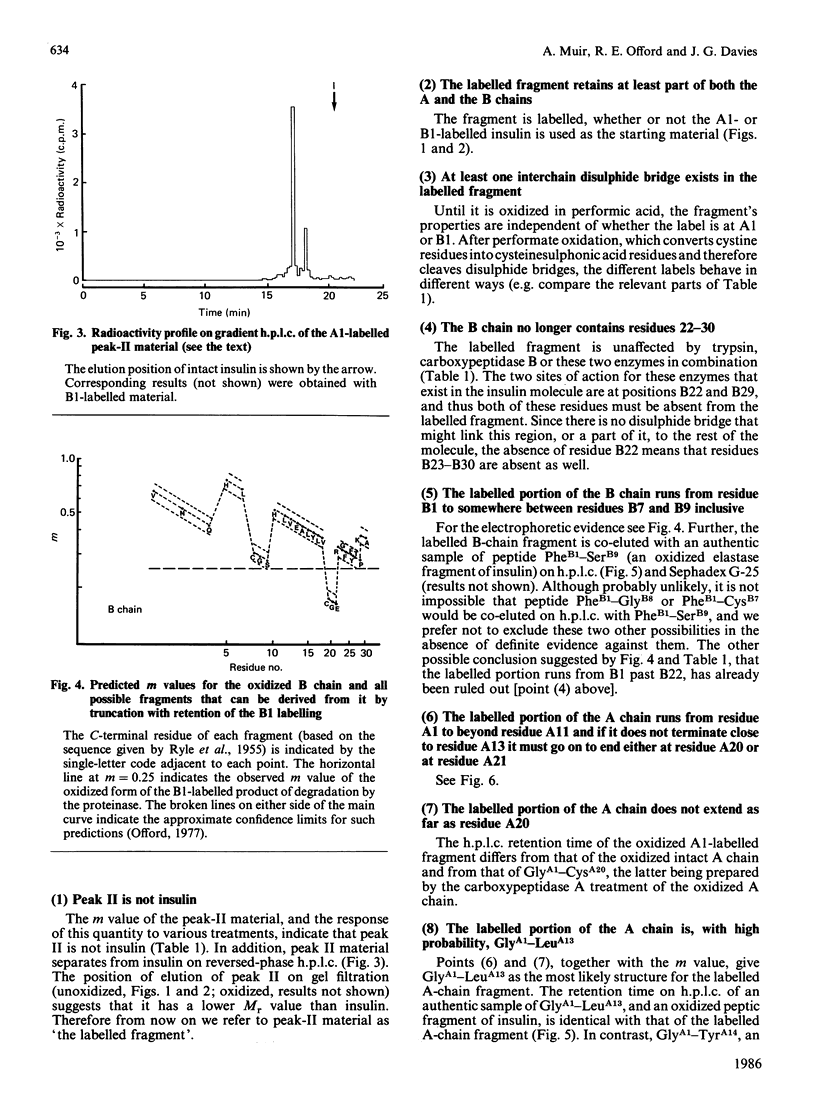
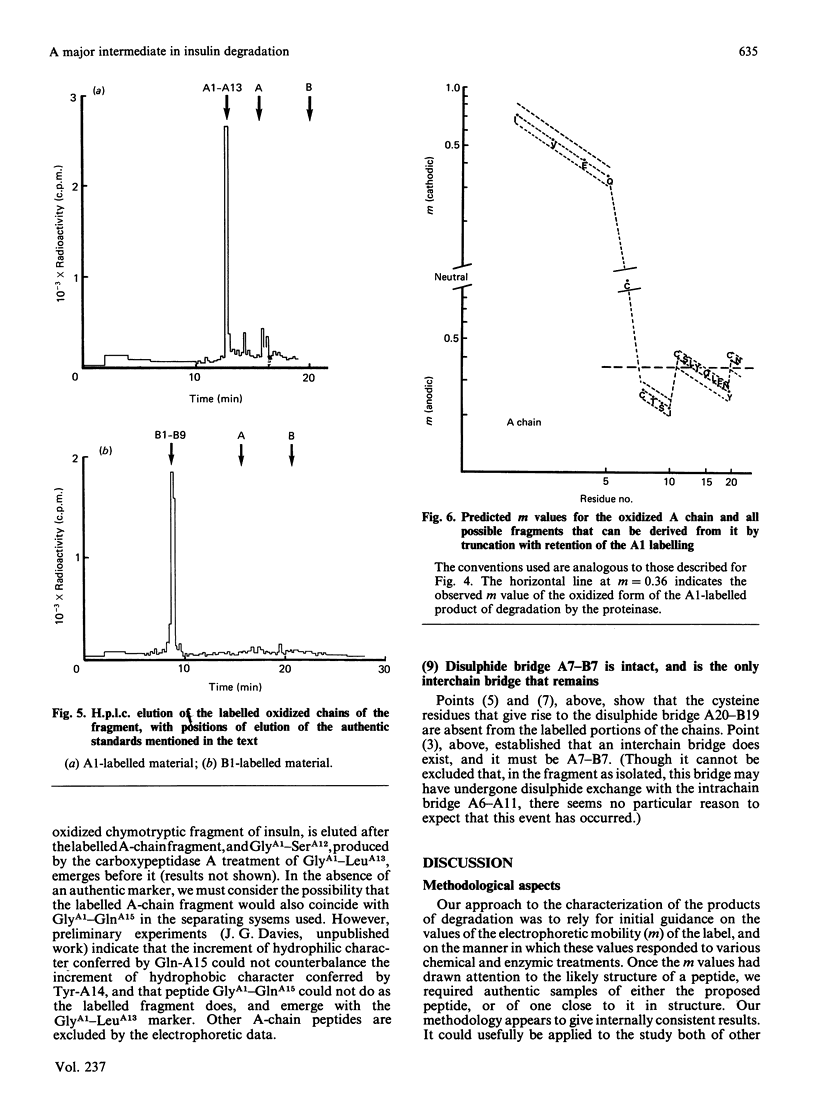
We have studied a major product in the degradation of insulin by insulin proteinase (EC 3.4.22.11). Semisynthetic [[3H]PheB1]insulin and [[3H]GlyA1]insulin were used in the experiments. The structure of the fragment was deduced by observing the chromatographic and electrophoretic migration of the label both before and after further digestion of the fragment with proteinases of known specificity, with and without additional treatment by performic acid. Ambiguities were resolved by studying the behaviour of authentic fragments of known structure, isolated and characterized after digestion of intact insulin by proteinases of known specificity. We conclude that a major product in the degradation of insulin by insulin proteinase consists of a truncated section of the A chain, joined by the disulphide bridge B7-A7 to a truncated section of the B chain. The A-chain fragment consists most probably of residues A1-A13, and the B-chain fragment consists most probably of residues B1-B9. The similarity between this fragment and that found by other workers when insulin is degraded by intact hepatocytes is significant in the light of proposals that insulin proteinase is a possible participant in the physiological degradation of insulin by target cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Tager H. S. Peptide intermediates in the cellular metabolism of insulin. J Biol Chem. 1982 Aug 10;257(15):9078–9085. [PubMed] [Google Scholar]
- Bailey C. J., Ramshaw J. A. The electrophoretic mobility of peptides on paper at pH 1.9. Biochem J. 1973 Dec;135(4):889–891. doi: 10.1042/bj1350889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin F. K., Duckworth W. C., Kitabchi A. E. Sites of cleavage of glucagon by insulin-glucagon protease. Biochem Biophys Res Commun. 1975 Nov 3;67(1):163–169. doi: 10.1016/0006-291x(75)90297-1. [DOI] [PubMed] [Google Scholar]
- Burghen G. A., Kitabchi A. E., Brush J. S. Characterization of a rat liver protease with specificity for insulin. Endocrinology. 1972 Sep;91(3):633–642. doi: 10.1210/endo-91-3-633. [DOI] [PubMed] [Google Scholar]
- Davies J. G., Offord R. E. The preparation of tritiated insulin specifically labelled by semisynthesis at glycine-A1. Biochem J. 1985 Oct 15;231(2):389–392. doi: 10.1042/bj2310389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W. C., Heinemann M. A., Kitabchi A. E. Purification of insulin-specific protease by affinity chromatography. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3698–3702. doi: 10.1073/pnas.69.12.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W. C., Heinemann M., Kitabchi A. E. Proteolytic degradation of insulin and glucagon. Biochim Biophys Acta. 1975 Feb 19;377(2):421–430. doi: 10.1016/0005-2744(75)90322-8. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C., Kitabchi A. E. Insulin metabolism and degradation. Endocr Rev. 1981 Spring;2(2):210–233. doi: 10.1210/edrv-2-2-210. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C., Runyan K. R., Wright R. K., Halban P. A., Solomon S. S. Insulin degradation by hepatocytes in primary culture. Endocrinology. 1981 Apr;108(4):1142–1147. doi: 10.1210/endo-108-4-1142. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C., Stentz F. B., Heinemann M., Kitabchi A. E. Initial site of insulin cleavage by insulin protease. Proc Natl Acad Sci U S A. 1979 Feb;76(2):635–639. doi: 10.1073/pnas.76.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J., Sonne O. Binding and receptor-mediated degradation of insulin in adipocytes. J Biol Chem. 1978 Nov 10;253(21):7857–7863. [PubMed] [Google Scholar]
- Goldstein B. J., Livingston J. N. An evaluation of the importance of lysosomal and neutral cytosol proteases in insulin degradation by adipocytes. Endocrinology. 1981 Mar;108(3):953–961. doi: 10.1210/endo-108-3-953. [DOI] [PubMed] [Google Scholar]
- Halban P. A., Karakash C., Davies J. G., Offord R. E. The degradation of semisynthetic tritiated insulin by perfused mouse livers. Biochem J. 1976 Nov 15;160(2):409–412. doi: 10.1042/bj1600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban P. A., Offord R. E. The preparation of a semisynthetic tritiated insulin with a specific radioactivity of up to 20 Curies per millimole. Biochem J. 1975 Nov;151(2):219–225. doi: 10.1042/bj1510219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misbin R. I., Almira E. C. The fate of insulin in rat hepatocytes. Evidence for the release of an immunologically active fragment. Diabetes. 1984 Apr;33(4):355–361. doi: 10.2337/diab.33.4.355. [DOI] [PubMed] [Google Scholar]
- Morishima T., Bradshaw C., Radziuk J. Measurement using tracers of steady-state turnover and metabolic clearance of insulin in dogs. Am J Physiol. 1985 Feb;248(2 Pt 1):E203–E208. doi: 10.1152/ajpendo.1985.248.2.E203. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Offord R. E. The use of logarithmic plots of electrophoretic mobilities of peptides. Methods Enzymol. 1977;47:51–69. doi: 10.1016/0076-6879(77)47008-3. [DOI] [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade D. S., Eaton R. P. The regulation of plasma ketone body concentration by counter-regulatory hormones in man. III. Effects of norepinephrine in normal man. Diabetes. 1979 Jan;28(1):5–10. doi: 10.2337/diab.28.1.5. [DOI] [PubMed] [Google Scholar]
- Steiner D. F. The Banting Memorial Lecture 1976. Insulin today. Diabetes. 1977 Apr;26(4):322–340. doi: 10.2337/diab.26.4.322. [DOI] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Binding and degradation of 125I-insulin by rat hepatocytes. J Biol Chem. 1975 Nov 10;250(21):8389–8398. [PubMed] [Google Scholar]


