Abstract
Background: Previous studies have reported that STAT1 (Signal Transducer and Activator of Transcription 1) is associated with multiple tumor progression. This study aimed to investigate the role and related mechanisms of STAT1 in bladder cancer. Methods: STAT1 expression in bladder cancer tissues and human bladder cancer cell lines was assessed by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The bladder cancer cell line T24 was transfected with overexpressing lentivirus targeting STAT1. Cell proliferation, invasion, and apoptosis were measured by Cell Counting Kit-8, Transwell assays, and flow cytometric analysis. Furthermore, RNA-Seq was performed to identify the downstream signaling pathways. Finally, the signaling pathway-related molecules were determined by RT-qPCR and western blot assays. Results: The overexpression of STAT1 inhibited bladder cancer cell proliferation and invasion while enhancing apoptosis. Moreover, the overexpression of STAT1 in bladder cancer cells delayed tumor tumorigenesis in vitro. Mechanistically, RNA-Seq analysis revealed that the JAK-STAT signaling pathway was up-regulated, especially SOCS1 (suppressor of cytokine signaling 1) and SOCS3 (suppressor of cytokine signaling 3) in STAT1-sufficient cells. Conclusions: These results indicate the potential of STAT1 as a therapeutic target in bladder cancer.
Keywords: Bladder cancer, STAT1, pathogenesis, SOCS1, SOCS3
Introduction
Bladder cancer has a high prevalence worldwide and is one of the most common urological cancers [1]. The incidence of bladder cancer is higher in men than in women. Moreover, smoking, age, gender, and occupational exposure have been found to be causative factors of bladder cancer, with smoking being one of the major risk factors [2,3]. Bladder cancer is categorized into two main types, namely non-muscle invasive bladder cancer and muscle-invasive bladder cancer [4,5]. The main symptoms include hematuria, urinary frequency, urgency, dysuria, and lower abdominal pain [4,6]. These symptoms may be caused by the tumor in the bladder compressing or irritating the mucosal tissues. Bladder cancer is a serious disease, and early detection and treatment are essential to improve survival [7,8].
This study revealed a correlation between the STAT1 gene, a member of the STAT family, and bladder cancer. The STAT family is a group of transcription factors that play a crucial role in cell signaling, especially in response to cytokines and interferons. The STAT family consists of seven members, STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6 [9,10]. Among them, STAT1 is an important cell signaling protein participating in the immune system and apoptosis. The JAK-STAT pathway is an essential cell signaling pathway involved in the regulation of cell proliferation, differentiation, apoptosis, and immune response [11]. STAT1 is a key member of the JAK-STAT pathway and plays a pivotal role in interferon signaling, which is particularly relevant to the study of cancer, immune-related diseases, and inflammatory diseases [12,13].
SOCS1 and SOCS3 are members of the SOCS family, which are key negative feedback regulators of cellular signaling and inhibit the cytokine signaling pathway to maintain immune homeostasis and prevent excessive immune response [14-16]. SOCS1 and SOCS3 are negative feedback regulators of the JAK-STAT pathway, which is involved in cell signaling; however, excessive or sustained activation may lead to undesirable effects [17]. Hence, SOCS1 and SOCS3 maintain the homeostasis of the pathway by inhibiting JAK-STAT signaling.
This study hypothesized that the STAT1 gene affects bladder cancer progression by regulating the JAK-STAT pathway, thereby influencing the expression of SOCS1 and SOCS3 genes.
Methods
Human tissue samples
Ten samples of cancer tissues and ten samples of pericarcinoma tissues were collected in the Second Affiliated Hospital of Kunming Medical University. Informed consent was obtained from all participating individuals. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University.
RNA extraction and real-time PCR
Total RNA was extracted from cells and tissues using the RNA Rapid Extraction and Purification Kit (TIANGEN, China), and the Reverse Transcription Kit (TaKaRa, Japan) was utilized to generate cDNA. cDNA was reverse transcribed by using the Super SYBR Green RT-qPCR Master Mix reagent (Vazyme, China), and gene expression was calculated using the 2-ΔΔ Ct method with GAPDH as an internal reference. The primer sequences are summarized in Supplementary Table 1.
Cell culture
Human ureteral epithelial immortalized cells (SV-HUC-1) and bladder cancer cell lines (5637, T24) were purchased from Procell Life Science & Technology. Human ureteral epithelial immortalized cells (SV-HUC-1) were maintained in Ham’s F-12K medium (Hyclone, USA), and the nuclei of 5637 cells T24 cells were maintained in RPMI-1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Hyclone, USA) and 1% penicillin and streptomycin (Hyclone, USA) 37°C, 5% CO2.
Vector construction and stable transfection
The overexpression lentivirus, control lentivirus, and small interfering si-STAT1 used in this experiment were designed and constructed by OBiO (Shanghai, China). The sequence of STAT1 gene was summarized in Supplementary Table 2. T24 cells were infected with the STAT1 overexpression lentivirus and a control lentivirus for 72 hours, and the cells that were not effectively infected were killed by adding and maintaining 1 μg/mL of puromycin (Hyclone, USA), resulting in a strain with stable expression that was ultimately maintained by puromycin.
Cell Counting Kit-8 (CCK-8) assay
The cell density was adjusted to 3×10^4 cells/ml, and 0.1 ml of cell suspension was dispensed into 96-well plates, setting up 5 replicate wells per group. The cells were incubated at 37°C with 5% CO2. After 24 h, 48 h, and 72 h, the supernatant was removed and each well was washed. Subsequently, a mixture of 10 µl of CCK-8 reagent (Beyotime, China) and 90 µl of complete medium (Beyotime, China) was added. The mixture was incubated for 2 h in CO2 and the absorbance (OD) of the cells in each well was measured at 450 nm.
Cell flow cytometry analysis
The cell suspension was collected and the cells were lysed. Fluorescently labeled annexin V-IF647 (Servicebio, China) were added according to the recommended dosage in the instruction manual, and the solution was mixed and incubated at 4°C under darkness for 30 minutes. An appropriate amount of cell staining buffer (or PBS containing 1% BSA) was added to resuspend the cells; the cell suspension was centrifuged at 300 g for 5 min, and the supernatant was discarded. Subsequently, 0.2 mL of cell staining buffer (or PBS containing 1% BSA) was added to resuspend the cells, which were detected and analyzed by flow cytometry.
Transwell cell invasion assay
About 150 to 200 µL of cell suspension (5×105 cells/mL) was added to the upper chamber and incubated for 24 hours. The non-migrating cells in the chamber were removed with a cotton swab and the membrane was fixed with methanol and stained with crystal violet. Then, the number of cells that migrated was counted under a microscope.
Antibodies and western blot
Cells were lysed with RIPA lysis buffer. Protein concentration was determined by the BCA method (Pierce). Protein samples were prepared in a 4× Sampling Buffer (Invitrogen, USA) at the desired concentration and the samples were denatured (95°C, 5 min). Aliquots of protein were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS/PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% skim milk and sequentially incubated with specific antibodies. Finally, chemiluminescent substrates (ECL) were used for visualization, and the grayscale bands of proteins were quantified using ImageJ software. Antibodies against STAT1 (1:1000), SOCS1 (1:1000), SOCS3 (1:1000), GAPDH (1:1000), and β-actin (1:1000) were purchased from Cell Signaling Technologies (CST, USA).
RNA-seq
Total RNA was isolated using TRIzol reagent (Invitrogen, USA) from Normal Control cells and over-expressed cells. The RNA unity was evaluated by Bioanalyzer 4200 (Agilent, USA). The RNA samples were processed for RNA library generation using IlluminaTruSeq RNA Sample Prep Kit (Cat #FC-122-1001). In addition, the 2×150 bp paired-end sequencing (PE150) was performed on the NovaSeq 6000 (LC-Bio Technology CO., Ltd., Hangzhou, China). Differentially expressed gene (DEG) analysis was performed between the two groups using the DESeq R package (1.18.1) with the criteria |log2(FoldChange)|>1 and adjusted P value <0.05. Gene Ontology (GO) and KEGG enrichment were implemented by using R package software.
Data analysis
Experiments were conducted in triplicate in this study, and quantitative data were expressed as mean ± SD. Statistical analyses were performed using GraphPad Prism 8 software (GraphPad Software, Inc., La Jolla, CA, USA) using one-way ANOVA or t-test. Significance levels at *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001 are indicated in the graphs. Non-significant results are indicated by “ns”.
Results
Expression of STAT1 in bladder cancer patients and bladder cancer cell lines
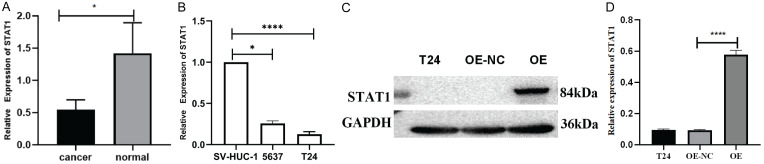
In this study, 10 samples of bladder cancer and their corresponding paracancerous tissues were collected for RT-qPCR experiments. Significantly reduced expression levels of STAT1 were observed in these samples compared with the paracancerous tissues (Figure 1A). This finding may indicate that STAT1 is involved in the pathogenesis and development of bladder cancer.
Figure 1.
STAT1 expression in patients and in bladder cancer cell lines. A. STAT1 mRNA levels in bladder cancer samples. B. STAT1 mRNA levels in cell lines. C, D. Western blot analysis of bladder cancer cell lines showing STAT1 protein overexpression (*P<0.05, ****P<0.0001).
Low STAT1 expression was found in bladder cancer tissues and cells, which are required for bladder cancer cell proliferation. Based on the above experimental results, RT-qPCR was performed to validate the expression level of STAT1 in bladder cancer cell lines (5637, T24) and human ureteral epithelial immortalized cells (SV-HUC-1) (Figure 1B). The experimental results revealed a significant differential expression in STAT1 in T24 cells, which showed the lowest expression levels among the evaluated cell lines. Therefore, T24 cells were selected for subsequent studies. These results emphasize the differential expression of STAT1 in bladder cancer cells, especially in T24 cells, and provide a strong basis for further studies. This may suggest the important role of STAT1 in the pathogenesis and development of bladder cancer, which is worthy of further study and research.
Overexpression of STAT1 in T24 cells
In this study, T24 cells were transfected with the STAT1-overexpressing lentivirus, and a stable cell line was successfully constructed. Western blot experiments detected a significant increase in the STAT1 protein expression level in samples transfected with the overexpression lentivirus (OE), indicating that STAT1 had been successfully overexpressed (Figure 1C, 1D). These results indicated the successful overexpression of STAT1 protein in T24 cells, enabling further investigation of the function and role of STAT1 in bladder cancer. The STAT1 overexpressing T24 cells were applied to explore the potential role and mechanism of STAT1 in bladder cancer development.
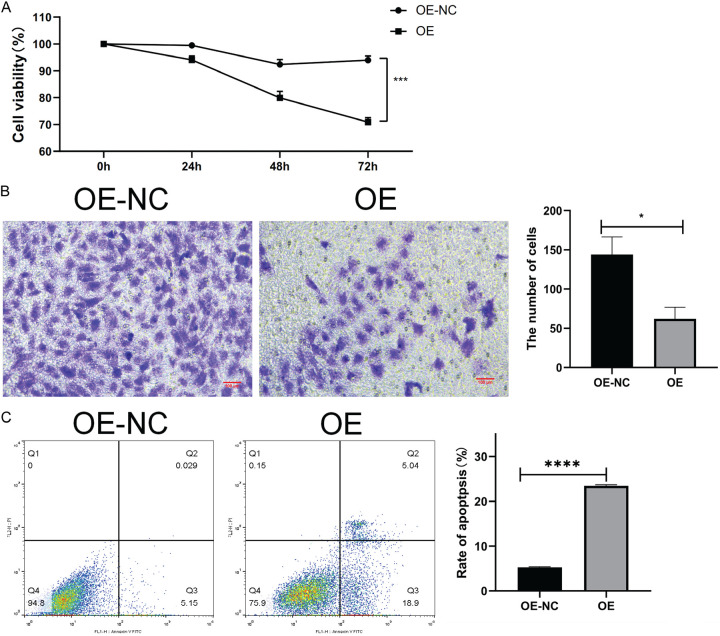
Overexpression of STAT1 inhibits the proliferation and invasion while promoting the apoptosis of T24 cells
The biological role of STAT1 was studied in CCK-8 experiments, which showed that overexpression of STAT1 significantly inhibited the proliferation ability of T24 cells. These findings suggested that overexpression of STAT1 exerts an inhibitory effect on T24 cells. STAT1 may play a negative regulatory role in T24 cell proliferation (Figure 2A). Moreover, overexpression of STAT1 exerted an inhibitory effect on T24 cell invasion, suggesting that high expression of STAT1 plays an important role in inhibiting cell invasion. These results emphasized the importance of STAT1 in T24 cells for cell invasion, and high expression of STAT1 may have an inhibitory effect on cell invasion (Figure 2B). Furthermore, apoptosis was significantly increased in T24 cells overexpressing STAT1, indicating that overexpression of STAT1 promotes apoptosis in T24 cells (Figure 2C).
Figure 2.
STAT1 overexpression suppresses T24 cell proliferation and invasion, and promotes apoptosis. A. T24 cells were subjected to CCK-8 assays. B. Transwell cell invasion assay was used to detect the invasive ability of T24 cells (100× magnification). C. Flow cytometric apoptosis assay was used to detect the apoptosis rate of T24 cells.
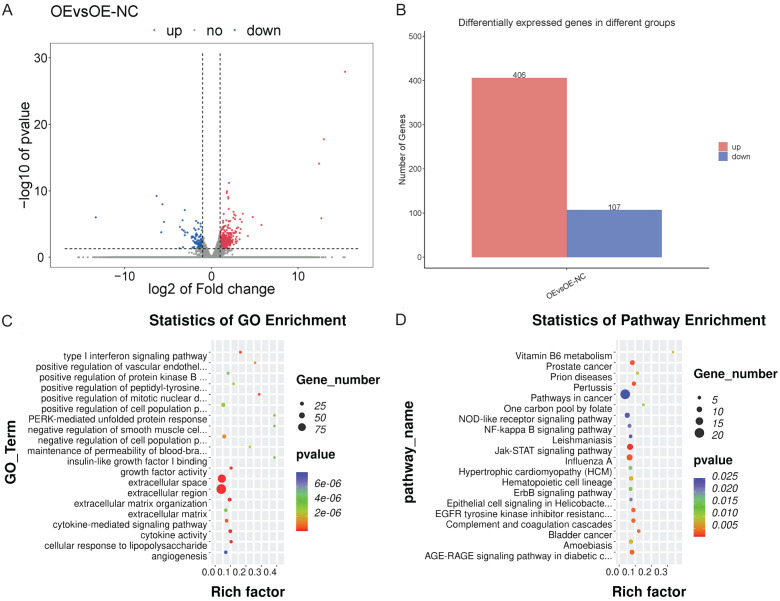
Overexpression in STAT1 activated the JAK-STAT signaling pathway
Global transcriptome changes caused by STAT1 overexpression were investigated by analyzing mRNA expression from the T24-STAT1-OE cells (OE group) and T24-STAT1-OE-NC cells (OE-NC group) using RNAseq. The significance thresholds log fold change in absolute value |log2FC|≥1 and P<0.05 were set for the analysis. Based on these criteria, 513 differentially expressed genes between the STAT1 overexpressing cells and the normal control cells, of which 406 genes were significantly up-regulated and 107 genes were significantly down-regulated (Figure 3A, 3B).
Figure 3.
Analysis of RNAseq data from STAT1 overexpression of T24 cells. A, B. Volcano and bar plot of 173 differentially expressed genes between NC cells and STAT1-overexpressing cells. C. GO enrichment analysis of upregulated and downregulated genes. D. KEGG pathway enrichment analysis of upregulated and downregulated genes (*P<0.05, ***P<0.001, ****P<0.0001).
The differentially expressed genes were subjected to GO enrichment analysis, revealing that the 513 differentially expressed genes were mainly associated with type I interferon signaling, extracellular region, extracellular space, cytokine activity, extracellular matrix organization, cellular response to lipopolysaccharide, growth factor activity, etc. (Figure 3C). KEGG enrichment analysis indicated that the genes were mostly associated with the JAK-STAT signaling pathway, prostate cancer, pertussis, influenza A, AGE-RAGE signaling pathway in diabetic complications, EGFR tyrosine kinase inhibitor resistance, complement and coagulation cascades, bladder cancer, and other pathways (Figure 3D). Among them, the JAK-STAT signaling pathway showed the strongest correlation (P<0.005).
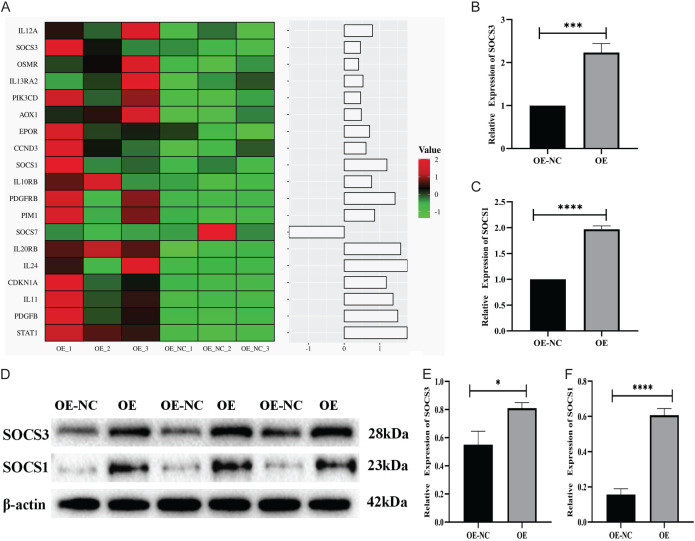
Cluster analysis of the genes related to the JAK-STAT signaling pathway showed that the expression of SOCS1 and SOCS3 was up-regulated following the up-regulation of STAT1 (Figure 4A). These results were further verified by RT-qPCR, revealing that the expression of SOCS1 and SOCS3 was also up-regulated in the STAT1 overexpression group (OE group) (Figure 4B, 4C). The WB experiment also indicated that the expression of SOCS1 and SOCS3 in the STAT1 overexpression group (OE group) was higher than that in the normal group (OE-NC group) (Figure 4D-F). The JAK-STAT signaling pathway was found to be up-regulated, with the upregulation of the STAT1 gene also affecting the upregulation of SOCS1 and SOCS3 in STAT1-sufficient cells.
Figure 4.
STAT1 overexpression induced tumor promotion in T24 cells via the JAK-STAT pathway. A. Heatmap showing the differentially expressed genes in the JAK-STAT signaling pathway. B, C. RT-qPCR validation of SOCS3 and SOCS1 mRNA expression in NC cells and over-expressed cells. D-F. SOCS3 and SOCS1 expression in NC cells and over-expressed cells detected by western blotting (*P<0.05, ***P<0.001, ****P<0.0001).
Therefore, STAT1 was hypothesized to inhibit the progression of bladder cancer by upregulating SOCS1 and SOCS3 in the JAK-STAT signaling pathway.
Discussion
STAT1 is an important signaling protein that is involved in a variety of cellular signaling pathways, including those related to apoptosis [18]. Previous studies have shown that STAT1 is associated with the progression of various tumors. For example, STAT1 inhibits the transcriptional activity of TRIM21 in gastric cancer, thereby acting as a cancer suppressor [19-21]. However, the function of STAT1 in bladder cancer remains elusive. In this study, bladder cancer samples were collected and analyzed by RT-qPCR, showing low expression of the STAT1 gene in bladder cancer tissues. Therefore, low expression of the STAT1 gene was hypothesized to be correlated with bladder cancer occurrence. Next, the function of STAT1 in the progression of bladder cancer was explored using STAT1 over-expressed cells in vitro.
The CCK8 assay verified that up-regulation of STAT1 inhibited the proliferation of T24 cells, indicating that low expression of STAT1 may increase the proliferative activity of tumor cells. The expression of the STAT1 gene was negatively correlated with the proliferation of T24 tumor cells; hence, up-regulation of the STAT1 gene might reduce the proliferation of tumor cells. Next, the apoptosis of T24 cells upon overexpression of the STAT1 gene was detected by Annexin V-IF647 assay. The results indicated significantly increased apoptosis of T24 cells in the overexpression group compared with the control group. STAT1 may play an important role in maintaining the balance between cell survival and apoptosis. Furthermore, the results of the Transwell assay showed that the invasive effect of T24 cells in the overexpression group was attenuated, suggesting that the invasiveness of T24 cells is related to the expression of the STAT1 gene. The reduction of T24 cell invasiveness may inhibit tumor spread, which is positively impacted for the treatment of bladder cancer. Our data demonstrated the tumor-inhibiting function of STAT1 in bladder cancer, suggesting that targeting STAT1 might be a potential approach for preventing bladder tumorigenesis and progression.
STAT1 overexpression was analyzed in bladder cancer cell lines by RNASeq to explore the mechanism underlying the inhibitory effects of STAT1 on carcinostasis. The data showed that the JAK-STAT pathway was notably upregulated when STAT1 was overexpressed. Further bioinformatics analysis revealed that the genes SOCS1 and SOCS3 were strongly associated with the JAK-STAT pathway. RT-qPCR and WB experiments confirmed an increased expression of SOCS1 and SOCS3 following STAT1 overexpression. Therefore, the anti-cancer effect of STAT1 was hypothesized to be mediated by the up-regulation of SOCS1 and SOCS3 expression, thereby up-regulating the JAK-STAT signaling pathway.
Signal transduction and activators of transcription (STATs) are a potential class of cytoplasmic transcription factors. Activation of STAT proteins is mediated primarily by conserved tyrosine phosphorylation of serine residues in their c-terminal transactivation domains via Janus kinases (JAKs) and mitogen-activated protein kinases (MAPKs), which allow activated STAT to dimerize and translocate to the nucleus [22]. In addition, STAT proteins play important roles in mediating a wide range of biological processes such as cell proliferation, survival, apoptosis, and differentiation by regulating the expression of target genes. Among the members of the STAT family, growing evidence indicates that STAT1 plays a key role in various forms of cell death [23-25]. The role of STAT1 in the biological process of apoptosis may serve as a target for tumor therapy. Furthermore, the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway has been shown to be involved in the cellular process of tumorigenesis and is regulated by a variety of cytokines, interferons, and growth factors [26,27]. Bladder cancer is a common urological tumor, and its pathogenesis involves multiple genetic and molecular factors. This study investigated the potential role of the STAT1 gene in the development of bladder cancer. Hong et al. [28] showed that STAT1 was associated with carcinostasis of bladder cancer, which is consistent with this study.
Moreover, the SOCS1 and SOCS3 genes have been shown to be tumor suppressors, and their deletion may activate oncogenic signaling pathways [29]. Most cytokines transmit signals, often using Janus kinases/activators of signal transduction and transcriptional pathways to regulate various cell biological processes [30]. However, the activation of this signaling pathway is also negatively regulated, which is mainly achieved by the suppressor of cytokine signaling (SOCS) proteins. SOCS proteins play an essential role in inhibiting excessive or persistent signaling, of which SOCS1 and SOCS3 are two important members. These SOCS proteins maintain the signaling balance by negatively regulating the Janus kinase/STAT signaling pathway through various mechanisms [31-34]. Meanwhile, the STAT1 gene is an important component of this pathway, which plays a key role in cytokine signaling and transcriptional pathways. Hence, STAT1 is closely linked to SOCS1 and SOCS3, which are collectively involved in regulating the balance of cell signaling and cellular biological processes. This interaction maintains the appropriate level of signaling within the cell, ensuring normal cell function and survival. These reports are consistent with our research.
Nevertheless, the limitations of the present study should be acknowledged. First, the study only confirmed the association between STAT1 and cancer cell proliferation, invasion, apoptotic progression, and metastasis; however, other biological processes, including the cell cycle, were not validated. In addition, this research did not examine the precise ways in which STAT1 is involved in the tumor microenvironment. Transgenic animal models should be used in future studies to better investigate the exact molecular mechanism of STAT1 in cancer suppression. In summary, our work found that STAT1 can control tumor progression and exerts anti-cancer functions in many malignant tumors. This finding suggests that STAT1 may be a potential target related to the progression of bladder cancer.
Acknowledgements
This work was supported by the Yunnan Provincial Urological Diseases Clinical Medical Center Project and the National Natural Science Foundation of China (82060464).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: an update. World J Urol. 2020;38:1895–1904. doi: 10.1007/s00345-019-02984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobo N, Afferi L, Moschini M, Mostafid H, Porten S, Psutka SP, Gupta S, Smith AB, Williams SB, Lotan Y. Epidemiology, screening, and prevention of bladder cancer. Eur Urol Oncol. 2022;5:628–639. doi: 10.1016/j.euo.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Jubber I, Ong S, Bukavina L, Black PC, Comperat E, Kamat AM, Kiemeney L, Lawrentschuk N, Lerner SP, Meeks JJ, Moch H, Necchi A, Panebianco V, Sridhar SS, Znaor A, Catto JWF, Cumberbatch MG. Epidemiology of bladder cancer in 2023: a systematic review of risk factors. Eur Urol. 2023;84:176–190. doi: 10.1016/j.eururo.2023.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Dyrskjot L, Hansel DE, Efstathiou JA, Knowles MA, Galsky MD, Teoh J, Theodorescu D. Bladder cancer. Nat Rev Dis Primers. 2023;9:58. doi: 10.1038/s41572-023-00468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holzbeierlein JM, Bixler BR, Buckley DI, Chang SS, Holmes R, James AC, Kirkby E, Mckiernan JM, Schuckman AK. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline: 2024 amendment. J Urol. 2024;211:533–538. doi: 10.1097/JU.0000000000003846. [DOI] [PubMed] [Google Scholar]
- 6.Garg T, Pinheiro LC, Atoria CL, Donat SM, Weissman JS, Herr HW, Elkin EB. Gender disparities in hematuria evaluation and bladder cancer diagnosis: a population based analysis. J Urol. 2014;192:1072–1077. doi: 10.1016/j.juro.2014.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobruch J, Oszczudlowski M. Bladder cancer: current challenges and future directions. Medicina (Kaunas) 2021;57:749. doi: 10.3390/medicina57080749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran L, Xiao JF, Agarwal N, Duex JE, Theodorescu D. Advances in bladder cancer biology and therapy. Nat Rev Cancer. 2021;21:104–121. doi: 10.1038/s41568-020-00313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YJ, Zhang C, Martincuks A, Herrmann A, Yu H. Stat proteins in cancer: orchestration of metabolism. Nat Rev Cancer. 2023;23:115–134. doi: 10.1038/s41568-022-00537-3. [DOI] [PubMed] [Google Scholar]
- 10.Verhoeven Y, Tilborghs S, Jacobs J, De Waele J, Quatannens D, Deben C, Prenen H, Pauwels P, Trinh XB, Wouters A, Smits ELJ, Lardon F, van Dam PA. The potential and controversy of targeting STAT family members in cancer. Semin Cancer Biol. 2020;60:41–56. doi: 10.1016/j.semcancer.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Xue C, Yao Q, Gu X, Shi Q, Yuan X, Chu Q, Bao Z, Lu J, Li L. Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer. Signal Transduct Target Ther. 2023;8:204. doi: 10.1038/s41392-023-01468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdogan F, Radu TB, Orlova A, Qadree AK, de Araujo ED, Israelian J, Valent P, Mustjoki SM, Herling M, Moriggl R, Gunning PT. JAK-STAT core cancer pathway: an integrative cancer interactome analysis. J Cell Mol Med. 2022;26:2049–2062. doi: 10.1111/jcmm.17228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Lopez McDonald MC, Kim C, Ma M, Pan ZT, Kaufmann C, Frank DA. The complementary roles of STAT3 and STAT1 in cancer biology: insights into tumor pathogenesis and therapeutic strategies. Front Immunol. 2023;14:1265818. doi: 10.3389/fimmu.2023.1265818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai L, Han Y, Yang Z, Zeng Y, Liang W, Shi Z, Tao Y, Liang X, Liu W, Zhou S, Xing Z, Hu W, Wang X. Identification and validation of SOCS1/2/3/4 as potential prognostic biomarkers and correlate with immune infiltration in glioblastoma. J Cell Mol Med. 2023;27:2194–2214. doi: 10.1111/jcmm.17807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshimura A, Ito M, Mise-Omata S, Ando M. SOCS: negative regulators of cytokine signaling for immune tolerance. Int Immunol. 2021;33:711–716. doi: 10.1093/intimm/dxab055. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura A, Aki D, Ito M. SOCS, SPRED, and NR4a: negative regulators of cytokine signaling and transcription in immune tolerance. Proc Jpn Acad Ser B Phys Biol Sci. 2021;97:277–291. doi: 10.2183/pjab.97.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopalli SR, Annamneedi VP, Koppula S. Potential natural biomolecules targeting JAK/STAT/SOCS signaling in the management of atopic dermatitis. Molecules. 2022;27:4660. doi: 10.3390/molecules27144660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marti-Rodrigo A, Alegre F, Moragrega AB, Garcia-Garcia F, Marti-Rodrigo P, Fernandez-Iglesias A, Gracia-Sancho J, Apostolova N, Esplugues JV, Blas-Garcia A. Rilpivirine attenuates liver fibrosis through selective STAT1-mediated apoptosis in hepatic stellate cells. Gut. 2020;69:920–932. doi: 10.1136/gutjnl-2019-318372. [DOI] [PubMed] [Google Scholar]
- 19.Huo C, Gu Y, Wang D, Zhang X, Tang F, Zhao B, Liu T, He W, Li Y. STAT1 suppresses the transcriptional activity of TRIM21 in gastric cancer. J Cancer Res Clin Oncol. 2023;149:15335–15348. doi: 10.1007/s00432-023-05307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Li Q, Yang J, Xu P, Xuan Z, Xu J, Xu Z. Cytosolic TGM2 promotes malignant progression in gastric cancer by suppressing the TRIM21-mediated ubiquitination/degradation of STAT1 in a GTP binding-dependent modality. Cancer Commun (Lond) 2023;43:123–149. doi: 10.1002/cac2.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palakurthi B, Fross SR, Guldner IH, Aleksandrovic E, Liu X, Martino AK, Wang Q, Neff RA, Golomb SM, Lewis C, Peng Y, Howe EN, Zhang S. Targeting CXCL16 and STAT1 augments immune checkpoint blockade therapy in triple-negative breast cancer. Nat Commun. 2023;14:2109. doi: 10.1038/s41467-023-37727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HS, Lee MS. STAT1 as a key modulator of cell death. Cell Signal. 2007;19:454–465. doi: 10.1016/j.cellsig.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Wang F, Luo F. The role of JAK/STAT pathway in fibrotic diseases: molecular and cellular mechanisms. Biomolecules. 2023;13:119. doi: 10.3390/biom13010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montero P, Milara J, Roger I, Cortijo J. Role of JAK/STAT in interstitial lung diseases; Molecular and cellular mechanisms. Int J Mol Sci. 2021;22:6211. doi: 10.3390/ijms22126211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farooqi AA, Turgambayeva A, Tashenova G, Tulebayeva A, Bazarbayeva A, Kapanova G, Abzaliyeva S. Multifunctional roles of betulinic acid in cancer chemoprevention: spotlight on JAK/STAT, VEGF, EGF/EGFR, TRAIL/TRAIL-R, AKT/mTOR and non-coding RNAs in the inhibition of carcinogenesis and metastasis. Molecules. 2022;28:67. doi: 10.3390/molecules28010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pencik J, Pham HT, Schmoellerl J, Javaheri T, Schlederer M, Culig Z, Merkel O, Moriggl R, Grebien F, Kenner L. JAK-STAT signaling in cancer: from cytokines to non-coding genome. Cytokine. 2016;87:26–36. doi: 10.1016/j.cyto.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weng H, Yuan S, Huang Q, Zeng XT, Wang XH. STAT1 is a key gene in a gene regulatory network related to immune phenotypes in bladder cancer: an integrative analysis of multi-omics data. J Cell Mol Med. 2021;25:3258–3271. doi: 10.1111/jcmm.16395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan MGM, Ghosh A, Variya B, Santharam MA, Ihsan AU, Ramanathan S, Ilangumaran S. Prognostic significance of SOCS1 and SOCS3 tumor suppressors and oncogenic signaling pathway genes in hepatocellular carcinoma. BMC Cancer. 2020;20:774. doi: 10.1186/s12885-020-07285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamiya T, Kashiwagi I, Takahashi R, Yasukawa H, Yoshimura A. Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler Thromb Vasc Biol. 2011;31:980–985. doi: 10.1161/ATVBAHA.110.207464. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura A. Regulation of cytokine signaling by the SOCS and Spred family proteins. Keio J Med. 2009;58:73–83. doi: 10.2302/kjm.58.73. [DOI] [PubMed] [Google Scholar]
- 32.Guthula LS, Yeh KT, Huang WL, Chen CH, Chen YL, Huang CJ, Chau LK, Chan MWY, Lin SH. Quantitative and amplification-free detection of SOCS-1 CPG methylation percentage analyses in gastric cancer by fiber optic nanoplasmonic biosensor. Biosens Bioelectron. 2022;214:114540. doi: 10.1016/j.bios.2022.114540. [DOI] [PubMed] [Google Scholar]
- 33.Stone L. Putting a SOCS in prostate cancer. Nat Rev Urol. 2019;16:147. doi: 10.1038/s41585-019-0151-0. [DOI] [PubMed] [Google Scholar]
- 34.Durham GA, Williams JJL, Nasim MT, Palmer TM. Targeting SOCS proteins to control JAK-STAT signalling in disease. Trends Pharmacol Sci. 2019;40:298–308. doi: 10.1016/j.tips.2019.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.