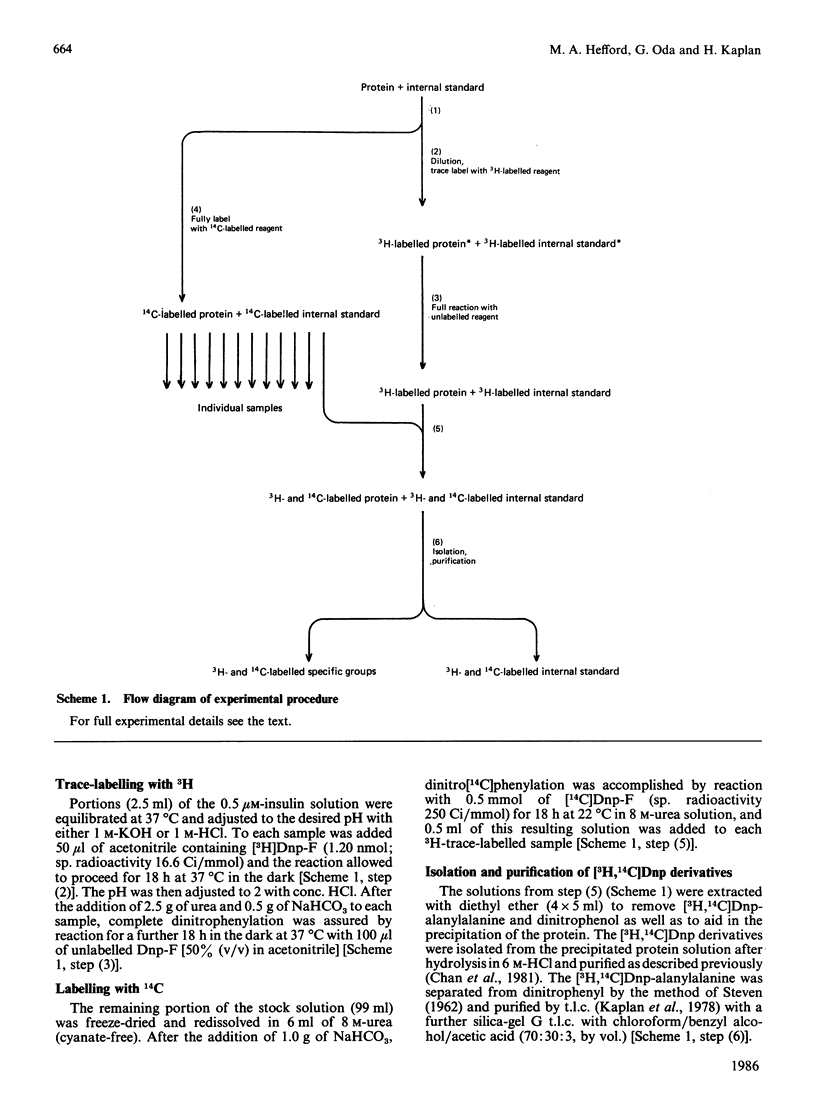
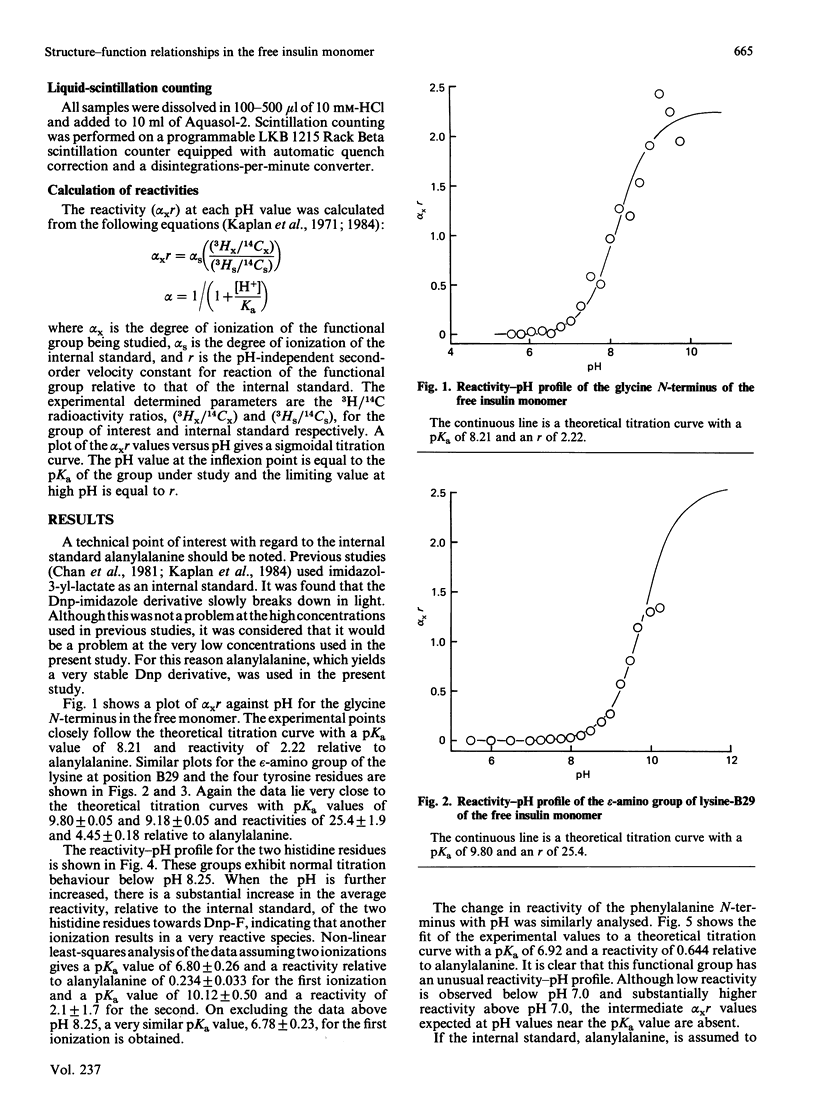
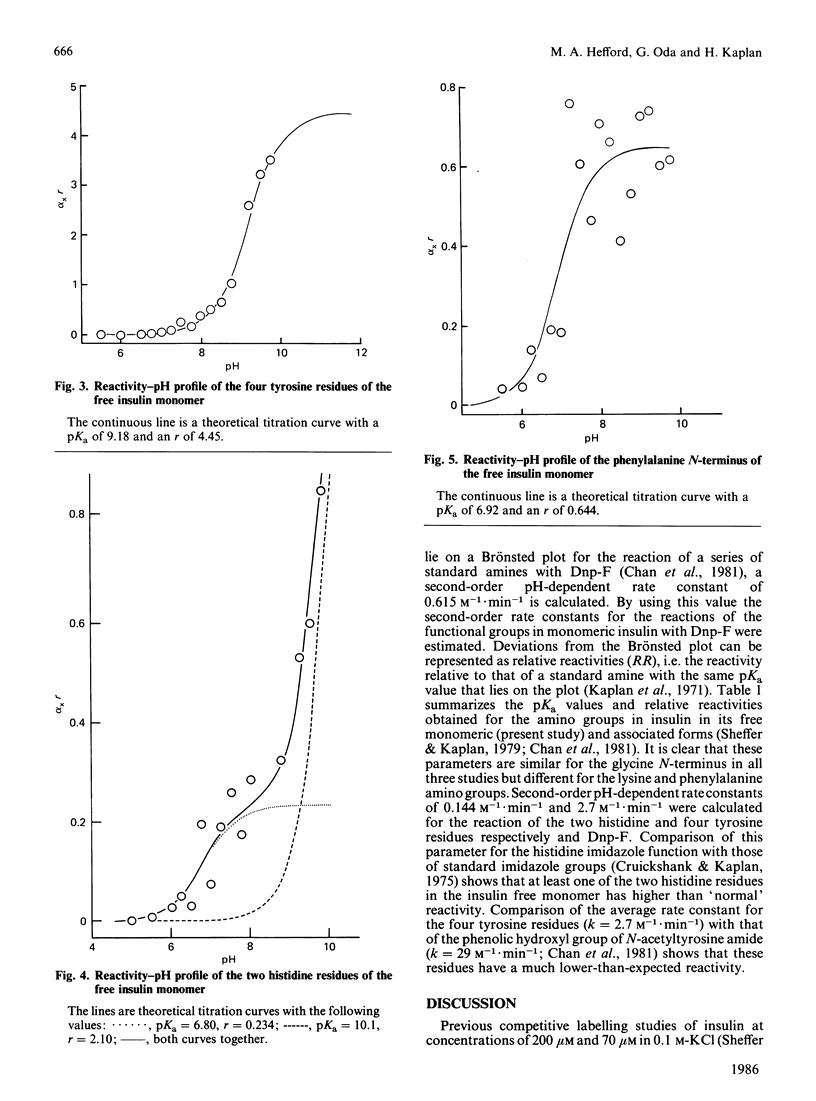
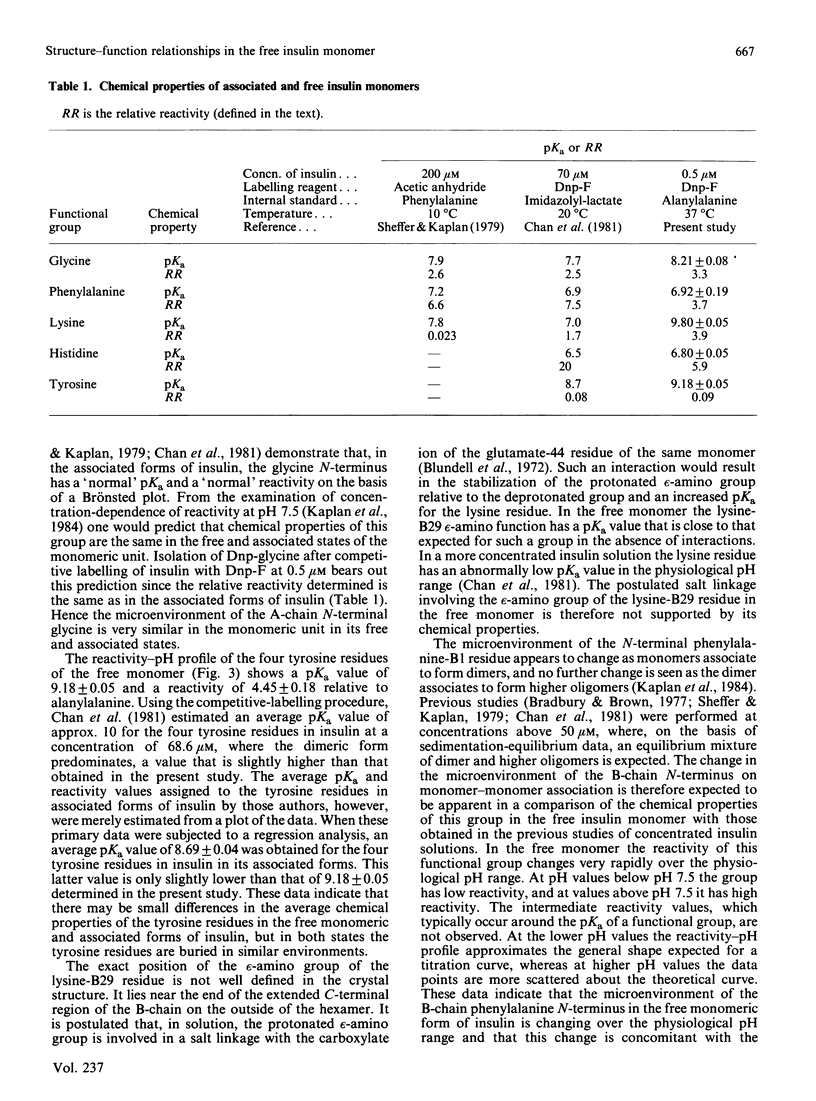
Abstract
The chemical properties of the functional groups of insulin were determined at a concentration (0.5 microM) where the predominant species of insulin is the free (unassociated) monomeric unit. The glycine N-terminus and the four tyrosine phenolic groups had the same properties as in the associated forms of insulin. On the other hand the lysine epsilon-amino group and the two histidine imidazole groups had substantially altered properties. Some alteration in the properties of the phenylalanine N-terminus was also observed. The reactivity-pH profile for the imidazole groups showed a second ionization with a pKa of 10.1 in addition to an ionization with a pKa of 6.8. On the basis of the X-ray-crystallographic structure of hexameric insulin the observed changes can be accounted for by disruption of monomer-monomer or dimer-dimer interactions in the associated states of insulin. It is concluded that the conformation of the monomeric unit of insulin is essentially the same in its free and associated states in solution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury J. H., Brown L. R. Nuclear-magnetic-resonance-spectroscopic studies of the amino groups of insulin. Eur J Biochem. 1977 Jun 15;76(2):573–582. doi: 10.1111/j.1432-1033.1977.tb11627.x. [DOI] [PubMed] [Google Scholar]
- Chan Y. K., Oda G., Kaplan H. Chemical properties of the functional groups of insulin. Biochem J. 1981 Feb 1;193(2):419–425. doi: 10.1042/bj1930419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Dodson G. G., Hodgkin D. C. Transmission of conformational change in insulin. Nature. 1983 Apr 7;302(5908):500–505. doi: 10.1038/302500a0. [DOI] [PubMed] [Google Scholar]
- Cruickshank W. H., Kaplan H. Properties of the histidine residues of indole-chymotrypsin. Implications for the activation process and catalytic mechanism. Biochem J. 1975 Jun;147(3):411–416. doi: 10.1042/bj1470411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G., Kaplan H. A competitive labeling method for the determination of the chemical properties of solitary functional groups in proteins. Biochemistry. 1975 Nov 18;14(23):5168–5175. doi: 10.1021/bi00694a023. [DOI] [PubMed] [Google Scholar]
- Goldman J., Carpenter F. H. Zinc binding, circular dichroism, and equilibrium sedimentation studies on insulin (bovine) and several of its derivatives. Biochemistry. 1974 Oct 22;13(22):4566–4574. doi: 10.1021/bi00719a015. [DOI] [PubMed] [Google Scholar]
- Hefford M. A., Evans R. M., Oda G., Kaplan H. Unusual chemical properties of N-terminal histidine residues of glucagon and vasoactive intestinal peptide. Biochemistry. 1985 Feb 12;24(4):867–874. doi: 10.1021/bi00325a009. [DOI] [PubMed] [Google Scholar]
- Hodgkin D. C., Dodson E., Dodson G., Reynolds C. Insulin. Biochem Soc Trans. 1983 Aug;11(4):411–417. doi: 10.1042/bst0110411. [DOI] [PubMed] [Google Scholar]
- Kaplan H., Cheng D. C., Oda G., Kates M. A new double-labelling procedure for determination of amino acid composition: application to bacteriorhodopsin. Can J Biochem. 1978 Jun;56(6):517–520. doi: 10.1139/o78-079. [DOI] [PubMed] [Google Scholar]
- Kaplan H., Hefford M. A., Chan A. M., Oda G. Chemical reactivity of the functional groups of insulin. Concentration-dependence studies. Biochem J. 1984 Jan 1;217(1):135–143. doi: 10.1042/bj2170135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H., Stevenson K. J., Hartley B. S. Competitive labelling, a method for determining the reactivity of individual groups in proteins. The amino groups of porcine elastase. Biochem J. 1971 Sep;124(2):289–299. doi: 10.1042/bj1240289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milthorpe B. K., Nichol L. W., Jeffrey P. D. The polymerization pattern of zinc(II)-insulin at pH 7.0. Biochim Biophys Acta. 1977 Dec 20;495(2):195–202. doi: 10.1016/0005-2795(77)90376-2. [DOI] [PubMed] [Google Scholar]
- Pekar A. H., Frank B. H. Conformation of proinsulin. A comparison of insulin and proinsulin self-association at neutral pH. Biochemistry. 1972 Oct 24;11(22):4013–4016. doi: 10.1021/bi00772a001. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Biswas S. B. Conformational dynamics of insulin in solution. Circular dichroic studies. Biochemistry. 1980 Oct 28;19(22):5043–5049. doi: 10.1021/bi00563a017. [DOI] [PubMed] [Google Scholar]
- STEVEN F. S. A sample chromatographic technique for the removal of dinitrophenyl-amino acids from excess dinitrophenyl-artifacts. J Chromatogr. 1962 Jul;8:417–418. doi: 10.1016/s0021-9673(01)99281-7. [DOI] [PubMed] [Google Scholar]
- Sheffer M. G., Kaplan H. Unusual chemical properties of the amino groups of insulin: implications for structure-function relationship. Can J Biochem. 1979 Jun;57(6):489–496. doi: 10.1139/o79-062. [DOI] [PubMed] [Google Scholar]
- Wood S. P., Blundell T. L., Wollmer A., Lazarus N. R., Neville R. W. The relation of conformation and association of insulin to receptor binding; x-ray and circular-dichroism studies on bovine and hystricomorph insulins. Eur J Biochem. 1975 Jul 15;55(3):531–542. doi: 10.1111/j.1432-1033.1975.tb02190.x. [DOI] [PubMed] [Google Scholar]


