Abstract
Introduction
Metabolic comorbidities are involved in the development and progression of noncommunicable diseases. There is convincing evidence that lifestyles are important contributors to metabolic comorbidities. This study measured the metabolic comorbidity score of South Asian adults and identified its relationship with lifestyles.
Methods
The authors studied 5 South Asian countries, including Afghanistan, Bangladesh, Bhutan, Nepal, and Sri Lanka, using the World Health Organization's STEPwise approach to noncommunicable disease risk factor surveillance data between 2014 and 2019. This was a nationally representative and cross-sectional survey on participants aged 15–69 years. The sample size was 27,616. The outcome was metabolic comorbidity score, calculated on the basis of total cholesterol, fasting plasma glucose, blood pressure, and abdominal obesity. Total metabolic comorbidity score of each participant varied between 0 and 8. It was then divided into 3 ranges: the lowest range (total metabolic comorbidity score <3), medium range (total metabolic comorbidity score ≥3 and ≤5), and the highest range (total metabolic comorbidity score ≥6). On the basis of the outcome of nonparametric receiver operating characteristics analysis, the medium and the highest ranges together were considered as higher metabolic comorbidity score. The lowest range was considered as lower metabolic comorbidity score. The higher metabolic comorbidity score was coded as 1, and the lower metabolic comorbidity score was coded as 0. Thus, the outcome variable, metabolic comorbidity score, became a binary variable. Exposures included physical inactivity (<150 minutes of medium-to-vigorous physical activity/week), high daily sedentary time (≥9 hours/day), use of tobacco (present or past smoking or daily use of smokeless tobacco products), and consumption of alcohol (at least once per month in the last 1 year). Binomial logistic regression model produced the OR with corresponding 95% CIs.
Results
The prevalence of higher metabolic comorbidity score was 34% among South Asian adults, 25% among the male respondents, and 41% among the female respondents. Participants who were physically inactive (OR=1.26; 95% CI= 1.17, 1.36), had high sedentary time (OR=1.24; 95% CI=1.11, 1.33), and consumed alcohol (OR=1.40; 95% CI=1.23, 1.53) showed higher metabolic comorbidity score than participants who were physically active, had low sedentary time, and did not consume alcohol respectively. However, the authors found an inverse association (OR=0.75; 95% CI=0.71, 0.81) between the use of tobacco and metabolic comorbidity score.
Conclusions
One third of South Asian adults had higher metabolic comorbidity score. Physical inactivity, daily sedentary hours, and minimal alcohol consumption were associated with higher metabolic comorbidity score.
Keywords: Metabolic comorbidity, lifestyle behavior, physical activity, sedentary behavior, alcohol consumption, South Asian adults
HIGHLIGHTS
-
•
A total of 34% of the respondents (male 41%, female 25%) had high metabolic comorbidity score.
-
•
A total of 60% of women aged ≥55 years had high metabolic comorbidity score.
-
•
Physical inactivity raised the odds of high metabolic comorbidity score by 26%.
-
•
High daily sedentary time raised the odds of high metabolic comorbidity score by 26%.
-
•
Alcohol consumption raised the odds of high metabolic comorbidity score by 40%.
INTRODUCTION
Metabolic disorders, including diabetes mellitus, hypertension, dyslipidemia, and obesity or being overweight, are involved in the development and progression of noncommunicable diseases (NCDs), which are the leading cause of mortality and morbidity worldwide.1 Multiple metabolic disorders often coexist, and this is defined as metabolic comorbidity.2 South Asia is one of the epicenters of the global diabetes pandemic.3 At the same time, obesity is predicted to double between 2010 and 2030 in South Asian countries.4 Hence, it is likely that the prevalence of metabolic comorbidities is on the rise in this region. However, despite considerable research on the prevalence of specific metabolic disorders, the prevalence of metabolic comorbidities has received less attention in the South Asian region. Evidence suggests that the risk of NCDs progressively increases with the number of metabolic comorbidities.2 Evaluating the population-level patterns and prevalence of metabolic comorbidities allows for more effective prevention and treatment strategies to mitigate the burden of NCDs.
Along with nonmodifiable risk factors such as aging, lifestyle factors are also important contributors to metabolic comorbidities.5 South Asian countries are undergoing rapid economic expansion and urbanization, causing dramatic changes in lifestyles.6,7 However, no studies have measured the metabolic comorbidity in relation to lifestyles among South Asian adults, but such studies are imperative, as opposed to the studies addressing individual metabolic risk factors in relation to lifestyles, because they offer insights into how lifestyles can improve holistic metabolic health, guiding the development of targeted public health interventions to mitigate the overall burden of NCDs, ultimately leading to improved health outcomes for both individuals and communities.
Therefore, the objectives of this study were to measure the metabolic comorbidity score of South Asian adults by considering the severity of each of the metabolic risk factors using nationally representative datasets and identifying its relationship with lifestyles.
METHODS
Study Sample
The World Bank South Asian region was the focus of this study. Of the 8 countries in this region, this study included 5 countries—Afghanistan, Bangladesh, Bhutan, Nepal, and Sri Lanka—on the basis of the availability of nationally representative data sets within the last 10 years. These countries varied in large degree by their sociodemographic characteristics (Appendix 1, available online).
The authors used WHO's STEPwise approach to NCD risk factor surveillance data. These are secondary survey data that were made available for research purpose only upon written request, and no ethical approval was required for this study. STEP data help countries to follow national NCDs status. STEP is a nationally representative, cross-sectional, and population-based household survey that included adult men and women aged between 18 and 69 years. The respondents must live in that household for at least 6 months and in the night before the survey. People who were too sick and mentally unfit or physically disable or incapable to give informed consent were also excluded from the survey. For each selected country, the authors used only the most recent survey data set if multiple STEP survey data sets were available. Appendix 2 (available online) shows the basic survey characteristics of all 5 STEP survey data sets. The authors excluded the participants (n=127) who were pregnant during interview from the final analysis to avoid bias in the association.
Measures
The outcome variable of this study was the metabolic comorbidity score of South Asian adults. This is a composite variable consisted of 4 metabolic risk factors: total cholesterol, fasting plasma glucose, blood pressure, and abdominal obesity. Table 1 shows the details of the measurement of metabolic comorbidity score. The authors used the definitions issued by relevant international organizations and defined 3 risk levels—low, medium, and high for each of the metabolic risk factors—and coded them as 0, 1, and 2, respectively. For total cholesterol (<200, ≥200 and ≤239, ≥240) and blood pressure (systolic/diastolic: <130 mmHg/80 mmHg, ≥130 mmHg/80 mmHg and ≤139 mmHg/89 mmHg, ≥140 mmHg/90 mmHg), the authors used the definitions issued by American Heart Association.8,9 For fasting plasma glucose (≥70 and ≤100, ≥101 and ≤125, ≥126), the authors used the definition issued by American Diabetes Association.10 For abdominal obesity (<94 cm [male] and <80 cm [female], ≥94 cm and ≤102 cm [male] and ≥80 cm and ≤88 cm [female], >102 cm [male] and >88 cm [female]), the authors used the definitions issued by WHO.11,12 Furthermore, participants who took medicine for controlling blood cholesterol or blood pressure or fasting plasma glucose or who took insulin were also placed in high-risk category for corresponding metabolic risk factor. For each participant, the total metabolic comorbidity score was calculated by adding the risk scores for each of the 4 metabolic risk factors.2,13 Total metabolic comorbidity score ranged between 0 and 8. It was then divided into 3 ranges: the lowest range (total metabolic comorbidity score <3), medium range (total metabolic comorbidity score ≥3 and ≤5), and the highest range (total metabolic comorbidity score ≥6). On the basis of the outcome of nonparametric receiver operating characteristics (ROC) analysis, the medium and the highest ranges together were considered as higher metabolic comorbidity score. The lowest range was considered as lower metabolic comorbidity score. The higher metabolic comorbidity score was coded as 1, and the lower metabolic comorbidity score was coded as 0. Thus, the outcome variable, metabolic comorbidity score, became a binary variable. Appendixes 3 and 4 (available online) describe the details of the nonparametric ROC analysis to determine the cut off point of lower versus higher metabolic comorbidity score.
Table 1.
Measurement of Metabolic Comorbidity Score of South Asian Adults
| Metabolic risk factors | Cut-off points | Risk levels | Risk score | Metabolic comorbidity score |
||
|---|---|---|---|---|---|---|
| Total | Ranges | Risk category | ||||
| Total cholesterola (mg/dL) | <200 | Low | 0 | 0–8 | Lowest (0–2) |
Lower |
| ≥200 and ≤239 | Medium | 1 | ||||
| ≥240 or medication | High | 2 | ||||
| Fasting plasma glucoseb (mg/dL) | ≥70 and ≤100 | Low | 0 | |||
| ≥101 and ≤125 | Medium | 1 | Medium (3–5) | Higher | ||
| ≥126 or medication | High | 2 | ||||
| Blood pressurea (mmHg) (systolic/diastolic) | <130/80 | Low | 0 | |||
| ≥130/80 and ≤139/89 | Medium | 1 | ||||
| ≥140/90 or medication | High | 2 | Highest (6–8) | |||
| Abdominal obesityc (waist circumference in cm) | <94 (male) and <80 (female) | Low | 0 | |||
| ≥94 and ≤102 (male) and ≥80 and ≤88 (female) | Medium | 1 | ||||
| >102 (male) and >88 (female) | High | 2 | ||||
Notes: Participants who were on medication to lower fasting plasma glucose, blood pressure, and total cholesterol in last 15 days were placed in corresponding high-risk category.
Defined by American Heart Association.
Defined by American Diabetes Association.
Defined by WHO.
The exposure variables of this study included 4 lifestyle risk factors: physical inactivity, daily sedentary time, use of tobacco, and alcohol consumption. According to WHO's guideline, the authors defined physical inactivity as <150 minutes/week of medium-to-vigorous physical activity. WHO Guideline Development Group found that if daily sedentary hours were between 7.5 and 9 hours, the risk of all-cause mortality, cardiovascular disease–related mortality, cancer mortality, incidence of cardiovascular disease, cancer, and Type 2 diabetes started to increase, and the associations became more pronounced if daily sedentary hours exceeded 9.5 hours.14 Thus in this study, if sedentary time was ≥9 hours per day, the authors considered it as high daily sedentary time.14 On the basis of the Surgeon General's reports on smoking and health, the authors defined the use of tobacco as current smoker or current user of smokeless tobacco products or past daily smoker or past daily user of smokeless tobacco products.15 Regarding alcohol consumption, a WHO statement, released in January 2023, stated that no level of alcohol consumption is safe when it comes to human health, which was supported by 2 other studies, published in Lancet in 2018.16, 17, 18 On the basis of these findings, the authors defined alcohol consumption as consuming alcohol at least 1 day per month in the past 1 year or stopped drinking alcohol owing to health issues. Appendix 5 (available online) provides the details of the exposure variables. The authors performed chi-square test, and each of the selected exposures showed significant relationship with metabolic risk score.
Covariates included participants’ sex, age, and education status; poverty headcount ratio at national poverty lines; and population density of each country. On the basis of previous literatures and chi-square test outcomes, the authors selected the covariates to include. In this study, sex had 2 categories: male and female. Sex was defined on the basis of sex assigned at birth following the visible external anatomy of a newborn.
Statistical Analysis
Multiple imputation by chained equation based on linear regression was performed to retrieve the missing values (10% of total sample) of fasting plasma glucose, total cholesterol, systolic and diastolic blood pressure, and waist circumference. Then, the authors checked consistency of metabolic comorbidity score over original and imputed data sets. Appendix 6 (available online) shows the outcome of consistency check. The authors estimated means and proportions and performed 2-sample t-test with equal variance (alpha=0.05) and chi-square test of independence, respectively, to check the statistical significance. The authors also performed direct age standardization of the rate of higher metabolic comorbidity score among South Asian countries and used the population structure of Central and Southern Asian region of United Nations in 2018 as the reference population (Appendix 7, available online). The authors checked the multicollinearity among the independent variables (mean variance inflation factor <10). Using binomial logistic regression model, the authors estimated the ORs and corresponding 95% CIs to assess the relationships between metabolic comorbidity score and lifestyles among adults in South Asia. Nonparametric ROC analysis was performed to check the accuracy of metabolic comorbidity score, and parametric ROC (area under curve≥0.70) analysis was performed to check the accuracy of multivariable models. Stata bootstrap technique was used for sensitivity analysis to check the robustness of the study outcomes. For the entire set of data management and statistical analysis, Stata/SE 15.0 (Stata Corporation, College Station, TX) was used in this study. Graphics were produced using statistics software Stata and R (version 3.2.3, R Foundation for Statistical Computing; Vienna, Austria).
RESULTS
This study included 27,616 adults over the period of 2014–2019 from 5 South Asian countries. Table 2 describes the characteristics of the study sample.
Table 2.
Characteristics of the Study Sample of South Asian Adults
| Characteristics | Total (N=27,616), mean (SD) or percentage (95% CI) | Male (n=11,874), mean (SD) or percentage (95% CI) | Female (n=15,742), mean (SD) or percentage (95% CI) |
|---|---|---|---|
| Age (years) | 40.2 (±0.2) | 41.5 (±0.2) | 39.3 (±0.2) |
| Fasting plasma glucose (mg/dL) | 91.0 (±0.5) | 91.3 (±0.6) | 90.8 (±0.5) |
| Total cholesterol (mg/dL) | 154.7 (±0.5) | 151.5 (±0.8) | 157.1 (±0.6) |
| Systolic blood pressure (mmHg) | 125.8 (±0.3) | 127.8 (±0.5) | 124.3 (±0.3) |
| Diastolic blood pressure (mmHg) | 81.9 (±0.1) | 81.8 (±0.4) | 82.1 (±0.1) |
| Mean waist circumference (cm) | 82.5 (±0.2) | 83.0 (±0.3) | 82.1 (±0.2) |
| Sex | 43.1 (42.4, 43.6) | 57.2 (56.4, 57.6) | |
| Education | |||
| No schooling | 35.4 (34.8, 36.0) | 29.7 (28.4, 30.0) | 40.1 (39.4, 40.9) |
| Primary or less | 26.7 (26.2, 27.2) | 28.5 (27.7, 29.3) | 25.3 (24.6, 26.0) |
| Secondary | 16.2 (15.8, 16.7) | 17.6 (16.9, 18.3) | 15.2 (14.6, 15.8) |
| Higher | 21.7 (21.2, 22.1) | 24.8 (24.0, 25.5) | 19.4 (18.7, 20.0) |
| Physically inactive | 15.7 (15.3, 16.5) | 12.1 (11.5, 12.7) | 18.5 (17.9, 19.1) |
| High sedentary time | 6.9 (6.6, 7.2) | 7.6 (7.1, 8.1) | 6.4 (6.0, 6.8) |
| Tobacco use | 33.6 (33.0, 34.1) | 53.9 (53.0, 54.8) | 18.2 (17.6, 18.8) |
| Alcohol consumption | 17.6 (17.1, 18.0) | 26.3 (25.5, 27.1) | 11.0 (10.5, 11.5) |
Notes: Tobacco use is defined as present or past smoking or daily use of smokeless tobacco products. Alcohol consumption is defined as consuming alcohol at least once in a month in the last 1 year. Physical inactivity is defined as <150 minutes of MVPS per week. High sedentary time is defined as sedentary time ≥9 hours per day. Difference of mean between groups were confirmed by 2 sample t-test and p<0.01. Difference of percentage between groups were confirmed by chi-square test of independence and p<0.01 for all the groups. Survey period: 2014–2019.
MVPS, medium-to-vigorous physical activity.
This study estimated the overall prevalence of higher metabolic comorbidity score in the South Asian region as 34%. The prevalence was 25% among male respondents and 41% among female respondents. The crude prevalence was around 30% in Bangladesh, Nepal, and Bhutan, whereas in Sri Lanka and Afghanistan, it was around 40%. The age standardized prevalence was 17%–19% in Bangladesh, Nepal, Bhutan, and Sri Lanka, whereas in Afghanistan, it was 26%. Furthermore, collapsing the metabolic comorbidity score into a binary variable might cause information loss about the distribution of metabolic comorbidity in these countries. Therefore, the authors provided the mean metabolic comorbidity score at each age group, overall and in each of the 5 countries separately in Appendix 8 (available online).
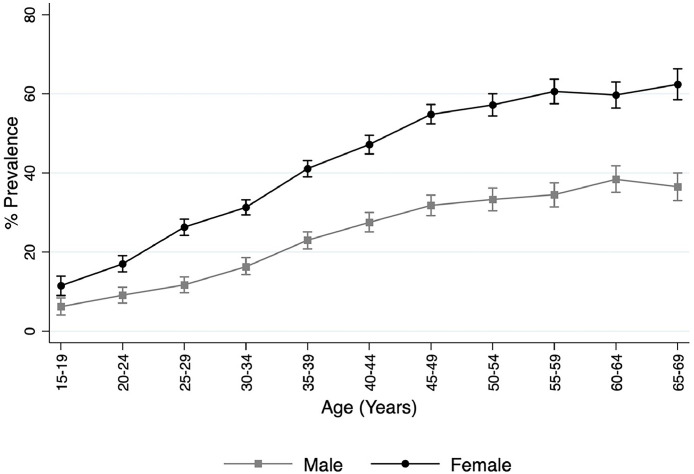
Figure 1 demonstrates the distribution of higher metabolic comorbidity score by age groups, among men, and among women. Higher metabolic comorbidity score was prevalent across all age groups, and the prevalence was higher in older age groups than in the younger age groups. The prevalence was higher among women than among men at each age groups. The gap between men's and women's prevalence widened with advancing age. Among the respondents aged ≥50 years, the prevalence was around 58% among women and 38% among men. Because South Asian people manifest metabolic diseases at an earlier age,19 the authors estimated the prevalence of higher metabolic comorbidity score by age groups in each country. Approximately 15% of the participants in each country aged <30 years had higher metabolic comorbidity score, with this number reaching 22% in Afghanistan. In the age group of 30–39 years, the prevalence of higher metabolic comorbidity score in each country was nearly 30%, with Afghanistan recording 39%. Similarly, in the age group of 40–49 years, the prevalence was nearly 40% in each country and 52% in Afghanistan. Finally, among participants aged 50–69 years, 2 of every 3 in Afghanistan and 1 of every 2 in Sri Lanka had higher metabolic comorbidity score. Appendix 9 (available online) shows the distribution of higher metabolic comorbidity score among South Asian adults by age groups in each country.
Figure 1.
Distribution of higher metabolic comorbidity score among South Asian adults by age and sex.
Notes: Higher metabolic comorbidity score: metabolic comorbidity score ≥3. Survey period: 2014–2019.
The authors did multivariable analysis on the total sample to assess the relationships between lifestyles and metabolic comorbidity score. The participants who were physically inactive (OR=1.26; 95% CI=1.17, 1.36), had high sedentary time (OR=1.24; 95% CI=1.11, 1.33), and consumed alcohol (OR=1.40; 95% CI=1.23, 1.53) showed higher metabolic comorbidity score than participants who were physically active, had low sedentary time, and did not consume alcohol, respectively. However, in this study, the authors found that the use of tobacco had a reverse association (OR=0.75; 95% CI=0.71, 0.81) with metabolic comorbidity score. Appendix 10 (available online) shows the country-specific ORs with 95% CIs of metabolic comorbidity score in connection with different lifestyle factors.
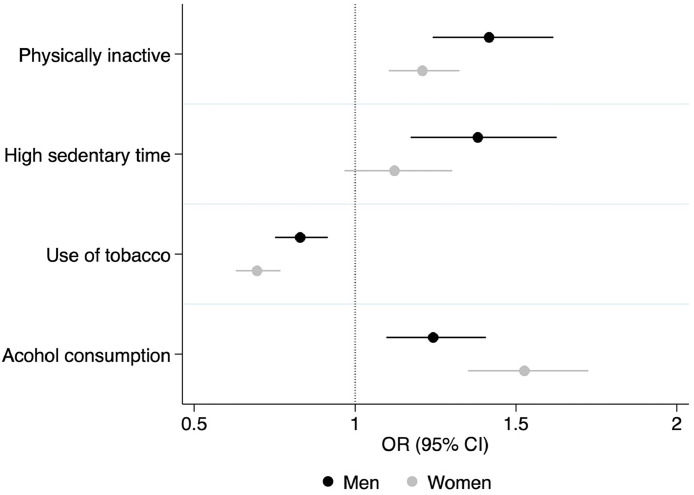
The authors also performed subgroup analysis of the relationship between lifestyle risk factors and metabolic comorbidity score for men and women, and the results are shown in Figure 2. Physical inactivity was associated with a higher metabolic comorbidity score among both men (OR=1.42; 95% CI=1.24, 1.62) and women (OR=1.20; 95% CI=1.1, 1.32). However, high daily sedentary time was linked with higher metabolic comorbidity score among men (OR=1.38; 95% CI=1.17, 1.63) but not among women (OR=1.12; 95% CI=0.97, 1.3). Alcohol consumption was associated with higher metabolic comorbidity score for both men (OR=1.24; 95% CI=1.10, 1.41) and women (OR=1.53; 95% CI=1.35, 1.72). However, tobacco use and metabolic comorbidity score were negatively correlated among both men (OR=0.83; 95% CI=0.75, 0.92) and women (OR=0.70; 95% CI=0.63, 0.78).
Figure 2.
Associations of lifestyle factors with metabolic comorbidity score among South Asian adults by sex.
Notes: Circular symbols represent ORs, and associated line symbols represent 95% CIs. Tobacco use is defined as present or past smoking or daily use of smokeless tobacco products. Alcohol consumption was defined as consuming alcohol at least once in a month in the last 1 year. Physical inactivity is defined as <150 minutes of medium-to-vigorous physical activity per week. High sedentary time is defined as sedentary time ≥9 hours per day. Reference groups included physically active participants, participants with normal daily sedentary time, and those with no consumption of alcohol and no use of tobacco. This figure was adjusted for education and age of each participant, country population density, and poverty headcount ratio at national poverty lines in each country. Higher metabolic comorbidity score is defined as total metabolic comorbidity score ≥3. Survey period: 2014–2019.
DISCUSSION
This observational study was conducted on adults across 5 South Asian countries to measure the metabolic comorbidity score of South Asian adults and to provide comprehensive evidence of associations between metabolic comorbidity score and lifestyle risk factors. The study found that around 1 in 3 South Asian adults had higher metabolic comorbidity score. Multivariable analysis revealed that physical inactivity, high sedentary time, and alcohol consumption were associated with higher metabolic comorbidity score, whereas the use of tobacco exhibited a reverse association with metabolic comorbidity score.
In this study, the authors found that physical inactivity was significantly associated with higher metabolic comorbidity score among both men and women, which was in line with previous study.20 One previous study showed that people in the South Asian region were particularly less active than those in other lower–middle-income regions (33% compared with 16%–26% of other lower–middle-income regions).21 Establishing national guidelines of physical activity, promotion of active commuting, and implementation of physical activity interventions might be beneficial to increasing the overall physical activity level of a population.22,23
This study found that the metabolic comorbidity score was higher among the participants with long daily sedentary time, which is in line with the findings of another previous study.24 Long sedentary time decreases the metabolic demands, increases the oxidative stress, stimulates the sympathetic nervous system, and subsequently decreases insulin sensitivity and vascular function.25 However, this study found that sedentary behavior was associated with higher metabolic comorbidity score among men but not among women. One possible reason might be that in South Asian countries, participation of women in the workforce is lower than that of men, and so occupational sitting time, which tends to be continuous, is less common among women than among men. There are no quantitative guidelines for sedentary behavior. To reduce daily sedentary time, American Dental Association recommended reducing and breaking up sitting time.26 Most importantly, interventions to increase physical activity should be targeted because physical activity can attenuate the adverse health outcome of long sedentary time.27
In this study, the authors set a very modest cut off to define alcohol consumption in reference to WHO's new release in 2023. Yet, the authors found that metabolic comorbidity score was considerably higher among the respondents who consumed alcohol, and this finding was in line with a previous study.28 Alcohol ingestion harms metabolic health through numerous mechanisms. The body can shut down all other metabolic pathways to focus more on metabolizing the alcohol instead.29 Alcohol can shut down fat burning for up to 12–36 hours depending on the individual and the dose, which can cause weight gain.29 Furthermore, this study revealed that the odds of higher metabolic comorbidity score was considerably higher among South Asian women than among South Asian men compared with their corresponding reference groups. Centers for Disease Control and Prevention explained that the body of women absorbs more alcohol and also takes longer time to metabolize alcohol than that of men.30
In this study, the authors found inverse associations between tobacco use and higher metabolic comorbidity score among South Asian adults across all age groups and both sexes. These findings are consistent with a prior study31 but discordant with other studies that reported positive associations between tobacco use and metabolic comorbidity.32,33 In response to the inverse association between tobacco use and higher metabolic comorbidity score in this study, the authors checked the relationships between tobacco use and each of the 4 metabolic risk factors (Appendix 11, available online). The results showed that the odds of abdominal obesity and Stage 2 hypertension were lower among tobacco users than among nonusers, and thus the association between tobacco use and higher metabolic comorbidity score became negative. Many previous studies also reported weight loss among smokers.34, 35, 36 However, the negative association between tobacco use and higher metabolic comorbidity score might also be associated with several other factors, including varying composition of tobacco products; differences between subjects studied; inadequate adjustment for confounders, such as SES; and dietary habits.
To the authors' knowledge, this is the first multicountry study in South Asian countries that analyzed metabolic comorbidity at population level and examined its association with lifestyle risk factors using nationally representative data sets. Furthermore, the survey data sets provided complete and up-to-date biochemical measurement data with standard measurement process and medication information, which are rare in lower- and middle-income countries (LMICs), and the findings of this study might be generalizable to other LMICs.
Limitations
However, this study has several limitations. First, while estimating the metabolic comorbidity score, to assess dyslipidaemia, the authors used only total cholesterol and could not include triglyceride and high-density lipoprotein owing to data unavailability. However, total cholesterol is a component of the Framingham risk score for cardiovascular diseases, and evidence suggests that a high total cholesterol level increases the risk of cardiovascular disease.37 Secondly, for assessing abdominal obesity, the authors used international cut off values rather than ethnicity-specific cut off values for the South Asian population. This is because international organizations such as WHO did not recognize South Asian ethnicity-specific cut off values owing to evidence gap.38 Moreover, because this is an observational study, there is possibility of reverse causality between lifestyles and higher metabolic comorbidity score.
CONCLUSIONS
The population-level estimates of higher metabolic comorbidity score in South Asian countries, that this study produced, might be helpful for the governments and policymakers to identify the at-risk population when planning NCD prevention programs and policies. This study might benefit the healthcare providers to calculate the metabolic comorbidity score, enabling a quick identification of the patient groups for lifestyle modification. It will also facilitate epidemiologic and clinical research of lifestyles and preventive treatment approaches for NCDs, particularly in LMICs.
One third of South Asian adults had higher metabolic comorbidity score. The prevalence was increasing with advancing age, being higher among women than among men. Metabolic comorbidity score was higher among participants who did not adhere to WHO's minimum recommendation of physical activity, exhibited daily 9 or more sedentary hours, and consumed alcohol at least once in a month over the last 1 year.
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to thank Dr. Ganan Devanathan, Department of Global Health Policy, The University of Tokyo for English proof reading of this manuscript.
Disclaimers: This original article is part of a PhD thesis at the University of Tokyo. Data sets are not publicly available. The data sets are accessible after registration and submission of written request from WHO's Global Health Observatory data repository.
Funding: None.
Declaration of interests: None.
CRediT AUTHOR STATEMENT
Sabera Sultana: Conceptualization, Methodology, Formal analysis, Software, Writing - original draft, data curation, visualization. Shuhei Nomura: Validation, Supervision, Writing - review and editing. Chris Fook Sheng NG: Validation, Supervision, Investigation. Masahiro Hashizume: Writing - review and editing, Investigation, Supervision, Project administration.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.focus.2024.100273.
Appendix. Supplementary materials
REFERENCES
- 1.Kibret KT, Backholer K, Peeters A, Tesfay F, Nichols M. Burdens of non-communicable disease attributable to metabolic risk factors in Australia, 1990–2019: joinpoint regression analysis of the Global Burden of Disease Study. BMJ Open. 2023;13(7) doi: 10.1136/bmjopen-2022-071319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An S, Moon S, Park SK. Association of metabolic comorbidity with myocardial infarction in individuals with a family history of cardiovascular disease: a prospective cohort study. BMC Public Health. 2022;22(1):1992. doi: 10.1186/s12889-022-14330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unnikrishnan R, Gupta PK, Mohan V. Diabetes in South Asians: phenotype, clinical presentation, and natural history. Curr Diab Rep. 2018;18(6):30. doi: 10.1007/s11892-018-1002-8. [DOI] [PubMed] [Google Scholar]
- 4.Tham KW, Abdul Ghani R, Cua SC, et al. Obesity in South and Southeast Asia-a new consensus on care and management. Obes Rev. 2023;24(2):e13520. doi: 10.1111/obr.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Marzo V, Silvestri C. Lifestyle and metabolic syndrome: contribution of the endocannabinoidome. Nutrients. 2019;11(8):1956. doi: 10.3390/nu11081956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boakye K, Bovbjerg M, Schuna J, et al. Urbanization and physical activity in the global Prospective Urban and Rural Epidemiology study. Sci Rep. 2023;13(1):290. doi: 10.1038/s41598-022-26406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casari S, Di Paola M, Banci E, et al. Changing dietary habits: the impact of urbanization and rising socio-economic status in families from Burkina Faso in sub-Saharan Africa. Nutrients. 2022;14(9):1782. doi: 10.3390/nu14091782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Circulation. 2019;139(25):e1082–e1143. doi: 10.1161/CIR.0000000000000625. https://DOI.ORG/10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Heart Association . American Heart Association; Dallas, TX: 2023. What is high blood pressure?https://www.heart.org/-/media/files/health-topics/answers-by-heart/what-is-high-blood-pressure.pdf Accessed Nov 15, 2023. [Google Scholar]
- 10.American Diabetes Association Professional Practice Committee; 2. Diagnosis and classification of diabetes: Standards of care in diabetes - 2024. Diabetes Care 2024; 47(supplement_1): S20-S42. 10.2337/dc24-S002. [DOI] [PMC free article] [PubMed]
- 11.WHO . WHO; Geneva, Switzerland: 2011. Waist circumference and waist-hip ratio: report of a WHO expert consultation Geneva, 8-11 December 2008.https://www.who.int/publications/i/item/9789241501491 [Google Scholar]
- 12.Owolabi EO, Ter Goon D, Adeniyi OV. Central obesity and normal-weight central obesity among adults attending healthcare facilities in Buffalo City Metropolitan Municipality, South Africa: a cross-sectional study. J Health Popul Nutr. 2017;36(1):54. doi: 10.1186/s41043-017-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Yang J, Su J, et al. Physical activity, sedentary time and their associations with clustered metabolic risk among people with type 2 diabetes in Jiangsu Province: a cross-sectional study. BMJ Open. 2019;9(8) doi: 10.1136/bmjopen-2018-027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO . WHO; Geneva, Switzerland: November 25, 2020. WHO guidelines on physical activity and sedentary behaviour.https://www.who.int/publications/i/item/9789240015128 [Google Scholar]
- 15.Center for Disease Control and Prevention, May 15, 2024, About Surgeon General’s Reports on smoking and tobacco usehttps://www.cdc.gov/tobacco-surgeon-general-reports/about/index.html?CDC_AAref_Val=https://www.cdc.gov/tobacco/sgr/50th-anniversary/pdfs/wynk-smoking.pdf. Accessed Sep 15, 2024.
- 16.Burton R, Sheron N. No level of alcohol consumption improves health. Lancet. 2018;392(10152):987–988. doi: 10.1016/S0140-6736(18)31571-X. [DOI] [PubMed] [Google Scholar]
- 17.GBD 2016 Alcohol Collaborators Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . WHO; Geneva, Switzerland: January 4, 2023. No level of alcohol consumption is safe for our health.https://www.who.int/europe/news/item/04-01-2023-no-level-of-alcohol-consumption-is-safe-for-our-health [Google Scholar]
- 19.Misra A, Soares MJ, Mohan V, et al. Body fat, metabolic syndrome and hyperglycemia in South Asians. J Diabetes Complications. 2018;32(11):1068–1075. doi: 10.1016/j.jdiacomp.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Sheng J, Abshire DA, Heiney SP, Wirth MD. Acculturation, physical activity, and metabolic syndrome in Asian American adults. J Transcult Nurs. 2022;33(6):675–684. doi: 10.1177/10436596221114150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health. 2018;6(10):1077–1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 22.Bauman AE, Reis RS, Sallis JF, et al. Correlates of physical activity: why are some people physically active and others not? Lancet. 2012;380(9838):258–271. doi: 10.1016/S0140-6736(12)60735-1. [DOI] [PubMed] [Google Scholar]
- 23.Craike M, Wiesner G, Hilland TA, Bengoechea EG. Interventions to improve physical activity among socioeconomically disadvantaged groups: an umbrella review. Int J Behav Nutr Phys Act. 2018;15(1):43. doi: 10.1186/s12966-018-0676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell BL, Smith AE, Rowlands AV, Parfitt G, Dollman J. Associations of physical activity and sedentary behaviour with metabolic syndrome in rural Australian adults. J Sci Med Sport. 2018;21(12):1232–1237. doi: 10.1016/j.jsams.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Dempsey PC, Larsen RN, Dunstan DW, Owen N, Kingwell BA. Sitting less and moving more: implications for hypertension. Hypertension. 2018;72(5):1037–1046. doi: 10.1161/HYPERTENSIONAHA.118.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contardo Ayala AM, Sudholz B, Salmon J, et al. The impact of height-adjustable desks and prompts to break-up classroom sitting on adolescents’ energy expenditure, adiposity markers and perceived musculoskeletal discomfort. PLoS One. 2018;13(9) doi: 10.1371/journal.pone.0203938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mañas A, Pozo-Cruz BD, Rodríguez-Gómez I, et al. Can physical activity offset the detrimental consequences of sedentary time on frailty? A moderation analysis in 749 older adults measured with accelerometers. J Am Med Dir Assoc. 2019;20(5):634–638.e1. doi: 10.1016/j.jamda.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Suliga E, Ciesla E, Lelonek M, Piechowska A, Gluszek S. Lifestyle elements and risk of metabolic syndrome in adults. PLoS One. 2022;17(9) doi: 10.1371/journal.pone.0275510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz K. Granite Mountain; Prescott Valley, AZ: June 16, 2022. Alcohol and fat metabolism: does drinking slow things down and make you gain weight?https://granitemountainbhc.com/blog/alcohol-and-fat-metabolism/ [Google Scholar]
- 30.Centers for Disease Control and Prevention; February 29, 2024. Excessive alcohol use is a risk to women’s health.https://www.cdc.gov/alcohol/fact-sheets/womens-health.htm [Google Scholar]
- 31.Moura ARDS, Paz SMRSD, Frota KMG, Carvalho CMRG. Lifestyle associated with risk of metabolic syndrome in adults and the elderly. Nutrition. 2022;99–100 doi: 10.1016/j.nut.2022.111647. [DOI] [PubMed] [Google Scholar]
- 32.Gharipour M, Sarrafzadegan N, Sadeghi M, et al. The metabolic syndrome and associated lifestyle factors among the Iranian population. Adv Biomed Res. 2015;4:84. doi: 10.4103/2277-9175.156645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding L, Xu Y, Wang LM, et al. Smoking and its relation to metabolic status among Chinese adults: analysis of a nationwide survey. Biomed Environ Sci. 2016;29(9):619–627. doi: 10.3967/bes2016.084. [DOI] [PubMed] [Google Scholar]
- 34.Harris KK, Zopey M, Friedman TC. Metabolic effects of smoking cessation. Nat Rev Endocrinol. 2016;12(5):299–308. doi: 10.1038/nrendo.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dare S, Mackay DF, Pell JP. Relationship between smoking and obesity: a cross-sectional study of 499,504 middle-aged adults in the UK general population. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor AE, Richmond RC, Palviainen T, et al. The effect of body mass index on smoking behaviour and nicotine metabolism: a Mendelian randomization study. Hum Mol Genet. 2019;28(8):1322–1330. doi: 10.1093/hmg/ddy434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lioyd-Jones DM, Braun LT, Ndumele CE, et al. Use of risk assessment tools to guide decision-making in the primary prevention of Atherosclerotic Cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. Am Heart Assoc Circ. 2019;139(25):e1162–1177. doi: 10.1161/CIR.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 38.Misra A. Ethnic-specific criteria for classification of body mass index: a perspective for Asian Indians and American Diabetes Association position statement. Diabetes Technol Ther. 2015;17(9):667–671. doi: 10.1089/dia.2015.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.