Abstract
In the first 2 wk after hatching, broiler chickens are vulnerable to enteric pathogens due to underdeveloped gastrointestinal and immune systems. Carvacrol has been reported to improve digestive and immune functions. This study aimed to optimize immune development of broiler chickens by delivering carvacrol in ovo. Effects of 2 in ovo treatments delivered at embryonic day (E)17.5 (saline or carvacrol) were evaluated at 3 stages (E19.5, hatch, and d 14 posthatch). Hatchability, performance parameters, lymphoid organ and yolk sac weights were determined. Histomorphology assessment was performed for jejunal samples at hatch and bursa of Fabricius samples at hatch and d 14. Gene expression of immune-relevant genes was determined for jejunal, bursal, and yolk sac samples over time. At hatch, BW was 0.85% lower (P = 0.02) after in ovo carvacrol delivery compared to the controls. Interactions between in ovo treatment and age were found for gene expression. At hatch, carvacrol treatment resulted in lower expression of proinflammatory cytokines IL-8 and IFN-γ in the yolk sac compared to the controls (P = 0.05 and < .001, respectively) suggesting a potential role for carvacrol-mediated immune modulation. At d 14, carvacrol treatment led to lower expression of proinflammatory cytokine IL-6 in the bursa compared to the controls (P = 0.002). In ovo carvacrol delivery led to bursal histomorphometric changes, including a larger cortex in the bursal follicles (P = 0.03), and a higher cortex/medulla ratio (P = 0.04) compared to the controls, indicating increased B-cell stimulation and maturation. Main effects were found for carvacrol treatment in the jejunum, with overall higher expression of proinflammatory mediators IL-1β and NF-κB, and anti-inflammatory IL-10 compared to the controls (P = 0.04, 0.02, and 0.02 respectively) from E19.5 to d 14. Age-related main effects showed various alterations in expression dynamics of immune-related genes across all tissues over time. Our findings suggest changes in immune parameters occur as the chicken develops, but these mostly do not interact with in ovo carvacrol treatment. In ovo carvacrol treatment alters immune activity of broiler chickens independent of age.
Key words: in ovo, carvacrol, immune development, broiler chicken
INTRODUCTION
Enteric pathogens can have a severe impact on the health and welfare of newly hatched chickens due to their immature gastrointestinal tract (GIT) and immune system (Lammers et al., 2010; Song et al., 2021). This, in turn, results in a high reliance on antibiotic treatment (Hamal et al., 2006). The use of antibiotics in livestock has been linked to the development of antimicrobial resistance which explains a high interest in developing alternative strategies to reduce antimicrobial dependence (Gadde et al., 2017a; Abreu et al., 2023). One of these strategies is to enhance the early development of the GIT and immune system to ensure that chickens can effectively handle pathogenic challenges particularly early in life.
In the field of poultry nutrition, the use of natural compounds found in essential oils has been showing beneficial effects on immune function (Brenes and Roura, 2010; Williams et al., 2020). Carvacrol is a natural bio-active compound found in essential oils such as oregano and thyme, with a wide range of biological activities, including antibacterial, antiviral, antioxidant, and anti-inflammatory properties (Sharifi-Rad et al., 2018). In broiler chickens, carvacrol has been shown to have immunomodulatory effects (Hashemipour et al., 2013; Pirgozliev et al., 2019; Rehman et al., 2021; Zheng et al., 2021).
Liu et al. (2019) showed that oral administration of carvacrol resulted in inhibition of the expression of inflammatory cytokines caused by a lipopolysaccharide challenge. It was reported that carvacrol downregulated the toll-like receptor (TLR) and nuclear factor κ-B (NF-κB) transcription pathway at d 15 posthatch (Liu et al., 2019a). However, supplementing carvacrol posthatching might be missing the window of opportunity to effectively optimize immune function before the critical early posthatching phase. In the first 14 d of the life of a chicken innate and adaptive immune responses are still impaired, making the prehatching phase a useful target period for applications aimed at improving immune development (Bar-Shira and Friedman, 2006; Kogut, 2009; Broom and Kogut, 2018). Fast-growing broiler chicken breeds spend around one-third of their life as embryo inside the egg, and during this time, different parts of the immune system are already developing and functional (Garcia et al., 2021). This makes in ovo interventions with compounds targeting immune development potentially valuable (Jha et al., 2019; Das et al., 2021).
The aim of the current study was to investigate the in ovo delivery of carvacrol in optimizing early immune development in broiler chickens. It was hypothesized that in ovo delivery of carvacrol may optimize immune development expressed by the degree of bursal differentiation, and expression of immune-relevant genes such as cytokines and immunoglobulins.
MATERIALS AND METHODS
This experiment was conducted under approval of the Animal Ethics Committee of the University of Queensland (2019/AE000463), in compliance with the Australian code for use of animals for scientific purposes (National Health and Medical Research Council, 2013).
Experimental Design
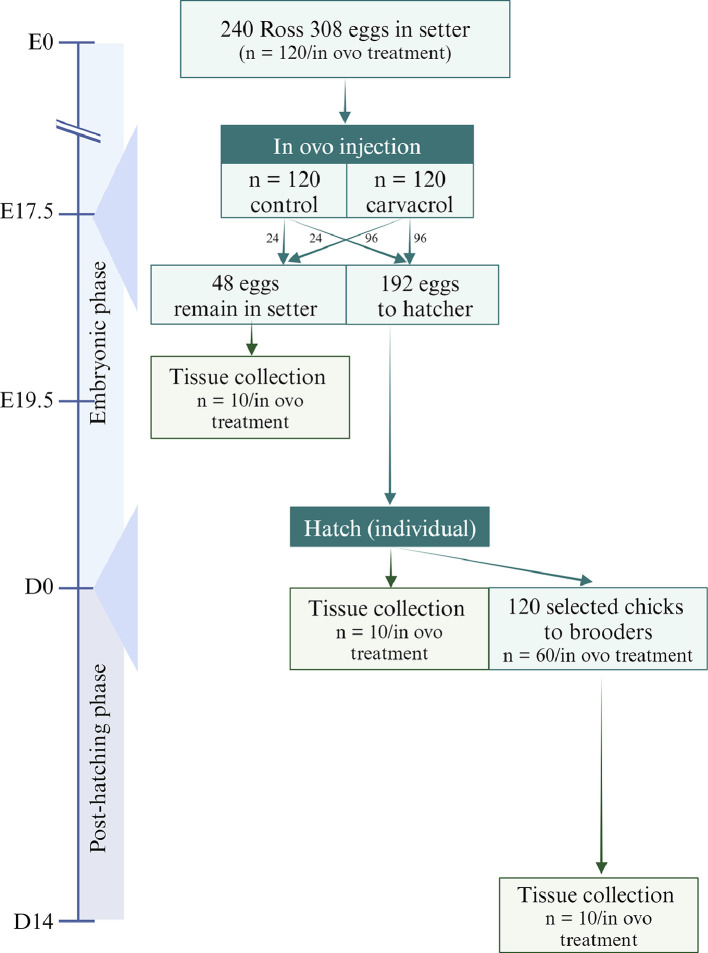
This experiment was set up as a 2 × 3 factorial arrangement with 2 in ovo delivery treatment groups at embryonic day (E)17.5 (control group injected with a 0.9% saline solution and a treatment group injected with 5µL carvacrol in 0.9% saline solution) and 3 stages of development for tissue collection after in ovo treatment, at E19.5, at hatch, and at day 14 posthatch (d 14) (Figure 1). The experimental solutions were injected into the amniotic fluid of fertile eggs at E17.5.
Figure 1.
Timeline and egg/chicken distribution. Created with BioRender.com.
Eggs, Chicks, and Housing
Ross 308 fertile eggs (n = 240, Figure 1) with an average weight of 61 g (SD = 1.6 g) from 1 broiler breeder flock, aged 38 wk, were obtained from a commercial hatchery (Woodlands, Beerwah, QLD, Australia) and transferred to the University of Queensland experimental chicken hatchery (St Lucia, Queensland, Australia). A setter (Ova-Easy 580 Advance Series II, Brinsea, FL, USA) containing 6 levels with 2 trays per level was used, making a total of 12 trays with a maximum capacity of 576 eggs. In this setter, eggs were incubated for 17.5 days until in ovo injection at a set incubator temperature of 37.8°C, a relative humidity of 57%, and a turning interval of 60 min over an angle of 90°. The start of incubation was defined as the moment the eggs were placed in the setter that had previously been set at the appropriate temperature and humidity. Before in ovo injection at E17.5 (see in ovo treatments), eggs were candled and dead embryos as well as infertile eggs were removed. Following injection, the eggs designated for sampling at E19.5 were placed back in the same setter, while the remaining eggs were transferred to a hatcher where they were allowed to hatch (Greatlander 6BH 6 Basket Hatcher, Australia). The settings of the setter were adjusted to a relative humidity of 70%, with no turning interval and an angle of 0° (horizontal). The hatcher consisted of 6 hatching baskets on top of each other with each basket partitioned into 32 single-egg compartments with windows for air flow. Each egg was allocated to 1 compartment, following the same randomized design used for the setter. Eggs were incubated until hatching at a set hatcher temperature of 37.8°C and relative humidity of 70%. From E20 onward, the hatcher was checked daily at 8 AM and 8 PM for hatched chicks and time of hatch was recorded, as well as hatchability (% of fertile eggs), navel quality (as described by Molenaar et al., 2010), chicken quality (1 = good, 2 = deformed and to be euthanized, 3 = dead on arrival). Sixty first-grade (quality score 1) chickens per in ovo treatment were transferred to cage brooders (Cimuka, Turkey) and were reared until 14 days of age. Five brooder units were used, each consisting of 5 cages on top of each other, of which the top 4 cages were used. Each cage (90 × 44 × 24 cm) was allocated to 6 chickens, where they had ad libitum access to water and feed (n = 10 cages/replicates per treatment). Brooder temperature was maintained at 35°C for the first 3 d and lowered to 30°C afterwards. For the first 3 d, 24 h of light was used, and from d 3 to d 14, chickens were provided with 6 continuous hours (11PM–5 AM) of daily darkness.
The body weight (BW) (per individual and averaged per cage) and feed intake (FI) (per cage) were measured on d 7 and d 14 posthatch. Feed conversion ratio (FCR) was calculated using body weight gain (BWG) and FI between d 0 and d 7, d 7 and d 14 and d 0 and d 14.
In Ovo Treatments
At E17.5, eggs were injected with saline or carvacrol solutions. Eggs were sterilized by wiping with 70% ethanol, whereafter a hole was drilled in the eggshell on the blunt side of the egg, using a multipurpose rotary tool (Ryobi EHT150, Ryobi, Hiroshima, Japan) with an arrow-shaped insert (Dremel High-Speed Cutter 6.4 mm, Dremel, Mount Prospect, Ill), while maintaining the membranes intact. For injection in the amniotic fluid, a 23G 1 ¼ (32 mm) precision needle was used. After injection, holes were sealed with beeswax and eggs were transferred to the hatcher (for tissue collection at hatch and d 14) or returned to the setter (for tissue collection at E19.5).
Eggs were injected with either 1000 µL of 0.9% sterile saline solution (NaCl 0.9% in water, Baxter, Deerfield, Ill, CAS: 7647-14-5) or a solution consisting of 5 µL carvacrol (Sigma Aldrich, St. Louis, MO, CAS: 499-75-2) in 1000 µL of 0.9% sterile saline. The dosage of carvacrol was determined based on the work performed by Niknafs et al., (2024), who found that up to 10 µL of oregano essential oil, containing approximately 75% carvacrol, did not negatively affect hatchability or other performance data. The carvacrol solution was prepared by mixing 2000 µL of carvacrol with 2000 µL of the nonionic surfactant polysorbate 80 (Tween 80, Sigma-Aldrich, St. Louis, MO, CAS: 9005-65-6) for solubilization. This was mixed properly by gently pipetting for 1 min. Afterwards, 2000 µL of 0.9% sterile saline solution was added and mixed with this carvacrol – polysorbate 80 solution by pipetting. This addition and mixing of 2000 µL saline were repeated 3 more times to a total volume of 12mL. Finally, the solution was brought to a total volume of 40 mL by adding 28 mL of sterile saline and shaking vigorously. This 5% carvacrol stock solution was used to make the experimental solutions of 0.5% carvacrol.
Sampling and Measurements
At E19.5, 10 eggs per treatment were randomly selected from the setters. The eggshell was removed, and embryonic BW was recorded. Embryos were euthanised using decapitation and the residual yolk weight (RSY) was recorded and expressed as the percentage of yolk free body weight. Approximately 5 mm of tissue was collected from the distal jejunum as well as half of the bursa. For yolk sac tissue collection, the yolk sac was suspended by lifting the point attached to the embryonic GIT (yolk stalk), thereby allowing gravity to weigh down the yolk sac, making it possible to collect a piece of tissue from the other side. Samples were rinsed with PBS and collected in RNAlater (Sigma Aldrich) and stored at −80°C until gene expression analysis.
At hatch, every 8th hatched chicken per treatment was decapitated, ensuring every chicken was between 0 and 12 h of age. The same sampling procedures for gene expression samples were followed as for E19.5. Additionally, jejunum and bursa samples were rinsed with PBS and fixed in formalin (10% Neutral Buffered Formalin, Hurst and Co) for histology. After 24 h, samples were washed with running tap water for 20 min. and stored in 70% ethanol for 48 h at room temperature until paraffin embedding.
At d 14 posthatch, 10 chickens per treatment (1 from each pen) were selected based on average weight per cage. The same procedures as for hatch were used, with the following exceptions: chickens were euthanized, using cervical dislocation and no yolk sac was weighed or collected. In addition, bursa and spleen weights were recorded and expressed as percentage of BW.
Histology
Jejunum and bursa samples were embedded in paraffin, sectioned at 6 µm and stained with hematoxylin and eosin. Slides were digitalized using a slide scanner (Zeiss AxioScan Z1; Zeiss, Oberkochen, Germany) and examined using Zeiss Zen 3.7 (Zeiss, Oberkochen, Germany) image processing software.
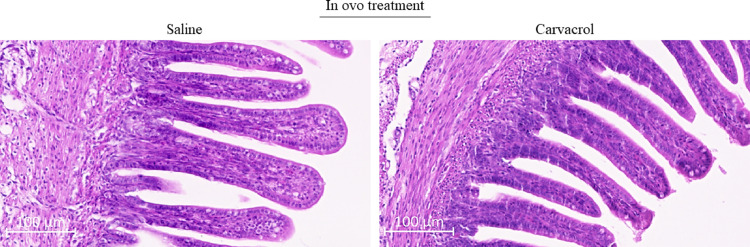
For the morphological assessment of the jejunum, 10 to 20 intact villi per chicken were measured and averaged per chicken. Due to sample loss of d 14 samples during processing, jejunum assessment was only performed on samples from hatched chickens (Figure 2). Villus height was measured from the tip of the villus to the villus crypt junction, and crypt depth was determined as the depth of the invagination between adjacent villi. Villus width was measured at the middle of each villus. The ratio villus height/crypt depth was determined for each villus by dividing villus height by crypt depth. For the determination of villus surface area, a villus was considered a cylindrical structure, using the formula of Nain et al. (2012):
Figure 2.
Morphological structure of the jejunum at hatch, following in ovo supplementation of saline or carvacrol at embryonic day (E) 17.5. No significant differences were found between treatments (Table 4).
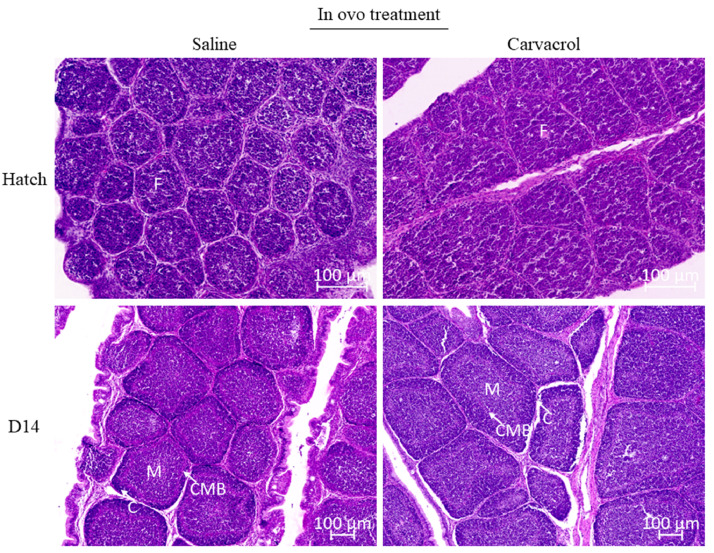
In the bursa of Fabricius, 20 intact bursal follicles per chicken were measured and averaged per chicken. A grid was applied, and only follicles at a crossing of the grid were used for morphological assessment. Follicle surface size was measured following the methods described by Correa Muniz et al., (2006) using Zeiss Zen 3.7 software, by tracing the outline of the follicle, and medulla size was measured as the inside of the follicle until the cortico-medullary border (Figure 3). Cortex size was measured by subtracting the size of the outside of the cortico-medullary border from the follicle size. Cortex/medulla ratio was determined by dividing cortex size by medulla size.
Figure 3.
Effects of in ovo carvacrol supplementation at embryonic day (E) 17.5 on the morphological structure of follicles in the bursa of Fabricius at hatch and d 14 (n = 10). F, follicle; C, cortex; M, medulla; CMB, cortico-medullary border. Cortex, medulla and corticomedullary border could not be distinguished at hatch. * P ≤ 0.05.
Gene Expression (qRT-PCR)
Total RNA was isolated from jejunal and bursa tissue samples, using RNeasy mini kit (Qiagen, Hilden, Germany) and from yolk sac tissue, using RNeasy Universal kit (Qiagen, Hilden, Germany). RNA concentrations were determined by Nanodrop 1000 (Thermo Scientific, Waltham, MA). Total RNA from each sample was reverse transcribed into cDNA, using a cDNA synthesis kit (Qiagen, Hilden, Germany). The cDNA was verified by 1.5% agarose gel electrophoresis.
For qRT-PCR 1 µL cDNA and 1 µL forward and reverse primers (4 nM) were added to 5 µL SYBR green-based mix (QuantiNova, Qiagen, Hilden, Germany) and filled up with 3 µL RNAse free water to a total volume of 10 µL. The primer sequences (Sigma Aldrich, St. Louis, MO) used for real-time PCR are listed in Table 1. The RT-PCR analysis was carried out on a Viia7 real-time PCR machine (Thermo Scientific, Waltham, MA). All reactions were analyzed in duplicate under the following conditions: 2 min 95°C, followed by 45 cycles of 5 s 95°C and 20 s 60°C. A melt curve was produced at the end of the run to determine single product amplification. The delta-delta Ct method (Livak and Schmittgen, 2001) was used to quantify qPCR results. This method involved comparing the Ct values of controls and treatments at different days (E19.5, hatch and d 14) to the control group at E19.5.
Table 1.
Primer sequences used for RT-qPCR analysis.
| Gene1 | Accession No. | Primer sequence (5′–3′) 2 | Reference |
|---|---|---|---|
| IL1B | NM_204524.2 | F: TGAGGGCACCACGCGCTTCGAGT | Liu et al. (2019a) |
| R: TAGCTTGTAGGTGGCGATGTTGAC | |||
| IL2 | AF033563 | F: TTCAAAATATCGAAAAGAACCTCAAG | Lammers et al. (2010) |
| R: CGGTGTGATTTAGACCCGTAAGAC | |||
| IL4 | AJ621249 | F: GTGCCCACGCTGTGCTTAC | Lammers et al. (2010) |
| R: AGGAAACCTCTCCCTGGATGTC | |||
| IL6 | NM_204628.2 | F: GTTCGCCTTTCAGACCTAC | Liu et al. (2019a) |
| R: ACCACTTCATCGGGATTTA | |||
| IL8L1 | NM_205018.2 | F: CACGTTCAGCGATTGAACTC | Santos et al. (2019) |
| R: GACTTCCACATTCTTGCAGTG | |||
| IL10 | AJ621614 | F: CGCTGTCACCGCTTCTTCA | Lammers et al. (2010) |
| R: TCCCGTTCTCATCCATCTTCTC | |||
| IFNG | Y07922 | F: GTGAAGAAGGTGAAAGATATCATGGA | Lammers et al. (2010) |
| R: GCTTTGCGCTGGATTCTCA | |||
| LITAF | XM_046927265.1 | F: CCCCTACCCTGTCCCACAA | Liu et al. (2019a) |
| R: TGAGTACTGCGGAGGGTTCAT | |||
| TGFB4 | M31160 | F: ACCTCGACACCGACTACTGCTT | Lammers et al. (2010) |
| R: ATCCTTGCGGAAGTCGATGT | |||
| NFKB | XM_046915553.1 | F: GAAGGAATCGTACCGGGAACA | Chiang et al. (2009) |
| R: CTCAGAGGGCCTTGTGACAGTAA | |||
| CD3D | NM_205512.2 | F: TGTTGTCGCCACTGTCTTGCTG | Song et al. (2021) |
| R: GTCCATCATTCCGCTCACCAAGG | |||
| BU1 | NM_205182.2 | F: GGCTGTTGTGTCCTCACTCATCT | Pal et al. (2020) |
| R: CACCACCGACATTGTTATTCCAT | |||
| IGMH | X01613.1 | F: GCATCAGCGTCACCGAAAGC | Lammers et al. (2010) |
| R: TCCGCACTCCATCCTCTTGC | |||
| IGGH | X07174.1 | F: ATCACGTCAAGGGATGCCCG | Lammers et al. (2010) |
| R: ACCAGGCACCTCAGTTTGG | |||
| IGAH | S40610 | F: GTCACCGTCACCTGGACTACA | Lammers et al. (2010) |
| R: ACCGATGGTCTCCTTCACATC | |||
| MUC2 | XM_040673077.2 | F: ATGCGATGTTAACACAGGACTC | Stefanello et al. (2020) |
| R: GTGGAGCACAGCAGACTTTG | |||
| BACT | X00182 | F: CAACACAGTGCTGTCTGGTGGTA | St. Paul et al. (2011) |
| R: ATCGTACTCCTGCTTGCTGATCC |
IL1B = Interleukin 1β; IL2 = Interleukin 2; IL4 = Interleukin 4; IL6 = Interleukin 6; IL8L1 = Interleukin 8-like 1; IL10 = Interleukin 10; IFNG = Interferon γ; LITAF = lipopolysaccharide induced Tumor necrosis factor; TGFB4 = Transforming growth factor β4; NFKB = Nuclear factor κ B; CD3D = CD3 δ subunit of T-cell Receptor Complex, present on T-cells and NK-cells; BU1 = Transmembrane protein of B-cells and a subset of macrophages; IGMH = Immunoglobulin M; IGGH = Immunoglobulin Y; IGAH = Immunoglobulin A; MUC2 = Mucin 2; BACT = β-actin.
F = forward primer; R = reverse primer.
Fold change in gene expression was then calculated using .
Afterwards, the E19.5 control group was assigned an arbitrary value of 1.00 and the other groups were expressed relative to this value.
Statistical Analyses
All qRT-PCR data were log transformed to obtain normal distribution. All data were analyzed using the statistical software package SAS 9.4 (SAS Institute Inc., Cary, NC).
For hatchability, BW at E19.5 and hatch, organ weights, hours until hatch, navel and quality scores, histological parameters, eggs or chickens were used as experimental units, while for BW at d 7 and d 14, BWG, FI, and FCR, cage was considered as the experimental unit.
A generalized linear mixed model (Proc Glimmix) procedure was used to analyze hatchability, navel score and quality score, with a binary distribution and a logit link function. A general linear mixed model (Proc Mixed) was used to analyze BW, organ %, hours until hatch, BWG, FI, FCR and all histological parameters. Model assumptions were approved on both the means and residuals. Data are expressed as LSmeans ± SEM and multiple comparisons between treatments were corrected for Tukey. Differences between treatments were considered significant at P ≤ 0.05.
The basic model used for all performance data was
| (1) |
where Yi = the dependent variable, µ = the overall mean, Treatmenti = in ovo treatment (i = saline or carvacrol injected) and ei = the residual error term.
For BW at E19.5 and hatch, egg weight at E0 was added to the model as a covariate. At E19.5 and hatch, tray within the setter was added to the model as random effect for all performance variables.
A general linear mixed model (Proc Mixed) was used to analyze gene expression fold change. The basic model used was:
| (2) |
where Y = the dependent variable, µ = the overall mean Treatmenti = in ovo treatment (i = saline or carvacrol injected), Dayj = the age of the chicken (j = E19.5, hatch or d 14), Treatment x Dayij = the interaction between the Treatment and Day, and eij = the residual error term. Individual chickens were considered as the experimental unit.
Additionally, gene expression fold change was analyzed comparing in ovo treatments (saline or carvacrol injected) per age (E19.5, hatch and d 14) using a Student's t-test (Proc T-Test).
RESULTS
Performance
On E19.5, no significant effects were found for the parameters BW and the percentage of residual yolk (P > 0.05; Table 2). At hatch, a difference in BW was found, with the carvacrol-injected chickens having a lower body weight compared to the control group (−0.8%; P = 0.02). No effects were found for the parameters hatchability, navel score, quality score or the percentage of residual yolk. At d 7, d 14 and between d 7 and d 14 no significant effects were found for BW, BWG, FI, FCR, bursa percentage, spleen percentage or spleen/bursa ratio. FCR between d 0 and d 14 tended to be lower (−0.8%) for the carvacrol injected chickens (P = 0.09). Between d 7 and d 14 a tendency was found for FI (P = 0.07), with the carvacrol injected chickens having a higher FI (+3.6%) than the control group.
Table 2.
Effects of in ovo delivery of carvacrol at embryonic day (E)17.5 in broiler eggs on hatchability, chicken quality, performance parameters and organ weights at E19.5, hatch, d 7, and d 14 posthatch.
| In ovo treatment |
|||||
|---|---|---|---|---|---|
| Control | Carvacrol | SEM | n | P-value | |
| E19.5 | |||||
| BW (g) | 44.53 | 44.56 | 0.89 | 10 | 0.97 |
| Residual yolk (% of BW) | 25.60 | 26.73 | 0.78 | 10 | 0.32 |
| Hatch | |||||
| Hatchability (%) | 98.9 | 95.8 | 1.65 | 96 | 0.21 |
| BW (g) | 46.04a | 45.65b | 0.12 | 96 | 0.02 |
| Hours until hatch (h) | 516 | 516 | 0.09 | 96 | 0.26 |
| Navel score1 | 1.30 | 1.40 | 0.07 | 96 | 0.22 |
| Quality score2 | 1.00 | 1.02 | 0.10 | 96 | 0.15 |
| Residual yolk (% of BW) | 14.95 | 14.94 | 0.60 | 10 | 0.99 |
| d 0–d 7 | |||||
| BW (g) | 197 | 198 | 2.98 | 10 | 0.84 |
| BWG (g) | 150 | 152 | 2.95 | 10 | 0.73 |
| FI (g) | 145 | 144 | 4.21 | 10 | 0.87 |
| FCR (g/g) | 0.97 | 0.94 | 0.02 | 10 | 0.32 |
| Bursa (% of BW) | 0.21 | 0.21 | 0.01 | 10 | 0.87 |
| Spleen (% of BW) | 0.07 | 0.07 | 0.01 | 10 | 0.73 |
| d 0–d 14 | |||||
| BW (g) | 540 | 546 | 7.57 | 10 | 0.58 |
| BWG (g) | 493 | 498 | 7.75 | 10 | 0.70 |
| FI (g) | 586 | 595 | 5.19 | 10 | 0.22 |
| FCR (g/g) | 1.18 | 1.17 | 0.01 | 10 | 0.09 |
| Bursa (% of BW) | 0.2 | 0.22 | 0.02 | 10 | 0.28 |
| Spleen (% of BW) | 0.07 | 0.09 | 0.01 | 10 | 0.20 |
| d 7–d 14 | |||||
| BWG (g) | 343 | 347 | 5.97 | 10 | 0.67 |
| FI (g) | 441 | 457 | 5.84 | 10 | 0.07 |
| FCR (g/g) | 1.29 | 1.30 | 0.02 | 10 | 0.97 |
LSMeans within a row lacking a common superscript differ (P ≤ 0.05).
Navel score: 1 = closed and clean navel area), 2 = black button up to 2 mm or black string, 3 = black button that exceeds 2 mm or open navel area. As described by Molenaar et al (2010).
Quality score: 1 = good, 2 = deformed and to be euthanized, 3 = dead on arrival.
Residual yolk, bursa and spleen weights were expressed as percentage of body weight (BW). Results are expressed as LSmeans ± pooled SEM with n as number of replicates per treatment.
Histology
The morphological assessment of the bursa of Fabricius at d 14 showed a larger bursal cortex size for the carvacrol injected chickens (Δ = 6663.06 µm²; P = 0.03), which also resulted in a higher cortex/medulla ratio (+22.4%; P = 0.04) (Table 3). At hatch, only follicle size was measured, because neither a clearly distinguishable cortico-medullary border nor cortex were visible. No significant differences were observed for bursal morphology at hatch (Table 3), nor for parameters measured in the jejunum, including villus height, crypt depth, ratio villus height/crypt depth and villus area (Table 4).
Table 3.
Effects of in ovo delivery of carvacrol at embryonic day (E) 17.5 on histomorphology parameters of bursal follicles in the bursa of Fabricius at hatch and d 14 posthatch in broilers.
| Follicle (µm²) |
Medulla (µm²) | Cortex (µm²) | C/M1 | ||
|---|---|---|---|---|---|
| Treatment | Hatch | d 14 | d 14 | ||
| Control | 13510.38 | 81610.01 | 37251.83 | 28437.47b | 0.85b |
| Carvacrol | 13713.29 | 91477.21 | 38809.75 | 35100.53a | 1.04a |
| SEM | 950.24 | 4294.09 | 2391.52 | 1934.94 | 0.06 |
| P-value | 0.88 | 0.12 | 0.65 | 0.03 | 0.04 |
LSMeans within a column lacking a common superscript differ (P ≤ 0.05).
C/M: Cortex/Medulla ratio.
Results are expressed as LSmeans ± pooled SEM.
Table 4.
Effects of in ovo delivery of carvacrol at embryonic day (E) 17.5 on histomorphology parameters in the jejunum at hatch in broilers.
| Treatment | Villus height (µM) | Crypt depth (µM) | Villus width (µM) | VH/CD1 | Villus area (µM²) |
|---|---|---|---|---|---|
| Control | 236.69 | 37.58 | 46.67 | 7.12 | 34667.06 |
| Carvacrol | 256.38 | 42.81 | 41.72 | 6.70 | 33809.35 |
| SEM | 14.52 | 2.62 | 2.17 | 0.49 | 2483.90 |
| P-value | 0.36 | 0.19 | 0.13 | 0.56 | 0.81 |
VH/CD: ratio Villus height/Crypt depth.
Results are expressed as LSmeans ± pooled SEM.
Gene Expression
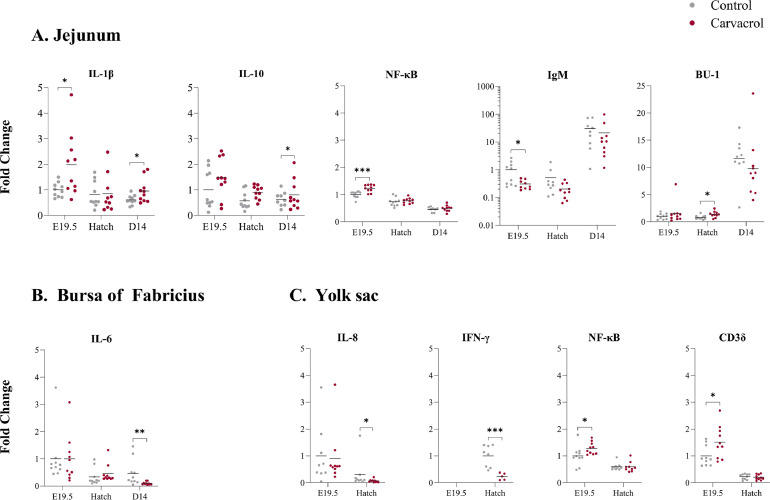
Main effects were found for treatment with in ovo carvacrol showing higher gene expression of IL-1β (P = 0.04), IL-10 (P = 0.02), NF-κB (P = 0.02) while IgM was lower (P = 0.008) compared to the saline control group (Table 5). While no interactions between treatment and age were found, comparisons per age resulted in significant differences between in ovo treatments indicating that in ovo delivery of carvacrol increased expressions of IL-1β at E19.5 (P = 0.02) and d 14 (P = 0.03) as well as expression of NF-κB at E19.5 (P = 0.001), expression of IL-10 at d 14 (P = 0.02) and expression of BU-1 at hatch (P = 0.02) (Figure 4). Furthermore, in ovo delivery of carvacrol decreased expression of IgM at E19.5 (P = 0.02).
Table 5.
Effects of in ovo delivery of carvacrol at embryonic day (E)17.5 on expression of immune-related genes (expressed as fold change relative to the saline-injected group at E19.5) in the jejunum of broiler chickens measured at E19.5, hatch and day 14.
| Treatment | Age | IL-1β | IL-2 | IL-4 | IL-8 | IL-10 | IFN-γ | TNF-α | TGF-β | NF-κB | CD3δ | BU-1 | IgM | IgY | IgA | Muc-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.81b | 0.82 | 0.55 | 4.28 | 0.74b | 0.89 | 2.51 | 0.64 | 0.73b | 1.40 | 4.48 | 10.67a | 2.28 | 29.86 | 63.48 | |
| Carvacrol | 1.26a | 0.94 | 0.67 | 4.70 | 1.06a | 1.05 | 2.62 | 0.61 | 0.83a | 1.33 | 4.23 | 7.23b | 1.43 | 25.93 | 87.73 | |
| SEM | 0.12 | 0.15 | 0.14 | 1.07 | 0.10 | 0.14 | 0.12 | 0.03 | 0.02 | 0.11 | 0.53 | 3.11 | 0.45 | 12.46 | 11.02 | |
| Control | E19.5 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Hatch | 0.82 | 0.51 | 0.20 | 4.52 | 0.63 | 0.50 | 1.97 | 0.44 | 0.73 | 0.52 | 0.81 | 0.51 | 0.32 | 0.30 | 22.33 | |
| d 14 | 0.61 | 0.95 | 0.44 | 7.31 | 0.57 | 1.18 | 4.54 | 0.48 | 0.46 | 2.70 | 11.64 | 30.50 | 5.51 | 88.29 | 167.11 | |
| Carvacrol | E19.5 | 1.98 | 0.98 | 1.40 | 1.03 | 1.48 | 0.87 | 1.14 | 1.08 | 1.23 | 1.08 | 1.55 | 0.30 | 1.50 | 1.48 | 1.46 |
| Hatch | 0.85 | 0.70 | 0.14 | 6.74 | 0.80 | 0.64 | 2.01 | 0.38 | 0.77 | 0.49 | 1.37 | 0.20 | 0.35 | 0.39 | 17.93 | |
| d 14 | 0.96 | 1.14 | 0.46 | 6.32 | 0.91 | 1.63 | 4.69 | 0.38 | 0.49 | 2.43 | 9.77 | 21.19 | 2.43 | 75.92 | 243.81 | |
| SEM | 0.21 | 0.26 | 0.24 | 1.85 | 0.17 | 0.24 | 0.22 | 0.05 | 0.04 | 0.19 | 0.92 | 5.39 | 0.78 | 21.58 | 19.09 | |
| P-value | ||||||||||||||||
| Treatment | 0.04 | 0.55 | 0.80 | 0.86 | 0.02 | 0.37 | 0.33 | 0.16 | 0.02 | 0.96 | 0.29 | 0.008 | 0.45 | 0.76 | 0.39 | |
| Age | <0.001 | 0.08 | <0.001 | <0.001 | 0.08 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Treatment × Age | 0.19 | 0.62 | 0.76 | 0.92 | 0.49 | 0.41 | 0.78 | 0.17 | 0.38 | 0.88 | 0.17 | 0.86 | 0.16 | 0.38 | 0.70 | |
IL-1β, 2, 4, 8, 10: Interleukin 1β, 2, 4, 8, 10; IFN-γ: Interferon γ; TNF-α: Tumor necrosis factor α; TGF-β: Transforming growth factor β; NF-κB: Nuclear factor κ B; CD3δ: CD3 δ subunit of T-cell Receptor Complex, present on T-cells and NK-cells; BU-1: Transmembrane protein of B-cells and a subset of macrophages; IgM, Y, A: Immunoglobulin M, Y, A; Muc-2: Mucin 2.
LSMeans within a column and factor lacking a common superscript differ (P ≤ 0.05).
The gene expression values of the control group at E19.5 were assigned an arbitrary value of 1.00 and the other groups were expressed relative to this value. Main effects for age are shown in Figure 4. Results are expressed as LSmeans ± SEM.
Figure 4.
Selected effects of in ovo delivery of carvacrol at embryonic day (E) 17.5 on expression of immune-related genes in the jejunum (A), bursa of Fabricius (B) and yolk sac (C) (expressed as fold change relative to the saline-injected group at E19.5) measured at E19.5, hatch and day 14 in broilers. The figures show only the parameters with significant differences between treatments at different ages. Results are expressed as the fold change of individual samples, relative to the saline-injected at E19.5. No expression of IFN-γ was detected in the yolk sac at E19.5. * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001.
In the bursa of Fabricius, an interaction between in ovo treatment and age was found for IL-6 (P = 0.002) (Table 6). At E19.5 and hatch, expression of IL-6 did not differ between the carvacrol injected and the control group but was reduced for both groups at hatch compared to E19.5. In contrast, at d 14 expression of IL-6 was lower in the carvacrol compared to the control group. Moreover, the interaction between treatment and age tended to affect IL-2 expression (P = 0.06). Comparisons per age resulted in a similar finding for IL-6, which was decreased by in ovo delivery of carvacrol at d 14 (P = 0.006) (Figure 4).
Table 6.
Effects of in ovo delivery of carvacrol at embryonic day (E) 17.5 on expression of immune-related genes (expressed as fold change relative to the saline-injected group at E19.5) in the bursa of Fabricius of broiler chickens measured at E19.5, hatch, and day 14.
| Treatment | Age | IL-1β | IL-21 | IL-4 | IL-6 | IL-8 | IL-10 | IFN-γ | TNF-α | TGF-β | NF-κB | CD3δ | BU-1 | IgM | IgY | IgA | Muc-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 2.77 | 2.12 | 1.11 | 0.60 | 1.26 | 2.24 | 0.68 | 0.88 | 0.95 | 0.96 | 0.73 | 1.03 | 1.19 | 20.27 | 2.34 | 2.08 | |
| Carvacrol | 3.09 | 1.79 | 0.95 | 0.52 | 1.18 | 1.49 | 0.57 | 0.88 | 1.01 | 1.03 | 0.74 | 1.13 | 1.24 | 16.01 | 2.48 | 1.47 | |
| SEM | 0.24 | 0.74 | 0.18 | 0.11 | 0.12 | 0.32 | 0.08 | 0.05 | 0.04 | 0.03 | 0.03 | 0.06 | 0.05 | 2.01 | 0.26 | 0.95 | |
| Control | E19.5 | 1.00 | - | 1.00 | 1.00a | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Hatch | 1.45 | 1.00 | 1.00 | 0.33c | 0.57 | 0.52 | 0.20 | 1.06 | 0.91 | 0.89 | 0.71 | 1.13 | 0.86 | 1.32 | 1.40 | 1.06 | |
| d 14 | 5.87 | 3.24 | 1.35 | 0.47bc | 2.21 | 5.21 | 0.84 | 0.60 | 0.94 | 0.99 | 0.47 | 0.95 | 1.69 | 58.49 | 4.63 | 4.18 | |
| Carvacrol | E19.5 | 1.26 | - | 1.16 | 1.00ab | 1.14 | 0.82 | 1.05 | 1.14 | 1.11 | 1.15 | 1.04 | 1.15 | 1.11 | 1.09 | 1.36 | 1.19 |
| Hatch | 2.05 | 2.43 | 0.87 | 0.46ac | 0.41 | 0.26 | 0.13 | 0.99 | 1.00 | 0.98 | 0.73 | 1.23 | 0.90 | 1.31 | 1.68 | 2.47 | |
| d 14 | 5.95 | 1.14 | 0.81 | 0.10d | 2.00 | 3.38 | 0.54 | 0.52 | 0.92 | 0.97 | 0.44 | 1.01 | 1.71 | 45.62 | 4.40 | 0.76 | |
| SEM | 0.42 | 1.04 | 0.31 | 0.19 | 0.22 | 0.55 | 0.14 | 0.09 | 0.07 | 0.06 | 0.05 | 0.10 | 0.09 | 3.49 | 0.45 | 1.64 | |
| P-value | |||||||||||||||||
| Treatment | 0.23 | 0.89 | 0.55 | 0.14 | 0.59 | 0.44 | 0.11 | 0.67 | 0.33 | 0.17 | 0.86 | 0.33 | 0.44 | 0.89 | 0.69 | 0.60 | |
| Age | <0.001 | 0.33 | 0.28 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.25 | 0.06 | <0.001 | 0.12 | <0.001 | <0.001 | <0.001 | 0.81 | |
| Treatment × Age | 0.50 | 0.06 | 0.26 | 0.002 | 0.53 | 0.59 | 0.26 | 0.25 | 0.69 | 0.32 | 0.81 | 0.87 | 0.77 | 0.43 | 0.55 | 0.89 | |
IL-1β, 2, 4, 6, 8, 10: Interleukin 1β, 2, 4, 6, 8, 10; IFN-γ: Interferon γ; TNF-α: Tumor necrosis factor α; TGF-β: Transforming growth factor β; NF-κB: Nuclear factor κ B; CD3δ: CD3 δ subunit of T-cell Receptor Complex, present on T-cells and NK-cells; BU-1: Transmembrane protein of B-cells and a subset of macrophages; IgM, Y, A: Immunoglobulin M, Y, A; Muc-2: Mucin 2.
No expression of IL-2 was detected at E19.5.
LSMeans within a column and factor lacking a common superscript differ (P ≤ 0.05).
The gene expression values of the control group at E19.5 (at hatch for IL-2) were assigned an arbitrary value of 1.00 and the other groups were expressed relative to this value. Main effects for age are shown in Figure 5. Results are expressed as LSmeans ± SEM.
In the yolk sac, an interaction between in ovo treatment and age was found for IL-8 (P = 0.05) (Table 7). At E19.5, the expression was similar between the carvacrol compared to the control group, while at hatch expression was lower for both, but significantly lower in the carvacrol compared to the control group. In addition, the interaction between treatment and age tended to affect expression of Cd3δ (P = 0.10) and NF-κB (P = 0.07). A main effect of carvacrol was observed showing a lower expression of IFN-γ compared to the control group (P ≤ .001). Comparisons per age resulted in similar findings for IL-8 and IFN-γ which were both decreased by in ovo delivery of carvacrol at hatch (P = 0.04 and P ≤ 0.001, resp.) (Figure 4). Additionally, at E19.5 in ovo delivery of carvacrol increased the expression of both NF-κB (P = 0.04) and CD3δ (P = 0.03).
Table 7.
Effects of in ovo delivery of carvacrol at embryonic day (E)17.5 on expression of immune-related genes in the yolk sac membrane (expressed as fold change relative to the saline-injected group at E19.5) of broiler chickens measured at E19.5, hatch and day 14.
| Treatment | Age | IL-1β | IL-2 | IL-4 | IL-8 | IFN-γ1 | TNF-α | TGF-β | NF-κB | CD3δ | BU-1 | IgM | Muc-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.76 | 0.63 | 0.91 | 0.65 | 1.00a | 0.79 | 0.80 | 0.80 | 0.61 | 0.67 | 0.61 | 0.78 | |
| Carvacrol | 0.70 | 0.57 | 0.72 | 0.49 | 0.23b | 0.83 | 0.84 | 0.94 | 0.86 | 0.64 | 0.76 | 0.71 | |
| SEM | 0.10 | 0.20 | 0.17 | 0.18 | 0.13 | 0.09 | 0.08 | 0.06 | 0.08 | 0.28 | 0.19 | 0.13 | |
| Control | E19.5 | 1.00 | 1.00 | 1.00 | 1.00a | - | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Hatch | 0.52 | 0.25 | 0.82 | 0.31b | 1.00 | 0.57 | 0.61 | 0.60 | 0.22 | 0.34 | 0.22 | 0.56 | |
| Carvacrol | E19.5 | 0.93 | 0.87 | 0.75 | 0.91a | - | 1.20 | 1.15 | 1.28 | 1.52 | 1.03 | 1.31 | 0.89 |
| Hatch | 0.48 | 0.26 | 0.68 | 0.07c | 0.23 | 0.46 | 0.53 | 0.60 | 0.20 | 0.25 | 0.21 | 0.54 | |
| SEM | 0.15 | 0.28 | 0.24 | 0.25 | - | 0.12 | 0.11 | 0.08 | 0.11 | 0.40 | 0.27 | 0.18 | |
| P-value | |||||||||||||
| Treatment | 0.59 | 0.92 | 0.12 | 0.14 | <0.001 | 0.88 | 0.90 | 0.12 | 0.34 | 0.87 | 0.16 | 0.90 | |
| Age | <0.001 | <0.001 | 0.23 | <0.001 | - | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.02 | 0.01 | |
| Treatment x Age | 0.98 | 0.66 | 0.71 | 0.05 | - | 0.35 | 0.27 | 0.07 | 0.10 | 0.49 | 0.45 | 0.61 | |
IL-1β, 2, 4, 8: Interleukin 1β, 2, 4, 8; IFN-γ: Interferon γ; TNF-α: Tumor necrosis factor α; TGF-β: Transforming growth factor β; NF-κB: Nuclear factor κ B; CD3δ: CD3 δ subunit of T-cell Receptor Complex, present on T-cells and NK-cells; BU-1: Transmembrane protein of B-cells and a subset of macrophages; IgM: Immunoglobulin M; Muc-2: Mucin 2.
No expression of IFN-γ was detected at E19.5.
LSMeans within a column and factor lacking a common superscript differ (P ≤ 0.05).
The gene expression values of the control group at E19.5 (at hatch for IFN-γ) were assigned an arbitrary value of 1.00 and the other groups were expressed relative to this value. Main effects for age are shown in Figure 6. Results are expressed as LSmeans ± SEM.
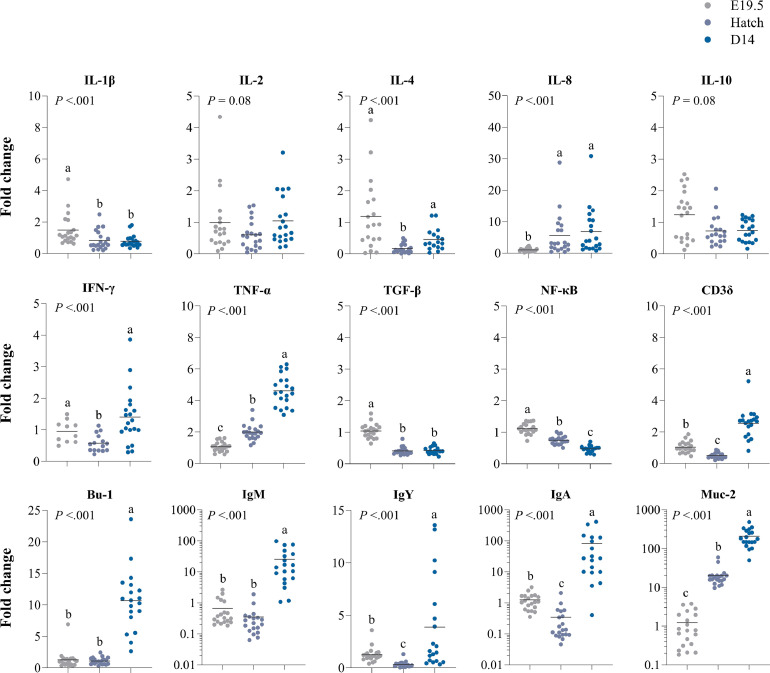
Main effects for age were found in all 3 tissues. In the jejunum, higher expressions over time were observed for IL-8 (P ≤ .001), TNF-α (P ≤ 0.001), Bu-1 (P ≤ 0.001), IgM (P ≤ 0.001), and Muc-2 (P ≤ 0.001) (Figure 5). In contrast, lower expressions over time were observed for IL-1β (P ≤ 0.001), NF-κB (P ≤ 0.001) and TGF-β (P ≤ 0.001). Expressions of IL-4 (P ≤ 0.001), IFN-γ (P ≤ 0.001) and IgY (P ≤ 0.001) were lowest at hatch but returned to similar levels as before hatch at d 14. The expressions of Cd3δ (P ≤ 0.001), and IgA (P ≤ 0.001) were lowest at hatch, but at d 14 surpassed expression of those genes compared to E19.5. In addition, age tended to affect IL-2 (P = 0.08) and IL-10 (P = 0.08). No expression of IL-6 was detected.
Figure 5.
Effects of age (embryonic day (E) 19.5, hatch or d 14) on gene expression of immune-related genes in the jejunum of broiler chickens. Results are expressed as the fold change of individual samples, relative to the saline-injected group at E19.5. a-c LSMeans lacking a common letter differ (P ≤ 0.05).
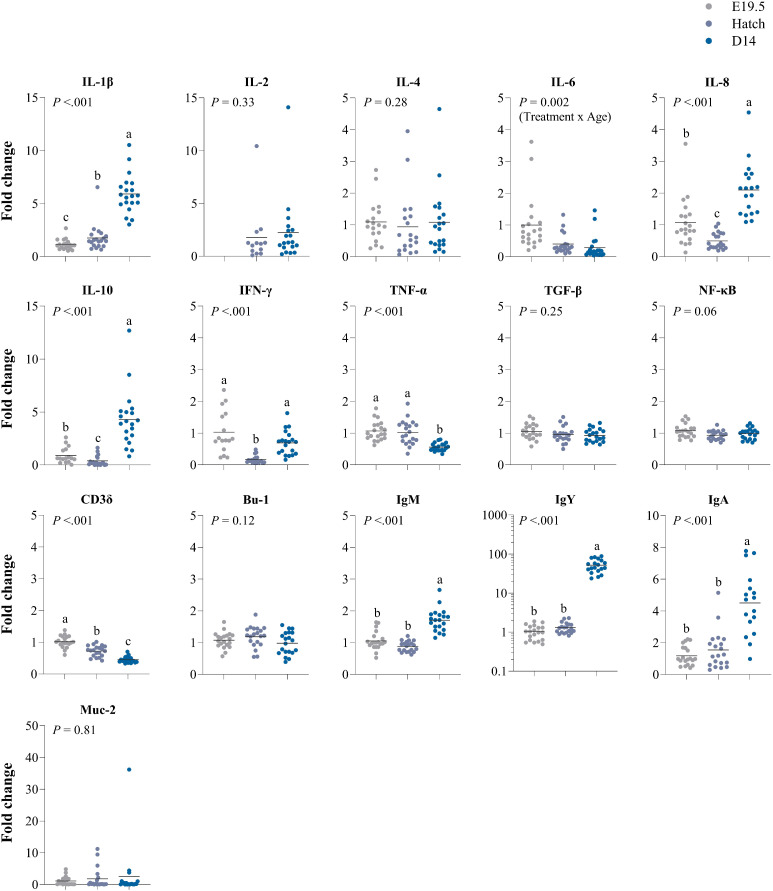
In the bursa, higher expressions over time were observed for IL-1β (P ≤ 0.001), IgM (P ≤ 0.001), IgY (P ≤ 0.001) and IgA (P ≤ 0.001), while expression of TNF-α (P ≤ 0.001) and Cd3δ (P ≤ 0.001) lowered over time (Figure 6). Expression of IFN-γ was lowest at hatch but returned to similar levels as before hatch at d 14 (P ≤ 0.001). IL-8 (P ≤ 0.001) and IL-10 (P ≤ 0.001) were lowest at hatch, but at d 14 surpassed expression of those genes compared to E19.5. Age tended to affect NF-κB (P = 0.06). Expression of IL-2 was only detected at hatch and d 14.
Figure 6.
Effects of age (embryonic day (E) 19.5, hatch or d 14) on gene expression of immune-related genes in the bursa of Fabricius of broiler chickens. Results are expressed as the fold change of individual samples, relative to the saline-injected group at E19.5. a-cLSMeans lacking a common letter differ (P ≤ 0.05).
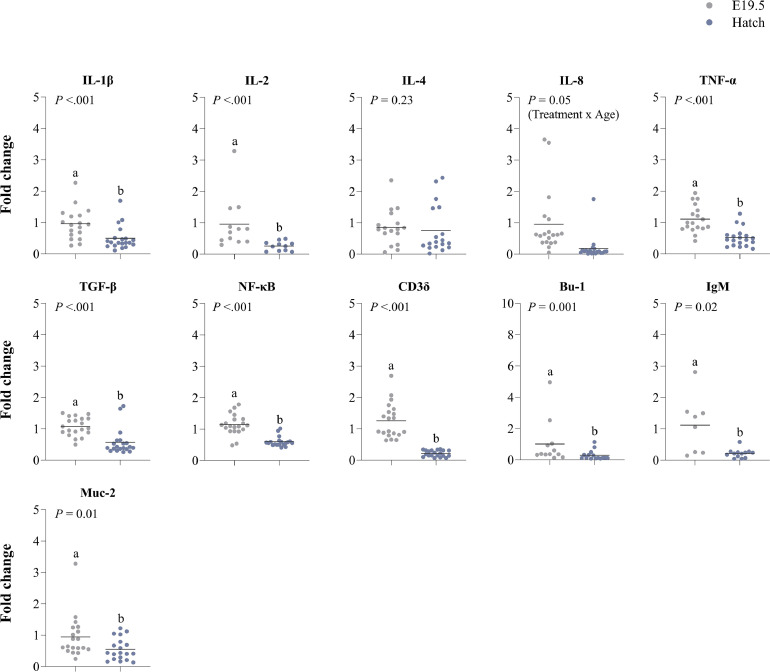
In the yolk sac, from E19.5 to hatch lower expressions were observed for IL-1β (P ≤ 0.001), IL-2 (P ≤ 0.001), IL-8 (P ≤ 0.001), TNF-α (P ≤ 0.001), TGF-β (P ≤ 0.001), NF-κB (P ≤ 0.001), CD3δ (P ≤ 0.001), Bu-1 (P = 0.01), IgM (P = 0.02), and Muc-2 (P = 0.01) (Figure 7). Expression of IFN-γ was only detected at hatch, and no expression of IL-6, IL-10, IgY or IgA was detected.
Figure 7.
Effects of age (embryonic day (E) 19.5 and hatch) on gene expression of immune-related genes in the yolk sac membrane of broiler chickens. Results are expressed as the fold change of individual samples, relative to the saline-injected group at E19.5. a,b Means lacking a common letter differ (P ≤ 0.05).
DISCUSSION
The current study aimed to investigate the impact of in ovo delivery of carvacrol on immune development in broiler chickens. In the jejunum, the expression levels of IL-1β, IL-10 and NF-κB from E19.5 to d 14 posthatch were higher in the carvacrol compared to the control group, suggesting that in ovo delivery of carvacrol may be associated with the activation of innate immune pathways. Carvacrol led to higher mRNA expression of proinflammatory cytokine IL-1β, together with higher expression of NF-κB. The cytokine IL-1β is produced as a result of NF-κB signaling pathway activation. In brief, after antigenic stimulation of TLRs, the resulting intracellular activation of NF-κB mediates the production of proinflammatory cytokines, such as IL-1β (Rehman et al., 2021). Previous research on posthatch supplementation of carvacrol found contradictory results (Liu et al., 2019a), however this was an observation after an immune challenge model based on lipopolysaccharide-induced proinflammatory state. Additionally, overall mRNA expression of immunoglobulin M (IgM) was lower after carvacrol delivery, which might have been a result of the activation of a proinflammatory response, thereby slowing down adaptive immune responses. The higher mRNA expression of regulatory cytokine IL-10 after the carvacrol treatment suggests that there might be a balancing effect on inflammatory responses, since IL-10 is known to downregulate inflammatory responses and shift adaptive responses from cell-mediated T-helper (Th)1 to a humoral Th2 response (Giansanti et al., 2007; Gadde et al., 2017b). Because the chicken undergoes major biological changes between E19.5 and d 14, treatments were also assessed based on age even though initially no evidence of treatment by age interactions were found. These results suggest that the observed increase in the expression of proinflammatory mediators IL-1β and NF-κB as well as the decrease in IgM mainly occur at E19.5, while the increased expression of anti-inflammatory cytokine IL-10 occurred at d 14. Similarly, when comparing in ovo treatments per age in the yolk sac, it was found that carvacrol increased expression of both NF-κB as well as CD3δ at E19.5, again suggesting that carvacrol may trigger proinflammatory responses (NF-κB) as well as T-cell or NK-cell stimulation (CD3δ) early after injection. Thus, the injection of carvacrol into the fertile egg triggered proinflammatory pathways during embryonic development. Since nutrients are required to sustain immune responses (Klasing, 2007), this may explain the observed subsequent temporary decrease in performance as shown by a lower BW at hatch. Given carvacrol's antimicrobial properties, it can be speculated that its impact on immune activation could be linked to bacterial modulation (Xu et al., 2008; Liu et al., 2019b). However, further research is necessary to confirm this observation.
In studies with mice, anti-inflammatory effects of carvacrol were found during inflammation involving IL-10 which played a key role in suppressing the inflammatory event. At the same time, production of proinflammatory cytokines IFN-γ and IL-6 was decreased, likely because of a lower TLR4/NF-κB pathway activation (Lima et al., 2013; Mahmoodi et al., 2019; Zhao et al., 2020). In broilers, posthatch feed supplementation with a combination of carvacrol and thymol also upregulated ileal IL-10 expression, while at the same time downregulating IL-6 (Li et al., 2023). This aligns with the capacity of IL-10 to inhibit inflammation, by downregulating production of proinflammatory cytokines, such as TNF-α, IL-1β and IL-6 (Ma, 2016). In the current study, no IL-6 could be detected in the jejunum, but in the bursa of Fabricius a pronounced decrease in IL-6 expression after in ovo carvacrol treatment was found at d 14 compared to the control group, and in the yolk sac IFN-γ was lower at hatch compared to the control group. It has been shown that a trigger such as heat stress was able to increase inflammatory pathways in broilers, including IL-6 mRNA expression in the bursa (Liu et al., 2021). Moreover, an increased IL-6 expression in the bursa of laying chickens was associated with infectious bursal disease virus or infectious bronchitis virus challenges (Xu et al., 2019; Tang et al., 2022). Thus, our results may indicate a decreased inflammatory state. In addition, this study showed that in ovo delivery of carvacrol increased bursal cortex/medulla ratio, reflected by a larger cortex size. A larger cortex could be the result of B-cell translocation from the medulla to the cortex, an indication of enhanced B-cell stimulation and migration to the peripheral bloodstream (Madej et al., 2015; Nagy et al., 2021). The cell types in the cortex were not identified, thus, the potential involvement of non–B-cell types cannot be discarded. Broiler chickens seem to develop adaptive immune responses between d 10 and 15 posthatch (Hamal et al., 2006; Lammers et al., 2010). Consequently, adaptive immune responses in the first 14 d posthatch are mostly of maternal origin. Thus, putting altogether, our results identified a 2-phase response to carvacrol triggering first an inflammatory response shortly after injection, specifically in the GIT, while then promoting a shift towards more anti-inflammatory and potentially adaptive responses 14 d after hatching.
The yolk sac is known to play multiple immune-related roles during embryonic development, while also having a direct connection with the GIT (van der Wagt et al., 2020; Wong and Uni, 2021). Previous research from our group showed that after in ovo delivery, carvacrol migrates mainly to the yolk sac content ready to be absorbed into the GIT (Meijer et al., 2024). Interestingly, in the current study an interaction was observed between treatment and age on mRNA expression of proinflammatory cytokine IL-8 consisting of a decreased expression of IL-8 with age from E19.5 to hatch in both control and carvacrol treatment groups, but with a more pronounced decrease for the carvacrol treatment. IL-8 is a chemokine produced following TLR activation (Kogut, 2002). The expression of proinflammatory cytokine IFN-γ was also lower at hatch compared to the control group, which is in line with previous research in mice splenocytes treated with carvacrol (Mahmoodi et al., 2019). IFN-γ is a type II interferon mainly produced by Th1 cells and is involved in macrophage and T-cell activation, leading to the development of a cell-mediated immune response (Giansanti et al., 2007). The expression of both cytokines (Il-8 and IFN-γ) was modulated following carvacrol treatment, which suggests that in ovo application of carvacrol may influence yolk sac immune pathways.
The absence of impact on performance parameters in the carvacrol treatment group may be due to carvacrol being supplemented only in ovo. Previous studies have shown benefits, especially during later life, when carvacrol was continuously supplemented posthatching in combination with thymol (Hashemipour et al., 2013; Li et al., 2023). The slight reduction in BW at hatch could be due to nutrient redirection towards immune function rather than growth, which may also explain trends of increased FI and decreased FCR observed posthatching, suggesting compensatory mechanisms.
Independent of carvacrol treatment, age affected gene expression patterns. Several genes were expressed higher as chicken development progressed (jejunum; IL-8, TNF-α, Bu-1, IgM, Muc-2 while expression of others decreased, indicating how immune development in the young chicken is a dynamic and ongoing process, which starts before hatching. However, caution is advised for the interpretation, as the embryos used for analyses at E19.5 remained in the setter, experiencing a slightly different environment compared to the chickens used for hatch and d 14, which were moved to the hatcher. Some of the expression patterns were similar between the jejunum and bursa (expression of IgM, IgY and IgA increased in both), while others showed opposite patterns (IL-1β decreased in the jejunum but increased in the bursa; TNF-α increased in the jejunum but decreased in the bursa; CD3δ increased in the jejunum but decreased in the bursa). Even though proinflammatory cytokines (IL-1β, TNF-α) as well as T-/NK-cell marker (CD3δ) patterns differed over time in both tissues, immunoglobulin gene expression increased in both. Immunoglobulin (IgM, IgY and IgA) expression increased over time in both tissues. Previous research in layers has indicated that during the peri-hatching phase only IgM is produced, and endogenous production of IgY and IgA starts after the first week of age (Lammers et al., 2010). Therefore, it is important to investigate if the gene expression of these immunoglobulins corresponds to their presence as free antibodies or as receptors bound to B-cells. Interestingly, expression of IFN-γ in the jejunum and bursa, as well as IL-8 in the bursa, showed a decrease at hatch, and returned to or exceeded embryonic levels at d 14, indicating that the process of hatching has a significant impact depressing the expression of these cytokines. Similarly, Ko et al. (2018) found a decrease at hatch in splenic BU-1-positive cells, which are indicative of B-cells, showing the potential impact of hatching on immune functioning. Potentially, the metabolic effort to hatch is more important and is therefore allocated more energy, resulting in a temporary suppression of inflammatory functioning. IFN-γ is known to stimulate and activate T-cells, essential for an effective immune response following vaccination. Marek's disease vaccine is commonly administered in ovo, and it has been shown that elevated IFN-γ levels increase the efficacy of the vaccine (Haq et al., 2011; Bavananthasivam et al., 2019). Therefore, the observed decrease in expression of IFN-γ may suggest that the efficacy of in ovo vaccination may be reduced around hatching.
In contrast, in the yolk all changes during development reflected a decrease in immune activity, which can be explained by the fact that during the peri-hatching phase the yolk sac undergoes a process of degradation, while immune organs in the chicken are developing (Wong and Uni, 2021).
Based on our findings, it can be concluded that (1) in ovo delivery of carvacrol modulates expression of genes related to immune responses during the peri-hatching phase until d 14 posthatch, and (2) major changes in immune-related gene expression take place between E19.5 and d 14 posthatch. For future research it is envisaged that the effects of in ovo administration of carvacrol might be potentiated under an immune challenge to mimic pathogen presence when the immune function is still under development. Unfortunately, serological data were not collected in this study, but future research should consider incorporating such analyses to further explain the regulatory effects of carvacrol on the immune response. Moreover, the gene expression of immunoglobulins at early age warrants more investigation, as well as the role of the yolk sac in the development of the immune response.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGMENTS
The financial support by AgriFutures Australia's Chicken Meat Program and Delacon Biotechnik GmbH (grant number PRJ-011584) is gratefully acknowledged. The authors thank Kym French and Cora Lau of UQ Biological Resources (Brisbane, Australia) for assisting with animal care and procedures during this study, as well as Allan Lisle for statistical advice.
Declaration of AI and AI-assisted technologies in the writing process: During the preparation of this work, the first author used ChatGPT to improve readability and language. After using this tool, all authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.104286.
Appendix. Supplementary materials
REFERENCES
- Abreu R., Semedo-Lemsaddek T., Cunha E., Tavares L., Oliveira M. Antimicrobial drug resistance in poultry production: Current status and innovative strategies for bacterial control. Microorganisms. 2023;11:953. doi: 10.3390/microorganisms11040953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shira E., Friedman A. Development and adaptations of innate immunity in the gastrointestinal tract of the newly hatched chick. Dev. Comp. Immunol. 2006;30:930–941. doi: 10.1016/j.dci.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bavananthasivam J., Alkie T.N., Matsuyama-Kato A., Hodgins D.C., Sharif S. Characterization of innate responses induced by in ovo administration of encapsulated and free forms of ligands of Toll-like receptor 4 and 21 in chicken embryos. Res. Vet. Sci. 2019;125:405–415. doi: 10.1016/j.rvsc.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Brenes A., Roura E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed. Sci. Technol. 2010;158:1–14. [Google Scholar]
- Broom L.J., Kogut M.H. Gut immunity: Its development and reasons and opportunities for modulation in monogastric production animals. Anim. Health. Res. Rev. 2018;19:46–52. doi: 10.1017/S1466252318000026. [DOI] [PubMed] [Google Scholar]
- Chiang H.-I., Berghman L.R., Zhou H. Inhibition of NF-kB 1 (NF-kBp50) by RNA interference in chicken macrophage HD11 cell line challenged with Salmonella enteritidis. Genet. Mol. Biol. 2009;32:507–515. doi: 10.1590/S1415-47572009000300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa Muniz E., Senador A., Miller F. Histomorphology of bursa of fabricius: Effects of stock densities on commercial broilers. Braz. J. Poult. Sci. 2006;8:217–220. [Google Scholar]
- Das R., Mishra P., Jha R. In ovo feeding as a tool for improving performance and gut health of poultry: A review. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.754246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health. Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gadde U., Oh S.T., Lee Y.S., Davis E., Zimmerman N., Rehberger T., Lillehoj H.S. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics. Antimicrob. Proteins. 2017;9:397–405. doi: 10.1007/s12602-017-9275-9. [DOI] [PubMed] [Google Scholar]
- Garcia P., Wang Y., Viallet J., Jilkova Z.M. The chicken embryo model: A novel and relevant model for immune-based studies. Front. Immunol. 2021;12:1–16. doi: 10.3389/fimmu.2021.791081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti F., Giardi M., Botti D. Avian cytokines: An overview. Curr. Pharm. Des. 2007;12:3083–3099. doi: 10.2174/138161206777947542. [DOI] [PubMed] [Google Scholar]
- Hamal K.R., Burgess S.C., Pevzner I.Y., Erf G.F. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poult. Sci. 2006;85:1364–1372. doi: 10.1093/ps/85.8.1364. [DOI] [PubMed] [Google Scholar]
- Haq K., Elawadli I., Parvizi P., Mallick A.I., Behboudi S., Sharif S. Interferon-γ influences immunity elicited by vaccines against very virulent Marek's disease virus. Antiviral Res. 2011;90:218–226. doi: 10.1016/j.antiviral.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. Metabolism and nutrition: Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013;92:2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- Jha R., Singh A.K., Yadav S., Berrocoso J.F.D., Mishra B. Early nutrition programming (in ovo and post-hatch Feeding) as a strategy to modulate gut health of poultry. Front. Vet. Sci. 2019;6:1–10. doi: 10.3389/fvets.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing K.C. Nutrition and the immune system. Br. Poult. Sci. 2007;48:525–537. doi: 10.1080/00071660701671336. [DOI] [PubMed] [Google Scholar]
- Ko K.H., Lee I.K., Kim G., Gu M.J., Kim H.Y., Park B.C., Park T.S., Han S.H., Yun C.H. Changes in bursal B cells in chicken during embryonic development and early life after hatching. Sci. Rep. 2018;8:16905. doi: 10.1038/s41598-018-34897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H. Dynamics of a protective avian inflammatory response: the role of an IL-8-like cytokine in the recruitment of heterophils to the site of organ invasion by Salmonella enteritidis. Comp. Immun. Microbiol. Infect. Dis. 2002;25:159–172. doi: 10.1016/s0147-9571(01)00035-2. [DOI] [PubMed] [Google Scholar]
- Kogut M.H. Impact of nutrition on the innate immune response to infection in poultry. J. Appl. Poult. Res. 2009;18:111–124. [Google Scholar]
- Lammers A., Wieland W.H., Kruijt L., Jansma A., Straetemans T., Schots A., den Hartog G., Parmentier H.K. Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev. Comp. Immunol. 2010;34:1254–1262. doi: 10.1016/j.dci.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Li L., Chen X., Zhang K., Tian G., Ding X., Bai S., Zeng Q. Effects of thymol and carvacrol eutectic on growth performance, serum biochemical parameters, and intestinal health in broiler chickens. Animals. 2023;13:2242. doi: 10.3390/ani13132242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.D., Song M.H., Yun W., Lee J.H., Kim H.B., Cho J.H. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poult. Sci. 2019;98:2026–2033. doi: 10.3382/ps/pey575. [DOI] [PubMed] [Google Scholar]
- Liu S.D., Song M.H., Yun W., Lee J.H., Kim H.B., Cho J.H. Effects of oral administration of essential oils on anti-immune stress, antimicrobial properties, and repairing the intestinal damage in broilers challenged by lipopolysaccharide. Can. J. Anim. Sci. 2019;99:377–383. [Google Scholar]
- Lima M.D.S., Quintans-Júnior L.J., De Santana W.A., Martins Kaneto C., Pereira Soares M.B., Villarreal C.F. Anti-inflammatory effects of carvacrol: Evidence for a key role of interleukin-10. Eur. J. Pharmacol. 2013;699:112–117. doi: 10.1016/j.ejphar.2012.11.040. [DOI] [PubMed] [Google Scholar]
- Liu W.C., Ou B.H., Liang Z.L., Zhang R., Zhao Z.H. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of Fabricius via modulating NF-κB signaling pathway in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma X. 1st ed. Springer; Dordrecht: 2016. Regulation of cytokine gene expression in immunity and diseases. [Google Scholar]
- Madej J.P., Stefaniak T., Bednarczyk M. Effect of in ovo-delivered prebiotics and synbiotics on lymphoid-organs’ morphology in chickens. Poult. Sci. 2015;94:1209–1219. doi: 10.3382/ps/pev076. [DOI] [PubMed] [Google Scholar]
- Mahmoodi M., Amiri H., Ayoobi F., Rahmani M., Taghipour Z., Ghavamabadi R.T., Jafarzadeh A., Sankian M. Carvacrol ameliorates experimental autoimmune encephalomyelitis through modulating pro- and anti-inflammatory cytokines. Life. Sci. 2019;219:257–263. doi: 10.1016/j.lfs.2018.11.051. [DOI] [PubMed] [Google Scholar]
- Meijer M.M.Y., van den Brand H., Niknafs S., Stark T., Navarro M., Khaskheli A.A., Roura E. Carvacrol in ovo delivery optimization and flow dynamics in broiler chicken eggs. Poult. Sci. 2024;103:443. doi: 10.1016/j.psj.2024.103443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar R., de Vries S., van den Anker I., Meijerhof R., Kemp B., van den Brand H. Effect of eggshell temperature and a hole in the air cell on the perinatal development and physiology of layer hatchlings. Poult. Sci. 2010;89:1716–1723. doi: 10.3382/ps.2010-00779. [DOI] [PubMed] [Google Scholar]
- Nagy, N., I. Oláh, and L. Vervelde. 2021. Structure of the avian lymphoid system. Pages 11–44 in Avian Immunology. Kaspers, B., Schat, K.A., Göbel, Th., Vervelde, L., eds. 3rd ed. Elsevier, Amsterdam.
- Nain S., Renema R.A., Zuidhof M.J., Korver D.R. Effect of metabolic efficiency and intestinal morphology on variability in n-3 polyunsaturated fatty acid enrichment of eggs. Poult. Sci. 2012;91:888–898. doi: 10.3382/ps.2011-01661. [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council (NHMRC) Australian code for the care and use of animals for scientific purposes. 8th edition. National Health and Medical Research Council; Canberra: 2013. [Google Scholar]
- Niknafs S., Meijer M.M.Y., Khaskheli A.A., Roura E. In ovo delivery of oregano essential oil activated xenobiotic detoxification and lipid metabolism at hatch in broiler chickens. Poult. Sci. 2024;103:321. doi: 10.1016/j.psj.2023.103321. https://linkinghub.elsevier.com/retrieve/pii/S0032579123008404 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A., Pal A., Mallick A.I., Biswas P., Chatterjee P.N. Molecular characterization of Bu-1 and TLR2 gene in Haringhata Black chicken. Genomics. 2020;112:472–483. doi: 10.1016/j.ygeno.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Pirgozliev V., Mansbridge S.C., Rose S.P., Lillehoj H.S., Bravo D. Immune modulation, growth performance, and nutrient retention in broiler chickens fed a blend of phytogenic feed additives. Poult. Sci. 2019;98:3443–3449. doi: 10.3382/ps/pey472. [DOI] [PubMed] [Google Scholar]
- Rehman M.S., Rehman S., Yousaf W., Hassan F.U., Ahmad W., Liu Q., Pan H. The potential of toll-like receptors to modulate avian immune system: exploring the effects of genetic variants and phytonutrients. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.671235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R.R., Awati A., Roubos-van den Hil P.J., van Kempen T.A.T.G., Tersteeg-Zijderveld M.H.G., Koolmees P.A., Smits C., Fink-Gremmels J. Effects of a feed additive blend on broilers challenged with heat stress. Avian. Pathol. 2019;48:582–601. doi: 10.1080/03079457.2019.1648750. [DOI] [PubMed] [Google Scholar]
- Sharifi-Rad M., Varoni E.M., Iriti M., Martorell M., Setzer W.N., del Mar Contreras M., Salehi B., Soltani-Nejad A., Rajabi S., Tajbakhsh M., Sharifi-Rad J. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018;32:1675–1687. doi: 10.1002/ptr.6103. [DOI] [PubMed] [Google Scholar]
- Song B., Tang D., Yan S., Fan H., Li G., Shahid M.S., Mahmood T., Guo Y. Effects of age on immune function in broiler chickens. J. Anim. Sci. Biotechnol. 2021;12:1–12. doi: 10.1186/s40104-021-00559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Paul M., Mallick A.I., Haq K., Orouji S., Abdul-Careem M.F., Sharif S. In vivo administration of ligands for chicken toll-like receptors 4 and 21 induces the expression of immune system genes in the spleen. Vet. Immunol. Immunopathol. 2011;144:228–237. doi: 10.1016/j.vetimm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Stefanello C., Rosa D.P., Dalmoro Y.K., Segatto A.L., Vieira M.S., Moraes M.L., Santin E. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front. Vet. Sci. 2020;6:1–10. doi: 10.3389/fvets.2019.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Qi J., Sun L., Zhao J., Zhang G., Zhao Y. Pathological effect of different avian infectious bronchitis virus strains on the bursa of Fabricius of chickens. Avian. Pathol. 2022;51:339–348. doi: 10.1080/03079457.2022.2063710. [DOI] [PubMed] [Google Scholar]
- van der Wagt I., de Jong I.C., Mitchell M.A., Molenaar R., van den Brand H. A review on yolk sac utilization in poultry. Poult. Sci. 2020;99:2162–2175. doi: 10.1016/j.psj.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A.R., Andersen-Civil A.I.S., Zhu L., Blanchard A. Dietary phytonutrients and animal health: Regulation of immune function during gastrointestinal infections. J. Anim. Sci. 2020;98:1–11. doi: 10.1093/jas/skaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E.A., Uni Z. Centennial Review: The chicken yolk sac is a multifunctional organ. Poult. Sci. 2021;100:821. doi: 10.1016/j.psj.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhou F., Ji B.P., Pei R.S., Xu N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008;47:174–179. doi: 10.1111/j.1472-765X.2008.02407.x. [DOI] [PubMed] [Google Scholar]
- Xu Z.Y., Yu Y., Liu Y., Ou C.B., Zhang Y.H., Liu T.Y., Wang Q.X., Ma J.Y. Differential expression of pro-inflammatory and anti-inflammatory genes of layer chicken bursa after experimental infection with infectious bursal disease virus. Poult. Sci. 2019;98:5307–5314. doi: 10.3382/ps/pez312. [DOI] [PubMed] [Google Scholar]
- Zhao W., Deng C., Han Q., Xu H., Chen Y. Carvacrol may alleviate vascular inflammation in diabetic db/db mice. Int. J. Mol. Med. 2020;46:977–988. doi: 10.3892/ijmm.2020.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K., Wu S., Lv Y., Pang P., Deng L., Xu H., Shi Y., Chen X. Carvacrol inhibits the excessive immune response induced by influenza virus A via suppressing viral replication and TLR/RLR pattern recognition. J. Ethnopharmacol. 2021;268 doi: 10.1016/j.jep.2020.113555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.