Abstract
Elevated atmospheric heat is considered as one of the bottlenecks for global wheat production. Screening potential wheat genotypes against heat stress and selecting some suitable indicators to assist in understanding thermotolerance could be crucial for sustaining wheat cultivation. Accordingly, 80 diverse bread wheat genotypes were evaluated in controlled lab condition by imposing a week-long heat stress (35/25 °C D/N) at the seedling stage. The response of heat stress was evaluated using multivariate analysis techniques on 20 morpho-physiological traits. Results showed significant variations in the studied traits due to the imposition of heat stress. Eleven seedling traits that contributed significantly to the genotypic variability were identified using principal component analysis (PCA). A substantial correlation between most of the selected seedling attributes was observed. Hierarchical cluster analysis identified three distinct clusters among the tested wheat genotypes. Cluster 1, consisting of 33 genotypes, exhibited the highest tolerance to heat stress, followed by Cluster 2 (18 genotypes) with moderate tolerance and Cluster 3 (29 genotypes) showing susceptibility. Linear discriminant analysis (LDA) approved that nearly 93 % of the wheat genotypes were appropriately ascribed to each cluster. The squared distance analysis confirmed the distinct nature of the clusters. Using multi-trait genotype-ideotype distance index (MGIDI), all 12 identified tolerant genotypes (BG-30, BD-468, BG-24, BD-9908, BG-32, BD-476, BD-594, BD-553, BD-488, BG-33, BD-495, and AS-10627) originated from Cluster 1. Selection gain in MGIDI analysis, broad-sense heritability, and multiple linear regression analysis together identified shoot and root dry and fresh weights, chlorophyll contents (a and total), shoot tissue water content, root-shoot dry weight ratio, and efficiency of photosystem II (PS II) as the most vital discriminatory factors explaining heat stress tolerance of 80 wheat genotypes. The identified genotypes with superior thermotolerance would offer resourceful genetic tools for breeders to improve wheat yield in warmer regions. The traits found to have greater contribution in explaining heat stress tolerance will be equally important in prioritizing future research endeavors.
Keywords: Thermotolerance, Triticum aestivum L., Photosystem II, Cluster analysis, Principal component analysis, MGIDI
1. Introduction
The world's food production is under constant risk due to the introduction of climate change associated with rising in atmospheric temperature. In a high-emission scenario, the mean global temperature is expected to increase by 3.3–5.7 °C by the end of the 21st century [1]. The appearance of supra-optimum temperature during crop production is a significant devastating impact of global climate change [2]. This elevated temperature has been affecting the productivity of many crops and wheat is not an exception of that. A collaborative research integrating the selection of heat-tolerant wheat genotypes with an in-depth understanding of vital physiological traits related to thermotolerance has the potential to significantly contribute to sustainable wheat production.
Among the cereals, the production of bread wheat (Triticum aestivum L.) secures the second position both globally and in Bangladesh [3,4]. The significance of wheat is vital as it solely supplies over one-fifth of required calories and proteins for the world [5]. In the near future, the productivity as well as availability of wheat will be the crucial for global food security and economy mainly to feed about 9.6 billion people by 2050 [6,7]. The above-optimum temperature at all stages of wheat growth negatively affects plant development [8] and causes physiological and biochemical damage [9].
Heat stress during early growth can hinder seedling development by disrupting enzymatic activity involved in seed metabolism. Additionally, the elevated abscisic acid level and reduced gibberellin activity due to heat stress inhibit seed germination and delays the germination process, respectively [10]. In addition to creating oxidative stress, which damages cells, heat stress slows the synthesis of hormones necessary for cell division and elongation. This ultimately restricts the absorption and movement of water and nutrients by the roots and shoots [11]. The disruption in cell wall structure and inhibition in expansion activity under elevated temperature restrict leaf growth and thus reducing the overall photosynthetic surface area [12]. The conformational changes, oxidative modifications, and proteolytic degradation resulted in inactivation of Rubisco under heat stress condition resulted in limiting carbon assimilation and cut down of plant growth and biomass accumulation [13].
High temperature alters the expression and activity of transporter proteins involved in nutrient absorption and transport, and damage the vascular tissue system, resulting in nutrient deficiencies and stunted growth [14]. Protein denaturation under elevated temperature is associated with unfolding and losing of functional conformation followed by toxicity to cells [15]. The kinetic energy of lipid molecules increases under heat stress, causing the membranes more fluid and permeable, making them highly susceptible to oxidative damage by ROS [16]. The reduction in biomass accumulation in seedlings is the consequence of the cumulative influence of high temperature stress on multiple physiological and biochemical processes. Heat stress diverts energy from growth processes, causing seedlings to exhibit reduced biomass and overall vigor due to the production of HSPs and antioxidant defenses [17]. The thylakoid lamellae, the site of the photochemical reactions of photosynthesis, is regarded as one of the foremost targets of heat stress mainly because of heat-induced ROS generation [18]. The photosystem II (PSII) is considered as the most heat-sensitive element of the photosynthetic electron transport chain, prone to heat-induced denaturation caused by dissociation of D1 protein from PSII reaction center [19]. The intrinsic photochemical efficiency of PSII can be recognized by a suitable chlorophyll fluorescence approach, namely the maximum photochemical efficiency of PSII photochemistry (Fv/Fm). High temperature causes a decline in Fv/Fm [20,21] which is the outcome of increased non-reducing PSII reaction centers followed by a decline in electron transport from QA to QB [22]. For detecting and quantifying the heat-induced alterations in the photosynthetic machinery, the chlorophyll a fluorescence is considered the most sensitive and reliable method [23]. A wide range of information concerning processes in PSII and thylakoid membranes has been provided by chlorophyll a fluorescence that leads an improvement regarding the understanding of photosynthesis [24]. The chlorophyll a fluorescence technique is a non-invasive and versatile method to monitor plant health, stress severity, and the efficiency of light-driven reactions in photosynthesis [25]. High temperature induced inhibition of PSII activity leads to breakdown of chlorophyll pigments [26] followed by negative impacts on photosynthetic transformation of solar energy to the energy of chemical bonds.
Heat stress also brings about reduction in chlorophyll content and enhanced reactive oxygen species (ROS) production [27], decreased relative water content [28], and destruction in organelles membrane [29]. Heat stress not only inhibits the functionality of vital chlorophyll biosynthesis enzymes like glutamyl-tRNA reductase and Mg-chelatase but also accelerates chlorophyll degradation through enhancing the activity of chlorophyllase and other degrading enzymes, leading to a greater reduction in light harvesting pigment content [30]. All these potential physiological phenomena significantly affect wheat productivity. For mitigating heat stress impacts, it is vital to screen out potential genotypes based on some indicators aiming to endure environmental challenges [31]. In addition, an upgraded understanding on morpho-physiological features linked to heat stress tolerance has rational implications for recognizing several tolerance mechanisms that will assist in ameliorating the adverse effects of heat stress on wheat [[32], [33], [34]]. In this aspect, the adoption of multivariate analysis technique is a better option not only to select potential genotypes but also to identify vital traits that principally govern the tolerance mechanisms under high temperature condition [8].
For exploring relationships, grouping, and selecting traits from a complex data set, multivariate analysis techniques are essential. These approaches are more representative, meaningful, and precise inferences compared to simpler methods [35,36]. The cluster analysis classifies genotypes for desirable traits according to their genetic similarity with minimal error [37]. Similar to cluster analysis, the principal component analysis (PCA) also explains the dissimilarity among genotypes and it has the ability to transform the information into a lesser set of variables that capture most of the original information [38,39]. PCA is a powerful technique for understanding the relationships between traits and analyzing their correlations [40]. For defining genotypic groups as prior sorting criteria, linear discriminant analysis (LDA) is frequently used. LDA identifies misclassification inaccuracy and calculates the remoteness between groups, and as a result can effectively screen out tolerant genotypes under stress conditions [41]. Moreover, selecting superior genotypes with higher genetic gain and desirable traits is crucial for successful breeding programs. To assist in such identification, the introduction of multi-trait genotype-ideotype distance index (MGIDI) plays an important role as this index considers multiple traits simultaneously, allowing researchers to assess both the strengths and weaknesses of the tested genotypes relative to an ideal breeding target [42].
Therefore, the general purpose of this investigative study is to assess heat tolerance in a wide number of wheat genotypes based on seedling performance. Precise objectives are outlined to- (i) evaluate the variations in morpho-physiological features of the wheat genotypes subjected to high temperature stress; (ii) categorize wheat genotypes into distinct clusters differing in heat tolerance using hierarchical clustering algorithm; and (iii) identify potential heat-tolerant genotypes and seedling traits associated with heat stress tolerance using diverse multivariate approaches.
2. Materials and methods
2.1. Plant materials and heat stress imposition
In this exploratory investigation, eighty wheat genotypes of diverse nature were collected from various sources. Among them, 9 mutant lines (mutagen: 1 % EMS) were collected from ACI Seed; 14 varieties and 2 advanced lines from Bangladesh Wheat and Maize Research Institute; 1 variety from Bangladesh Institute of Nuclear Agriculture; and rest of the 54 wheat accessions collected from Plant Genetic Resource Center of Bangladesh Agricultural Research Institute. Table S1 lists the sources and types of 80 wheat genotypes used in the investigation. A two-factor completely randomized design (CRD) with 3 replicates was used for the study. Two levels of temperature treatment as ‘control’ (25/15 °C D/N) and ‘heat stress’ (35/25 °C D/N), according to Khatun et al. [43], was applied to 80 wheat genotypes at seedling stage.
Seeds of uniform sized of each wheat genotypes were selected prior to sowing. The selected seeds were first disinfected with 80% ethanol for 5 min, rinsed thoroughly with sterile distilled water, and then soaked in distilled water for another 10 min. Before that, the germination trays (21cm × 15cm × 4.5 cm) were filled up with 1.5 kg of sterile sand moistened with distilled water. Later on, for the specified control and heat stress conditions, 90 seeds from each genotype were placed in two germination trays. The trays were kept under normal room temperature condition (28–33 °C) for five days.
Five days after seed sowing, the percent germination in each germination tray was determined as n/N × 100 (where n represents the total number of germinated seed; N represents the total number of seeds sown) (Table S1). The wheat seedlings were subsequently thinned to 30 per tray. After that, the germination trays with established seedlings were set in a plant growth facility (GC-560H, Firstek Scientific, Taiwan) with the following settings: 25/15 °C (D/N) temperature (control), relative humidity (RH) 75–80%, 14 hours of photoperiod maintained by 200 μmol m−2 s−1 of photosynthetic photon flux density (PPFD).
Each germination tray was provided with 50 mL of half-concentrated Hoagland's solution on every alternated day as a source of nutrients. The control temperature was continued up to 17 days of sowing. On 18th day after seed sowing, one set of germination tray was transferred to another growth facility of same nature and heat stress (35/25 °C D/N) was imposed therein for seven days (18–24 days after sowing). A digital temperature and humidity meter (HD-306, HTC Instruments, Taiwan) was used to monitor the growth chamber temperature and humidity. The experimental setup and the visual impacts of heat stress on wheat genotypes are provided in Fig. S1. The control and heat stress treatments imposed on wheat genotypes were terminated 24 days after seed sowing, and data were recorded subsequently.
2.2. Measurement of seedling traits
The seedling traits like lengths and dry and fresh weights of shoots and roots were recorded from 10 seedlings in each replicate. The length of shoots and roots was determined by measuring from the root-shoot junction to the respective leaf and root apices. Dry weights of shoots and roots were measured after oven drying (DSO-300D, Digisystem Laboratory Instruments Inc., Taiwan) at 80 °C for 24 h. The root dry weight over the shoot dry weight was used to determine the root-shoot ratio. Tissue water content (TWC), an expression of the amount of water present per unit of shoot and root fresh weight, was determined following the formula proposed by Mickky et al. [44].
The formula of Hellal et al. [45] was used to measure the seedling vigor index (SVI).
The relative water content of leaf (LRWC) was determined in accordance of Meher et al. [46]. Briefly, fresh leaf samples (at least of 0.5 g) from both control and heat-stressed plants were soaked in 50 mL deionized water for 4 h. Afterward, weights of the turgid leaf samples were recorded using a digital weighing balance (AJ-620E, Shinko Denshi Co. Ltd., Japan) after carefully removing the surface water using blotting paper. The leaf samples were dried at 80 °C until their weight stabilized. The dry weight of each sample was then determined.
Cell membrane stability (CMS) was assessed using the slightly modified protocol described by Rasool et al. [47]. Briefly, 0.1 g leaf samples from both control and heat-treated plants were washed separately in double-distilled water, cut into uniform squares. Then the leaf chips were transferred in test tubes containing 10 mL of deionized water and then incubated for 30 min at 40 °C in a hot water bath. Afterward, 200 μL of solution was taken from each test tube, and the initial conductivity (C1) was recorded using a compact electrical conductivity meter (LAQUAtwin-EC-22, Horiba Scientific, Japan). Subsequently, the same test tubes were placed in a boiling water bath for 15 min. After cooling, the final conductivity (C2) was measured. The CMS was then calculated individually for respective growing conditions as proposed by Mohi-Ud-Din et al. [48].
The germination trays with wheat seedlings were allowed to dark-adapt for 30 min before the measurement of chlorophyll a fluorescence attributes as proposed by Khan et al. [49]. The minimum (Fo) and maximum (Fm) fluorescence of dark-adapted leaves were recorded using a fluorometer (Junior-PAM, Heinz Walz GmbH, Germany) with a low measuring beam intensity of 125 μmol m−2 s−1 from the adaxial surface of fully expanded lowermost leaf. The variable fluorescence (Fv) was calculated from the difference of Fm to Fo. The fluorescence attributes were estimated according to Sperdouli et al. [50].
Heat stress induced thylakoid membrane damage (TMD) was measured by comparing the Fv/Fm values of control and heat-treated plants. The equation suggested by Moradpour et al. [31] was employed to calculate TMD as follows:
The same leaves used for recording chlorophyll a fluorescence attributes were then collected to determine the chlorophylls and carotenoids according to the procedure described by Porra et. el [51]. Briefly, 100 mg of fresh leaf sample from both control and heat-treated plants was placed in 10-mL glass vials with 5 mL of 80% acetone and then it was preserved at 4 °C in the complete darkness for 24 hours. Afterward, 1 mL of supernatant was transferred in a quartz cuvette and the absorbance was read at 663, 646, and 470 nm wavelengths corresponding to chlorophyll a (chl a), chlorophyll b (chl b) and carotenoids (car), respectively, with a UV–VIS spectrophotometer (Genesys 10S UV–VIS, Thermo Fisher Scientific, USA). Acetone (80%) was used as blank. The leaf pigment contents (chl a, chl b, total chlorophyll [chl T], and car) were quantified following the method described by Mohi‐Ud‐Din et al. [9] and expressed as mg g−1 fresh weight.
The ratios of chlorophyll a to b (chl a:b) and chlorophyll to carotenoids (chl:car) were calculated by dividing chl a by b and chl T by car, respectively.
For the measurement of seedling traits, the fully developed leaves from ten seedlings were pooled to create a single biological replicate. This process was repeated twice more using separate sets of seedlings for a total of three replicates. For the recording of fluorescence attributes and pigment contents, six fully expanded lowermost leaves were collected. Two leaves were pooled to create one biological replicate. This pooling was repeated three times to obtain a total of three replicates. For all the measurements and assays, samples were collected separately from control and heat-treated seedlings.
The collected data on seedling traits were expressed as relative values (RV). The RV was calculated based to the formula prescribed in Wahab et al. [52] as follows:
2.3. Statistical technique and data analysis using machine learning systems
Every form of statistical analysis was performed using R-4.0.3 for win (http://CRAN.R-project. org/) (accessed on January 12, 2023) in Rstudio-1.3.1093 (https://rstudio.com/) (accessed on January 12, 2023). The analysis of variance (ANOVA) of the studied traits was performed under 2-factor (temperature regimes × genotypes) condition in the general linear model (GLM) in the R package ‘lme4’ [53]. The library ‘agricolae’ [54] was adopted to compare the mean differences by Tukey's HSD test. Statistical significance was determined at a p-value threshold of less than 0.05.
The graphical representation via boxplots and radar plot were adopted to illustrate the impact of heat stress on wheat seedling and relative magnitude of change in the seedling traits due to a-week long heat stress treatment, respectively. Boxplots visually summarize multiple variable distributions, aiding in detecting relationships, comparing central tendency and variability, and aiding in exploratory data analysis and hypothesis testing [55]. Radar plots allow for the simultaneous representation of multiple variables in a complex dataset, providing insights into patterns and trends across different observations [56]. The R packages ‘ggplot2’, ‘ggpubr’, and ‘reshape2’ were used to create boxplot and ‘fsmb’ package along with ‘reshape2’ were implied to develop radar plot [57,58]. Principal component analysis (PCA), a versatile tool of multivariate analysis, offers insights into the structure of complex datasets not only by reducing the dimensionality of the datasets while remaining most of the variability but also identifies the key components driving the variation [59]. The relative value (RV) of the studied seedling traits were used for computing PCA. The R packages ‘ggplot2’, ‘gridExtra’, ‘factoextra’, ‘ggbiplot’ and ‘corrplot’ were used to extract the Eigen value and to visualize the PCA variable plot [60]. The principal components (PCs) with eigenvalues >1 was considered as significant and the contribution (%) of all seedling traits in those significant PCs was evaluated to select the key traits, having impact on thermotolerance, for succeeding multivariate approaches (Fig. S2).
Correlation analysis not only helps identify patterns and dependencies in datasets by quantifying linear relationships between variables but also provides a holistic view of interdependencies and aids in variable selection [61]. The Pearson's correlation coefficients were computed separately for both growing conditions. The R package ‘metan’ was employed to visualize the correlation using the function corr_coef [62]. The ideal number of clusters was identified using the gap statistic algorithm implemented in the fviz_nbclust function of the ‘factoextra’ R package (Fig. S3) [63]. The hierarchical clustering of genotypes, a valuable tool of multivariate analysis, aids in not only categorizing genotypes with similar genetic makeup and performances but also assist in further studying the trait associated analysis like principal component analysis [64]. The RVs were used to create a heatmap and hierarchical clusters with Euclidean distance and Ward's minimum variance method (WardD2) using the ‘pheatmap’ and ‘ggplot2’ libraries [65]. The genotypes were grouped into definite clusters with distinct dissimilarities in the produced cluster heatmap, and the genotypes within the clusters exhibited a high degree of similarity.
LDA is a powerful multivariate analysis method used to improve classification accuracy by finding linear combinations of features in complex datasets, reducing dimensionality, and discriminating between groups [66]. The R packages ‘psych’ and ‘MASS’ were adopted to perform LDA using RV [67]. Mahalanobis distance is a crucial matrix in multivariate analysis, providing insights into data relationships, distributions, similarity between points, and clustering observations [68]. The Mahalanobis squared distance (D2) among the clusters was estimated by using a cluster validation package ‘clv’ [69]. The Multi-trait Genotype-Ideotype Distance Index (MGIDI), a novel technique suggested by Olivoto and Nardino [42], can be used to compare a genotype's proximity to an ideotype across many traits, making it easier to pick genotypes with desired attributes. The RVs were used to compute MGIDI using the ‘metan’ R package [62]. Multiple linear regression (MLR) enhances the selection of influential variables through exploring, quantifying, and interpreting the relationships among multiple variables [70]. MLR was estimated with the aid of R package ‘datarium’ by placing shoot dry weight (SDW) as dependent variable and rest of the selected seedling traits as independent variables [71].
3. Results
3.1. Variability among the seedling traits
While tested in two growing conditions (control and heat stress), 80 wheat genotypes showed highly significant (p ≤ 0.001) variation in all seedling traits (Table S1). With the exception for chl a:b, the effect of growing conditions and its interaction effect with genotypes were significant at least at p ≤ 0.05 (Table S2). A box plot was used to visualize the descriptive statistics of the seedling traits (Fig. 1). Due to heat stress, the seedling traits decreased significantly over the control condition except chl a:b. Heat stress decreased shoot length (SL), shoot fresh weight (SFW), shoot dry weight (SDW), and shoot tissue water content (STWC) by 11, 42, 23, and 10%, respectively, over control, whereas 9, 59, 29, and 23% decreases were recorded in root length (RL), root fresh weight (RFW), root dry weight (RDW), and root tissue water content (RTWC), respectively (Fig. 1). The seedling vigor index (SVI) and root-shoot ratio (RSR) reduced by 12 and 5%, respectively due to heat stress. The leaf relative water content (LRWC) and cell membrane stability (CMS) were decreased by 13 and 15%, respectively, due to heat stress (Fig. 1). The maximum energy conversion potential of PSII (Fv/Fo) was more affected due to heat stress and reduced by 20% over the control while a little decline (5%) was noticed in the maximum photochemical efficiency of PSII photochemistry (Fv/Fm). High temperature stress greatly declined the concentration of chl a, chl b, chl T, and car content by 25, 23, 24, and 16%, respectively, while the chl a:b and chl:car were reduced by 3 and 11%, respectively due to heat stress over the control (Fig. 1).
Fig. 1.
The descriptive statistics of the seedling attributes of wheat genotypes under distinct temperature conditions are displayed in the box plots. Different asterisk(s) on the boxes indicate significant difference between growth conditions. ∗∗∗, ∗∗, and ∗ indicate significant at p ≤ 0.001, 0.01, and 0.05, respectively according to Tukey's HSD test; ns = non-significant. The respective trait mean is shown by the black circle, while the median is represented by the horizontal line within the box. The lower and upper bounds of the box, lower and upper whisker denotes Q1 (first quartile/25th percentile), Q3 (third quartile/75th percentile), (Q1−1.5IQR) and (Q3 + 1.5IQR), respectively. IQR—interquartile range. The distribution of the 80 observations is shown by the slate-colored dots in the boxes. SL—shoot length; RL—root length; SVI—seedling vigor index; SFW—shoot fresh weight; RFW—root fresh weight; SDW—shoot dry weight; RDW—root dry weight; STWC—shoot tissue water content; RTWC—root tissue water content; RSR—root-shoot weight ratio; LRWC—leaf relative water content; CMS—cell membrane stability; Fv/Fo— efficiency of the water-splitting complex on the donor side of PSII; Fv/Fm—maximum photochemical efficiency of PSII photochemistry; chl a—chlorophyll a content; chl b—chlorophyll b content; chl a:b—chlorophyll a to b ratio; chl T —Total chlorophyll content; car—carotenoid content; chl:car—chlorophyll to carotenoid ratio; FW—fresh weight.
3.2. Principal component analysis
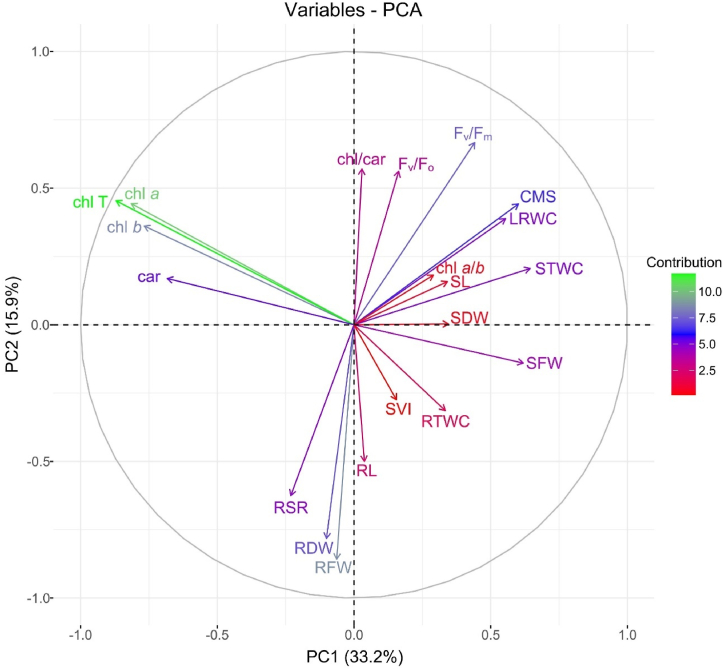
Principal component analysis (PCA), a potential tool of multivariate data analysis, is deliberately used to study and simplify complex and substantial datasets. In our study, the PCA was computed to enhance the discriminatory power for categorizing the measured traits based on their relationships under heat stress conditions. In the present study, a total of 20 principal components (PCs) were acquired, but only first six PCs with eigenvalues >1 were considered as significant (data not shown). These six PCs explained about 84% of the genotypic variability, as influenced by observed traits. Among the significant PCs, the first two components explained about 49% of the total variability (Fig. 2). The first PC (PC1) in PCA-variable plot exhibited about 33% of the total variability, and the seedling traits like SFW, STWC, CMS, LRWC, chl a:b, and RTWC contributed positively to PC1, while the pigment attributes like chl a, chl b, chl T, and car had negative contributions towards PC1 (Fig. 2). The second PC (PC2) accounted for about 16% of total variability and was principally explained by fluorescence attributes like Fv/Fo, Fv/Fm and chl:car on the way to positive direction. Almost all root related traits contributed negatively towards PC2 (Fig. 2). To gain an overall understanding, the contributions of seedling traits to the first six significant principal components (PCs) were evaluated. Traits with a contribution of greater than 5% to the overall variability were then selected for further analysis using multivariate approaches. These traits included chl a, chl b, chl T, Fv/Fo, Fv/Fm, SFW, RFW, SDW, RSR, RDW, and STWC (Fig. S2).
Fig. 2.
PCA-variable plot showing the position of seedling traits. A vector's length and color intensity in the plot specify the representation quality and traits contribution to the principal components, respectively. Positive or negative interactions of the seedling attributes are displayed by the angles between the vectors produced from the plot's central point. Details of seedling traits are presented in Fig. 1. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Correlation analysis
The correlation coefficients were employed to measure the degree of association among the seedling traits. The greater amount of stronger significant correlations among the studied traits were observed under control compared to the heat stress conditions (Fig. 3A and B). Under control conditions, SFW exhibited a positive and association with majority of seedling traits, except for fluorescence features and RSR (Fig. 3A). However, under heat stress, SFW maintained a significant and positive association with fluorescence features (Fv/Fo and Fv/Fm) and a negative association with RSR (Fig. 3B). RFW showed a greater number of significant correlations with most seedling traits under control conditions compared to heat stress. SDW followed the similar pattern of SFW under control conditions, however, it did not maintain any association with fluorescence features under heat stress conditions. RDW correlated with SDW, SFW, fluorescence features, RFW, and RSR under control conditions, while under heat stress, RDW only maintained positive associations with SFW, RFW, and RSR (Fig. 3). The STWC maintained a positive relationship with pigment contents, SFW, and RFW under control conditions, while showed a positive correlation with RFW, SFW, and fluorescence features under heat stress. RSR exhibited the most unique pattern in its association with seedling traits under both growing conditions. It consistently showed a significant negative association with pigment content, but this effect was stronger under heat stress. Interestingly, RSR maintained significant relationships with fluorescence features and RDW under both temperature conditions. However, under heat stress, it additionally showed negative associations with SFW and SDW. The fluorescence features maintained a positive association with RSR in all treatment states. However, under heat stress, they additionally showed a positive association with SFW. Pigment contents consistently displayed a positive association with SFW, RFW, SDW, and STWC, while maintaining a negative relationship with RSR under control and heat stress (Fig. 3). Interestingly, chl T and chl a maintained a greater number of significant associations compared to chl b under heat stress conditions (Fig. 3).
Fig. 3.
The Pearson correlation coefficient values are displayed in the correlation heatmap for the selected seedling traits under control (A) and heat stress (B) conditions. The blue values are positive and the red ones are negative. It has a range of −1 to 1, whereby −1 denotes an absolute negative linear relationship, 1 indicates a perfect positive linear relationship, and 0 specifies no relationship at all between studied variables. Details of seedling traits are presented in Fig. 1. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The majority of the seedling traits selected by PCA exhibited significant correlations among themselves across growing conditions (Fig. 2, Fig. 3). While root-related traits like RFW and RDW contributed negatively to PCA, they maintained highly significant positive associations with SFW, SDW, and pigment attributes under heat stress conditions, except for RSR (Fig. 2, Fig. 3B).
3.4. Hierarchical clustering of genotypes and traits
The cluster heatmap consisted of three clusters for the genotypes and three groups based on the variability available in the studied seedling traits (Fig. 4; the genotypes belonging to each cluster are listed in Table S3). The most similar genotypes were allotted in the clusters, whereas the seedling traits strongly associated with themselves were placed in groups of the clustered heatmap. SFW and STWC formed Group 1. Group 2 contained majority of the seedling traits, including fluorescence features, pigments contents, RFW, RDW, and RSR. SDW solely formed the Group 3. Cluster 1 contained the maximum number of genotypes (33) followed by Cluster 3 (29) and Cluster 2 (18). Interestingly, the allotment of wheat genotypes in Cluster 1 appears to be strongly influenced by all seedling traits, even though these traits were placed in different trait groups (Fig. 4).
Fig. 4.
Heatmap and cluster analysis (method = wardD2 and distance = Euclidean) based on PCA-selected traits influencing genotypic variability in heat tolerance revealed association between genotypes and seedling traits. The relative values (RV) attained from the PCA-selected traits of the wheat genotypes were subjected to cluster analysis. Three clusters were acquired at the genotype level (cluster-1, 2 and 3) and three groups (group-1, 2 and 3) were obtained at the trait level. The intensity of the relative values for the studied attributes is expressed by various color tones. Results indicated that Cluster 1 exhibited the highest potential for heat stress tolerance due to superior performance across most seedling traits, followed by Clusters 2 and 3. Table S3 displays the genotypes list for each cluster. Details of seedling traits are presented in Fig. 1; BG—BARI Gom, AS—ACI Seed. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The average RV of all seedling trait was highest in Cluster 1 (Table 1). Trait mean values for individual genotypes and relative values (RVs) within clusters are presented in Table S4 and Table S5, respectively. Thylakoid membrane damage (TMD) was determined for the genotypes of each cluster. Cluster 1 genotypes showed the lowest TMD (4.51%), followed by Clusters 3 (6.11%) and 2 (8.36%) (Table 1).
Table 1.
Comparative profiles of the three hierarchically clustered groups of 80 genotypes of wheat (the means of the relative values [RV] for genotypes of individual cluster are represented by the cluster figures).
| Seedling traits | Average RV of clusters |
||
|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | |
| Number of genotypes | 33 | 18 | 29 |
| Shoot fresh weight (SFW) | 0.726 | 0.362 | 0.579 |
| Root fresh weight (RFW) | 0.707 | 0.388 | 0.425 |
| Shoot dry weight (SDW) | 0.829 | 0.747 | 0.714 |
| Root dry weight (RDW) | 0.925 | 0.647 | 0.624 |
| Shoot tissue water content (STWC) | 0.967 | 0.715 | 0.935 |
| Root-shoot weight ratio (RSR) | 1.130 | 0.867 | 0.880 |
| Efficiency of the water-splitting complex on the donor side of PSII (Fv/Fo) | 0.834 | 0.748 | 0.775 |
| Maximum photochemical efficiency of PSII photochemistry (Fv/Fm) | 0.955 | 0.916 | 0.939 |
| Chlorophyll a content (chl a) | 0.859 | 0.794 | 0.582 |
| Chlorophyll b content (chl b) | 0.882 | 0.834 | 0.625 |
| Total chlorophyll content (chl T) |
0.866 |
0.803 |
0.594 |
| Thylakoid membrane damage (TMD) (%) | 4.51 | 8.36 | 6.11 |
3.5. Genotypic variability in the extracted clusters
The results obtained from cluster analysis revealed that the genotypes in Cluster 1 performed substantially better under heat stress condition followed by Cluster 2, while the genotypes of Cluster 3 performed poorly under the same temperature regime (Fig. 5 and Table 2). SFW and RFW were less affected by heat stress in Cluster 1 (reduced by 28 and 37%, respectively), while a drastic reduction was noticed in Cluster 2 by 69 and 70%, respectively. The reduction pattern of SFW and RFW in Cluster 3 was not aligned with other clusters and showed a moderately lower reduction in SFW (43%), but a greater reduction in RFW (69%).
Fig. 5.
Spider plot demonstrating the changes in studied attributes of the genotypes of various clusters as affected by high temperature stress. The values are given as a percentage of the control. Details of seedling traits are presented in Fig. 1.
Table 2.
Mean values of assessed seedling characteristics under both growing conditions and their variation (%) due to heat stress from control of three clusters.
| Cluster | Treatment | SFW | RFW | SDW | RDW | STWC | RSR | Fv/F0 | Fv/Fm | chl a | chl b | chl T |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 | Control | 314.78 | 57.47 | 50.36 | 16.30 | 0.837 | 0.328 | 3.315 | 0.766 | 0.795 | 0.300 | 1.251 |
| Heat | 226.79 | 36.14 | 41.79 | 15.09 | 0.809 | 0.368 | 2.763 | 0.731 | 0.691 | 0.262 | 1.089 | |
| % change | (−)27.95a | (−)37.13a | (−)17.01a | (−)7.39a | (−)3.33a | (+)12.19a | (−)16.66b | (−)4.50a | (−)13.17a | (−)12.41a | (−)12.95a | |
| Cluster 2 | Control | 316.65 | 82.13 | 51.12 | 19.99 | 0.828 | 0.392 | 2.882 | 0.730 | 0.676 | 0.309 | 1.127 |
| Heat | 98.67 | 24.67 | 36.99 | 12.24 | 0.585 | 0.337 | 2.146 | 0.670 | 0.530 | 0.246 | 0.889 | |
| % change | (−)68.84c | (−)69.96b | (−)27.63b | (−)38.78b | (−)29.25b | (−)14.01b | (−)25.56b | (−)8.28b | (−)21.58b | (−)20.30b | (−)21.17b | |
| Cluster 3 | Control | 295.11 | 87.87 | 49.51 | 22.07 | 0.827 | 0.446 | 3.769 | 0.786 | 0.756 | 0.291 | 1.197 |
| Heat | 167.35 | 26.87 | 35.57 | 12.75 | 0.772 | 0.380 | 2.939 | 0.739 | 0.440 | 0.180 | 0.708 | |
| % change | (−)43.29b | (−)69.42b | (−)28.15b | (−)42.23b | (−)6.57a | (−)14.87b | (−)22.02b | (−)6.04ab | (−)41.79c | (−)38.36c | (−)40.82c |
According to Tukey's HSD, mean % change values in a column with different superscript letter(s) are statistically different at p ≤ 0.05. The (+) and (−) signs indicate the percent increase and decrease, respectively regarding the effect of heat stress treatment over control. Details of seedling traits are presented in Fig. 1.
The genotypes of Cluster 1 were least affected in terms of SDW with a reduction of 17% while greater reduction was noticed in Clusters 2 and 3 with an average decline of 28% (Fig. 5 and Table 2). The effect of heat stress was less in the case of RDW in Cluster 1 (7% decrease); however, a major reduction was noticed in the rest of the clusters, with a mean decline of 40%. The shoot tissue water content (STWC) was little affected by heat stress in Cluster 1 (3% decrease), followed by Cluster 3 (7%) while a profound reduction occurred in Cluster 2 (29%) under heat stress over control. Contrarily, the RSR increased by 12% in Cluster 1 due to heat stress over control, while it reduced in the other clusters with an average of 14% (Fig. 5 and Table 2).
The degree of variation and heat stress sensitivity was very little in case of Fv/Fm, whereas Fv/Fo was less affected in Cluster 1 (17%) than rest of the clusters (an average of 24%) (Fig. 5 and Table 2). Heat stress affected the pigment contents (viz. chl a, chl b, and chl T) in similar magnitudes. Cluster 1 exhibited the least decline in pigment content (an average decrease of approximately 13%), followed by Cluster 2 (approximately 21%) (Fig. 5 and Table 2) The genotypes of Cluster 3 were severely affected in terms of pigment contents under heat stress (a mean decline of about 40%). Cluster 1 comprised of all released wheat varieties except Pavon-76, along with 15 accessions and 2 mutants. Cluster 2 and 3 contained a similar diversity of accessions and mutants, with Cluster 3 including the additional category of advanced lines.
3.6. Linear discriminant analysis
Linear discriminant analysis (LDA) is a technique used to reclassify genotypes based on previous classification criteria and to minimize the dimensionality of a dataset while preserving maximum information. Table S6 represents the coefficients of the studied traits with the linear discriminant functions (LD), and the coefficients are ordered by their absolute size. The LD1 explained about 58% of total variation, and the seedling trait chl T was ranked first with the maximum coefficient value (37.52). The coefficient values of other traits like chl a (−34.86), Fv/Fm (17.21), chl b (−10.92), Fv/Fo (4.12), RDW (−7.90), and SDW (4.42) also specified their leading role in explaining the variation under LD1.
On the other hand, 42% of total variation is explained by LD2, and was markedly contributed by Fv/Fm (22.53), chl T (9.48), STWC (9.31), chl a (−9.28), chl b (−3.52), and RDW (2.07 (Table S6). From the stepwise linear discriminant analysis, it can be concluded that seedling traits like chl T, chl a, Fv/Fm, chl b, Fv/Fo, STWC, RDW, and SDW played the most critical role to explain the variability of 80 diverse wheat genotypes under heat stress conditions.
3.7. Validation of genotype clustering using LDA
After performing cluster analysis, the wheat genotypes placed in individual clusters were confirmed with the aid of the prognostic capability of LDA. On the basis of LDA, wheat genotypes present in the preceding clusters were examined, matched, and re-allocated misclassified genotypes into the true groups (Table 3). About 97, 89, and 90% of the wheat genotypes were appropriately allotted in Clusters 1, 2, and 3, respectively, as per the results obtained from the LDA. The mean correctness of the genotypes in assigning various clusters was 93% (Table 3).
Table 3.
Matrix of classification based on LDA for three groups of 80 wheat genotypes (columns representing the anticipated category and rows showing the observed category).
| Clusters | True Groups |
Total No. Observed | ||
|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | ||
| Cluster 1 | 32 | 1 | 3 | 36 |
| Cluster 2 | 0 | 16 | 0 | 16 |
| Cluster 3 | 1 | 1 | 26 | 28 |
| Total Number | 33 | 18 | 29 | 80 |
| Number Corrects | 32 | 16 | 26 | 74 |
| % correctness | 97.0 | 88.9 | 89.7 | 92.5 |
3.8. Mahalanobis distance matrix
The LDA was also adopted to determine the Mahalanobis squared distance (D2) among the clusters. Clusters 1 and 2 were the least distant, with 7.63 units, indicated that these clusters were most similar in this exploratory experiment, followed by greater distance of Clusters 2 and 3 (9.41 units) in the distance matrix (Table 4). The genotypes those performed poorly under heat stress were allotted in Cluster 3.
Table 4.
Pairwise Mahalanobis squared distances (D2) among three clusters of wheat genotypes.
Distances that are significantly different from zero at a 95 % confidence level.
3.9. Selection of heat-tolerant genotypes using MGIDI
The multi-trait genotype-ideotype distance index (MGIDI) was calculated using the selected traits from PCA to identify the heat-tolerant genotypes. Interestingly, a very significant effect of genotypes (p < 0.05) was found for all selected traits (data not shown). Broad-sense heritability (h2) was extended from 0.53 (RSR) to 0.97 (STWC) for different traits, with an average of 0.773 (Table 5). These high heritability estimates suggest that these traits are good candidates for wheat improvement through selection. Among the selected traits, RFW, SFW, RDW, chl a, chl T, STWC, SDW, chl b, Fv/Fo, and RSR showed greater genetic gains, ranging from 5.4% for Fv/Fo to 50.9% for RFW (Table 5). Generally, the MGIDI resulted in a higher total gain, i.e., 147.4% for all variables assumed to be increased in the present study. Factor analysis (FA) grouped the 11 traits into five distinct factors (Table 5): FA1− SDW; FA2− chl a, chl b, and chl T; FA3− Fv/Fo and Fv/Fm; FA4− RFW, RDW, and RSR; and FA5− SFW and STWC.
Table 5.
Estimated genetic gain for the PCA-selected seedling characteristics in the MGIDI.
| Factor | Traits | Goal | Broad-sense heritability (h2) | Selection gain (%) |
|---|---|---|---|---|
| FA1 |
Shoot dry weight (SDW) |
Increase |
0.741 |
6.8 |
| FA2 | Chlorophyll a (chl a) | Increase | 0.662 | 9.5 |
| FA2 | Chlorophyll B (chl b) | Increase | 0.554 | 6.3 |
| FA2 |
Total chlorophyll (chl T) |
Increase |
0.646 |
9.2 |
| FA3 | Efficiency of the water-splitting complex on the donor side of PSII (Fv/Fo) | Increase | 0.936 | 5.4 |
| FA3 |
Maximum photochemical efficiency of PSII photochemistry (Fv/Fm) |
Increase |
0.960 |
1.8 |
| FA4 | Root fresh weight (RFW) | Increase | 0.892 | 50.9 |
| FA4 | Root dry weight (RDW) | Increase | 0.698 | 16.9 |
| FA4 |
Root-shoot weight ratio (RSR) |
Increase |
0.527 |
6.0 |
| FA5 | Shoot fresh weight (SFW) | Increase | 0.921 | 26.7 |
| FA5 | Shoot tissue water content (STWC) | Increase | 0.967 | 7.9 |
The MGIDI identified twelve heat-tolerant and -stable genotypes from this investigation: BG-30, BD-468, BG-24, BD-9908, BG-32, BD-476, BD-594, BD-553, BD-488, BG-33, BD-495, and AS-10627 (Fig. 6A). Interestingly, all these selected genotypes originated from Cluster 1, the highest performing group identified earlier from hierarchical cluster analysis (Fig. 4). Additionally, genotype BG-26, while not selected by MGIDI, was very close to the selection threshold and also belongs to Cluster 1. This highlights BG-26 as a potentially interesting candidate for further evaluation. Fig. 6B presents the relative strengths and weaknesses of studied genotypes, as determined by the contribution of each factor to the MGIDI value for each genotype. Genotypes (BD-594, AS-10627, BD-553, BD-488, BG-33, BD-476, and BD-495) with minimal contribution from SDW (FA1) in MGIDI analysis likely excel under heat stress due to optimized seedling growth (Fig. 6B, Table 5). Conversely, genotypes (BD-468, BG-30, BG-24, BD-594, and BD-488) with minimal contribution from pigment content (FA2) in the MGIDI analysis likely possess efficient light capture mechanisms (Fig. 6B, Table 5). Similarly, minimal contribution from fluorescence features (FA3) for genotypes BG-33, BD-468, BD-594, and BD-553 might indicate better thylakoid membrane function (Fig. 6B, Table 5). Minimal contribution from root traits (FA4) for genotypes BG-24, BD-553, and BG-30 suggests efficient root-shoot coordination (Fig. 6B, Table 5). Finally, genotypes (AS-10627, BG-24, BD-9908, BG-32, BD-553, BD-488, and BD-495) with minimal contribution from SFW and STWC (FA5) in the MGIDI analysis likely exhibit improved water retention in shoots (Fig. 6B, Table 5).
Fig. 6.
MGIDI analysis for genotype ranking in increasing order (A). The red color highlights the top-ranked and selected genotypes. Based on the selection pressure, the cut point is represented by the central red circle. The percentage of each factor on the calculated MGIDI index represents the genotype's strengths and weaknesses (B). The more closely a factor's characteristics resemble the ideotype, the less the proportion of the factor's explanation (nearer the outside border). The theoretical value, assuming equal contributions from all components, is indicated by the dashed line. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.10. Multiple linear regression
Multiple linear regression analysis was executed to assess the effect of the examined seedling traits on shoot dry matter accumulation (SDW). The SDW was significantly impacted by other seedling traits under heat stress conditions compared to the control group (Table 6). Results indicated that SFW, RFW, RDW, STWC, RSR, and chl T significantly influenced the SDW in both growing conditions, while SDW was substantially affected by chl a under heat stress (Table 6).
Table 6.
Multiple linear regression to evaluate the effect on seedling traits on shoot dry weight (SDW) under both growing conditions (n = 80).
| Seedling traits | Control |
Heat stress |
||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | Std. error | P | R2 | Coefficient | Std. error | P | R2 | |
| Intercept | 144.6 | 16.7 | <0.001 | 0.977 | 52.84 | 12.74 | <0.001 | 0.968 |
| SFW | 0.103 | 0.009 | <0.001 | 0.027 | 0.012 | 0.03 | ||
| RFW | 0.03 | 0.009 | 0.002 | −0.101 | 0.032 | 0.002 | ||
| RDW | 1.15 | 0.155 | <0.001 | 2.53 | 0.148 | <0.001 | ||
| STWC | −132.8 | 12.8 | <0.001 | −13.33 | 5.65 | 0.021 | ||
| RSR | −0.52 | 8.57 | <0.001 | −75.32 | 6.18 | <0.001 | ||
| Fv/Fo | 2.91 | 1.68 | 0.09 | 3.69 | 2.16 | 0.091 | ||
| Fv/Fm | −40.0 | 27.8 | 0.19 | −35.37 | 26.55 | 0.187 | ||
| chl a | 2.95 | 1.52 | 0.06 | 5.16 | 2.02 | 0.013 | ||
| chl b | 2.37 | 3.78 | 0.53 | −2.17 | 4.75 | 0.65 | ||
| chl T | 8.002 | 2.94 | 0.008 | 7.69 | 3.11 | 0.016 | ||
4. Discussion
A week-long heat stress significantly affected the morpho-physiological attributes of wheat genotypes, with almost all seedling traits exhibiting decreases compared to the control (Fig. 1). These findings align with previous reports on wheat exposed to elevated temperatures [72,73]. To elucidate heat stress tolerance mechanisms in wheat seedlings and identify promising genotypes for further research, selecting key traits through a well-considered multivariate approach is crucial at the seedling stage.
Principal component analysis (PCA) is a valuable statistical technique to lessen the dimensionality and gathering expressive information from a vastly connected complex dataset [74]. It also offers a data-driven approach to exploring relationships between genotypes and traits under heat stress, as demonstrated in various crops [72,75]. In PCA biplots, the angle between trait vectors indicates their correlation. Acute angles (<90°) characterize positive correlations, obtuse angles (>90°) indicate negative correlations, and the right angles (90°) suggest no correlation between the traits. PCA biplot emerged as a successful tool for evaluating crop tolerance to various abiotic stresses [72,76,77]. Investigating the intrinsic patterns and relationships among variables is a key application of PCA. This capability assists researchers to identify potential redundancies and irrelevant features within a dataset. This study employed PCA to identify seedling traits significantly influencing heat stress tolerance. Six significant principal components (eigenvalues >1) were identified in PCA capturing most of the variation in the seedling trait data. By focusing on traits contributing at least 5% to these components, we effectively selected relevant traits for further analysis (Fig. S2). PCA-identified traits guided the selection of the top-performing and tolerant genotypes using hierarchical cluster analysis (Fig. 4) and the multi-trait genotype-ideotype distance index (MGIDI) (Fig. 6).
Correlation studies reveal the type and strength of relationships between traits, aiding plant breeders in selecting desirable varieties with preferred characteristics [78]. Our study found significant associations among most seeding traits, demonstrating their combined influence on wheat plant responses to heat stress. This aligns with previous report suggesting that selecting a single trait can have unintended impact on others [79].
In the present study, enhanced accumulation of biomass (dry weights of shoot and root) and tissue water content appear to be crucial factors for genotypes grouped in the superior-performing Cluster 1. This aligns with the established principle that tolerance to stressful environments is often linked to dry matter accumulation in seedlings [75]. The ability of seedlings to maintain dry weight under heat stress further strengthens this connection, serving as a good indicator of thermotolerance [80]. Our study revealed that Cluster 1 genotypes exhibited minimal reduction in fresh and dry weights of root and shoot, indicating their superior ability to mitigate heat stress impacts compared to Clusters 2 and 3 (Fig. 5, Table 2). Heat stress disrupts the root-shoot weight ratio (RSR) in plants, likely due to a shift in resource allocation to cope with elevated temperatures. This shift occurs because heat stress reduces photosynthetic activity, limiting the carbohydrates available for root growth while favoring shoot maintenance [18]. Our study confirmed this, revealing that heat stress significantly reduced RSR in relatively susceptible genotypes (Fig. 5, Table 2), emphasizing their vulnerability in resource allocation under stress. In contrast, genotypes in Cluster 1 exhibited a higher RSR compared to other clusters, suggesting these genotypes possess a potential advantage in resource allocation under heat stress. A greater RSR suggests that root growth is less impacted than shoot growth in these resilient genotypes.
The water-splitting efficiency on the donor side of PSII (Fv/Fo) and maximum photochemical efficiency of PSII photochemistry (Fv/Fm) are commonly used to assess heat-induced impairment to PSII [31,81]. This study revealed significant declines in Fv/Fo and Fv/Fm values, with a more pronounced decrease in Fv/Fo compared to Fv/Fm, indicating the higher sensitivity of the former feature [50]. A decreased Fv/Fm remains a crucial indicator for assessing damage in chloroplasts, particularly within thylakoid membranes under abiotic stress conditions [82]. Thylakoid lamellae, the sites of photosynthetic reactions, are severely damaged under heat stress due to impairment of PSII reaction centers, particularly D1 protein, which undergoes structural alterations [83,84]. In the current study, Cluster 1 genotypes exhibited higher Fv/Fm and Fv/Fo values compared to Clusters 2 and 3, strongly indicating less damage in the thylakoid membrane (TMD) (Table 1). Genotypes with lower TMD can maintain physiological active during heat stress [31] and undergo crucial chloroplast adjustments, including altered thylakoid organization, grana stacking disruption, swelling [85,86].
Plant pigment systems, including chlorophyll a, b, and total chlorophyll, serve as indicators of stress tolerance levels [87,88]. Disruption of the thylakoid membrane due to heat stress decreases chlorophyll contents [9]. Additionally, elevated temperatures can increase the activity of enzymes like peroxidase and chlorophyllase, further accelerating chlorophyll degradation [9,30,89,90]. Our study confirmed these findings, recording significant decreases in chlorophyll content across all genotypes due to heat stress (Table 2). However, genotypes in Cluster 1 exhibited a lower reduction in chlorophyll content compared to other clusters, suggesting their potential for improved thermotolerance.
Hierarchical cluster analysis is an efficient method used by researchers to categorize genotypes based on their similarity across various stress tolerance indices [91,92]. In the present investigation, the analysis revealed distinct clusters with varying stress tolerance potentials. Cluster 1 genotypes exhibited maximum heat stress tolerance potential, likely due to contributions from most of the seedling traits (Fig. 4, Fig. 5). In contrast, Cluster 2 excels in shoot and root dry matter accumulation and pigment contents. However, Cluster 3 genotypes are characterized by moderate reduction in shoot fresh and dry mass contents, chlorophyll fluorescence features, and shoot tissue water content. Linear discriminant analysis (LDA) is a powerful tool that specifically targets enhancing the distinction between different classes by identifying the features that optimally discriminate between them [99]. This makes LDA a valuable tool for validating the effectiveness of clustering performed with methods like hierarchical clustering. In our study, we employed LDA to assess the quality of the clusters, particularly focusing on the placement of genotypes within the clusters. Our results were highly encouraging, with LDA achieving overall accuracy of 93% in assigning genotypes to their respective clusters, indicating minimal misclassification errors in the initial clustering. Furthermore, genotypes belonging to Cluster 1, identified as the best performing group, exhibited an even higher accuracy of 97% with LDA, further strengthening the confidence in its placement.
Cluster analysis has several limitations that can hinder the selection of tolerant genotypes. These include subjectivity interpretation, inadequate dimensionality reduction, challenges in handling missing data, lack of statistical rigor, and the inability to account for interaction effects [93]. To overcome these limitations, integrating other analytical techniques is crucial. For example, combining cluster analysis with quantitative indices like the multi-trait genotype-ideotype distance index (MGIDI) can facilitate the identification of tolerant genotypes. MGIDI enables an inclusive assessment of genotypes across multi-trait simultaneously, reducing the risk of overlooking crucial traits. MIGIDI involves collecting data on multiple traits for each genotype, defining an ideotype based on optimal values, normalizing and weighting traits, calculating remoteness between genotypes and the ideotype, and ranking genotypes based on distance to the ideotype. The smallest distances are preferred to represent the perfect combination of traits. This quantitative tool evaluates multiple traits to select tolerant genotypes with optimal stress tolerance, while simultaneously comparing genotypes to an ideotype, thereby enhancing breeding efficiency by curtailing time and supplies requisite to develop stress-tolerant varieties [94]. For the MGIDI analysis, all PCA-selected traits were assigned equal weights in this study, assuming positive values were optimal. This approach was justified by the substantial influence of these traits on genotype variability as determined by PCA. All selected traits met the desired goals, and MGIDI analysis indicated positive selection gains for all traits studied (Table 5). In the present study, genotypes in Cluster 1 displayed the highest heat stress tolerance potential due to the favorable contribution of most seedling traits (Fig. 4, Fig. 5). This finding was further supported by MGIDI analysis, which directly and precisely identified twelve highly heat-tolerant and stable genotypes (BG-30, BD-468, BG-24, BD-9908, BG-32, BD-476, BD-594, BD-553, BD-488, BG-33, BD-495, and AS-10627) (Fig. 6). Notably, all these genotypes originated from the top performing Cluster 1 (Fig. 4, Fig. 6). These findings highlight the prospects of the selected genotypes, suggesting their suitability for prioritization in breeding programs. Their superior performance under heat stress conditions makes them strong candidates for further evaluation and advancement, as they are more likely to exhibit the desired performance under warmer environments. The greater predictability, stability, broad-sense heritability (h2), and selection gain of traits can enhance breeders' ability to evaluate the effectiveness of selection strategies [[95], [96], [97]]. MGIDI analysis revealed that traits like STWC, Fv/Fo, SFW, RFW, SDW, RDW, chl a, and chl T exhibited higher h2 and selection gain, indicating their potential as breeding targets (Table 5).
A crucial prerequisite for assessing plant's overall performance is increased biomass output, measured by dry matter accumulation, especially under stressful conditions [98]. The inclusion of shoot dry weight (SDW) in both hierarchical clustering and MGIDI analysis underscores the importance of this vital trait. Further supporting this notion, multiple linear regression analysis revealed that SDW was significantly influenced by traits such as SFW, RFW, RDW, STWC, RSR, chl a, and chl T under heat stress condition (Table 6). Despite its non-significant relationship with biomass, Fv/Fo could be a potential marker for thermotolerance due to its high h2 and modest selection gain (Table 5). Notably, it exhibited a significantly positive association with STWC under heat stress (Fig. 3B), a crucial factor for membrane fluidity, a prerequisite for heat stress tolerance [99]. Although RSR showed low h2 in this study, its modest selection gain and significant association with SDW suggest its potential as a target for wheat improvement (Table 5, Table 6). Collectively, a comparative analysis of h2, selection gain, and multiple linear regression analysis identified RFW, SFW, RDW, SDW, RSR, STWC, chl a, chl T, and Fv/Fo as key seedling traits for effectively discussing heat stress tolerance.
5. Conclusions
In conclusion, our study demonstrated that heat stress significantly impacted seedling performance. Multivariate analysis approaches proved to be powerful tools for this exploratory investigation. PCA effectively unmasked the seedling traits that exerted the greatest influence to the genotypic variability. Most of the PCA-selected traits were significantly correlated with each other. Hierarchical cluster analysis enabled us to categorize the genotypes according to their tolerance to heat stress. The genotypes in Cluster 1 exhibited the highest level of heat stress tolerance relative to clusters 2 and 3. LDA confirmed a high classification accuracy, with 93% of genotypes correctly assigned to their respective hierarchical clusters. MGIDI analysis successfully identifying twelve highly heat-tolerant and stable genotypes: BG-30, BD-468, BG-24, BD-9908, BG-32, BD-476, BD-594, BD-553, BD-488, BG-33, BD-495, and AS-10627, all of them originated from the tolerant Cluster 1. The multiple linear regression analysis revealed that root and shoot fresh and dry weights, tissue water content, root-shoot weight ratio, and pigment contents had significant association with seedling biomass. The selected tolerant genotypes and identified crucial traits (RFW, SFW, RDW, SDW, RSR, STWC, chl a, chl T, and Fv/Fo) explaining heat stress tolerance derived from this experiment will assist breeders in developing heat-tolerant wheat genotypes for warmer environments. These heat-tolerant genotypes hold promise for developing practical solutions in sustainable agriculture, potentially mitigating the consequences of climate change on wheat production. To fully harness the potential of these thermotolerant genotypes, collaborative efforts among researchers, breeders, and policymakers are highly necessary.
Funding
We gratefully acknowledge the partial funding granted by the Bangabandhu Science and Technology Fellowship Trust, under the Ministry of Science and Technology, Government of the People's Republic of Bangladesh, for this study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Md. Mehedi Hasan: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Conceptualization. Md. Abdul Baset Mia: Writing – review & editing, Supervision, Resources, Project administration, Investigation, Conceptualization. Jalal Uddin Ahmed: Writing – review & editing, Supervision, Methodology, Conceptualization. M. Abdul Karim: Writing – review & editing, Supervision, Methodology. A.K.M. Aminul Islam: Writing – review & editing, Validation, Supervision. Mohammed Mohi-Ud-Din: Writing – review & editing, Visualization, Methodology, Investigation, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research article is a component of the first author's doctoral dissertation. We thankfully admit the invaluable support provided by the Advanced Plant Physiology Laboratory under the Department of Crop Botany of Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur-1706, Bangladesh, for facilitating the completion of this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e38623.
Contributor Information
Md. Abdul Baset Mia, Email: miabaset@bsmrau.edu.bd.
Mohammed Mohi-Ud-Din, Email: mmu074@bsmrau.edu.bd.
Appendix A. Supplementary data
The following is the supplementary data to this article.
References
- 1.IPCC . In: Climate Change 2021: the Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Masson-Delmotte V., Zhai P., Pirani A., Connors S.L., Péan C., Berger S., Caud N., Chen Y., Goldfarb L., Gomis M.I., Huang M., Leitzell K., Lonnoy E., Matthews J.B.R., Maycock T.K., Waterfield T., Yelekçi O., Yu R., Zhou B., editors. Cambridge University Press; Cambridge, United Kingdom and New York, NY, USA: 2021. Summary for policymakers; pp. 3–32. [DOI] [Google Scholar]
- 2.Zabel F., Müller C., Elliott J., Minoli S., Jägermeyr J., Schneider J.M., Franke J.A., Moyer E., Dury M., Francois L., Folberth C., Liu W., Pugh T.A.M., Olin S., Rabin S.S., Mauser W., Hank T., Ruane A.C., Asseng S. Large potential for crop production adaptation depends on available future varieties. Glob. Chang. Biol. 2021;27:3870–3882. doi: 10.1111/gcb.15649. [DOI] [PubMed] [Google Scholar]
- 3.BBS Yearbook of agricultural statistics-2021, 33 rd series. 2022. www.bbs.gov.bd
- 4.Mohi-Ud-Din M., Rohman M.M., Alam M.A., Hasanuzzaman M., Islam T. Wheat variety carrying 2NvS chromosomal segment provides yield advantage through lowering terminal heat–induced oxidative stress. Protoplasma. 2023;260:63–76. doi: 10.1007/s00709-022-01759-w. [DOI] [PubMed] [Google Scholar]
- 5.Erenstein O., Jaleta M., Mottaleb K.A., Sonder K., Donovan J., Braun H.-J. vol. 11. Springer Springer Nature; Switzerland AG, Gewerbestrasse: 2022. Global trends in wheat production, consumption and trade; pp. 47–66. (Wheat Improv. Food Sec. In a Chang. Cli.). 6330 Cham, Switzerland. [DOI] [Google Scholar]
- 6.Grote U., Fasse A., Nguyen T.T., Erenstein O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021;4:1–17. doi: 10.3389/fsufs.2020.617009. [DOI] [Google Scholar]
- 7.Akter N., Islam M.R. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017;37(37):1–17. doi: 10.1007/s13593-017-0443-9. [DOI] [Google Scholar]
- 8.Khan A., Ahmad M., Shah M.K.N., Ahmed M. Performance of wheat genotypes for morpho-physiological traits using multivariate analysis under terminal heat stress. Pakistan J. Bot. 2020;52(6):1981–1988. doi: 10.30848/PJB2020-6(30). [DOI] [Google Scholar]
- 9.Mohi‐ud‐din M., Siddiqui M.N., Rohman M.M., Jagadish S.V.K., Ahmed J.U., Hassan M.M., Hossain A., Islam T. Physiological and biochemical dissection reveals a trade‐off between antioxidant capacity and heat tolerance in bread wheat (Triticum aestivum L.) Antioxidants. 2021;10:351. doi: 10.3390/antiox10030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Divya B., Sandeep G., Meenakshi Amit, Yogesh K. Effects of high-temperature stress on crop plants. Res. J. Biotechnol. 2023;18:7 157–172. doi: 10.25303/1807rjbt1570172. [DOI] [Google Scholar]
- 11.Mishra S., Spaccarotella K., Gido J., Samanta I., Chowdhary G. Effects of heat stress on plant-nutrient relations: an update on nutrient uptake, transport, and assimilation. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms242115670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samalova M., Gahurova E., Hejatko J. Expansin-mediated developmental and adaptive responses: a matter of cell wall biomechanics? Quant. Plant Biol. 2022;3:e11. doi: 10.1017/qpb.2022.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijewardene I., Shen G., Zhang H. Enhancing crop yield by using rubisco activase to improve photosynthesis under elevated temperatures. Stress Biol. 2021;1:2. doi: 10.1007/s44154-021-00002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-García M.P., Conesa C.M., Lozano-Enguita A., Baca-González V., Simancas B., Navarro-Neila S., Sánchez-Bermúdez M., Salas-González I., Caro E., Castrillo G., del Pozo J.C. Temperature changes in the root ecosystem affect plant functionality. Plant Commun. 2023;4 doi: 10.1016/j.xplc.2022.100514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masson P., Lushchekina S. Conformational stability and denaturation processes of proteins investigated by electrophoresis under extreme conditions. Molecules. 2022;27:6861. doi: 10.3390/molecules27206861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav M.R., Choudhary M., Singh J., Lal M.K., Jha P.K., Udawat P., Gupta N.K., Rajput V.D., Garg N.K., Maheshwari C., Hasan M., Gupta S., Jatwa T.K., Kumar R., Yadav A.K., Vara Prasad P.V. Impacts, tolerance, adaptation, and mitigation of heat stress on wheat under changing climates. Int. J. Mol. Sci. 2022;23:2838. doi: 10.3390/ijms23052838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasanuzzaman M., Nahar K., Alam M., Roychowdhury R., Fujita M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013;14:9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan M.U., Rasool T., Iqbal C., Arshad A., Abrar M., Abrar M.M., Habib-ur-Rahman M., Noor M.A., Sher A., Fahad S. Linking plants functioning to adaptive responses under heat stress conditions: a mechanistic review. J. Plant Growth Regul. 2022;41:2596–2613. doi: 10.1007/s00344-021-10493-1. [DOI] [Google Scholar]
- 19.Ferguson J.N., McAusland L., Smith K.E., Price A.H., Wilson Z.A., Murchie E.H. Rapid temperature responses of photosystem II efficiency forecast genotypic variation in rice vegetative heat tolerance. Plant J. 2020;104:839–855. doi: 10.1111/tpj.14956. [DOI] [PubMed] [Google Scholar]
- 20.Gao C.H., Sun M., Anwar S., Feng B., Ren A.X., Lin W., Gao Z.Q. Response of physiological characteristics and grain yield of winter wheat varieties to long-term heat stress at anthesis. Photosynthetica. 2021;59:4 640–651. doi: 10.32615/ps.2021.060. [DOI] [Google Scholar]
- 21.Mahdavi S., Arzani A., Maibody S.A.M.M., Mehrabi A.A. Photosynthetic and yield performance of wheat (Triticum aestivum L.) under sowing in hot environment. Acta Physiol. Plant. 2021;43:106. doi: 10.1007/s11738-021-03278-2. [DOI] [Google Scholar]
- 22.Sharma D.K., Torp A.M., Rosenqvist E., Ottosen C.-O., Andersen S.B. QTLs and potential candidate genes for heat stress tolerance identified from the mapping populations specifically segregating for Fv/Fm in wheat. Front. Plant Sci. 2017;8:1668. doi: 10.3389/fpls.2017.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swoczyna T., Kalaji H.M., Bussotti F., Mojski J., Pollastrini M. Environmental stress - what can we learn from chlorophyll a fluorescence analysis in woody plants? A review. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.1048582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalaji H.M., Schansker G., Brestic M., Bussotti F., Calatayud A., Ferroni L., Goltsev V., Guidi L., Jajoo A., Li P., Losciale P., Mishra V.K., Misra A.N., Nebauer S.G., Pancaldi S., Penella C., Pollastrini M., Suresh K., Tambussi E., Yanniccari M., Zivcak M., Cetner M.D., Samborska I.A., Stirbet A., Olsovska K., Kunderlikova K., Shelonzek H., Rusinowski S., Bąba W. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017;132:13–66. doi: 10.1007/s11120-016-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.G. Schansker, Chlorophyll fluorescence and photosynthesis: Simple experiments with the JUNIOR-PAM chlorophyll fluorometer, 2020. Heinz Walz GmbH, Eichenring 6, 91090 Effeltrich, Germany. www.walz.com.
- 26.Muhammad I., Shalmani A., Ali M., Yang Q.-H., Ahmad H., Li F.B. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 2021;11 doi: 10.3389/fpls.2020.615942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar R.R., Tasleem M., Jain M., Ahuja S., Goswami S., Bakshi S., Jambhulkar S., Singh S.D., Singh G.P., Pathak H., Viswanathan C., Praveen S. Nitric oxide triggered defense network in wheat: augmenting tolerance and grain-quality related traits under heat-induced oxidative damage. Environ. Exp. Bot. 2019;158:189–204. doi: 10.1016/j.envexpbot.2018.11.016. [DOI] [Google Scholar]
- 28.Iqbal H., Yaning C., ur Rehman H., Waqas M., Ahmed Z., Raza S.T., Shareef M. Improving heat stress tolerance in late planted spring maize by using different exogenous elicitors. Chil. J. Agric. Res. 2020;80(1):30–40. doi: 10.4067/S0718-58392020000100030. [DOI] [Google Scholar]
- 29.Medina E., Kim S.-H., Yun M., Choi W.-G. Recapitulation of the function and role of ROS generated in response to heat stress in plants. Plants. 2021;10:371. doi: 10.3390/plants10020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu S., Ding Y., Zhu C. Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 2020;11:375. doi: 10.3389/fpls.2020.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moradpour M., Abdullah S.N.A., Namasivayam P. The impact of heat stress on morpho-physiological response and expression of specific genes in the heat stress-responsive transcriptional regulatory network in Brassica oleracea. Plants. 2021;10:1064. doi: 10.3390/plants10061064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langridge P., Reynolds M. Breeding for drought and heat tolerance in wheat. Theor. Appl. Genet. 2021;134:1753–1769. doi: 10.1007/s00122-021-03795-1. [DOI] [PubMed] [Google Scholar]
- 33.Rehman H.U., Tariq A., Ashraf I., Ahmed M., Muscolo A., Basra S.M.A., Reynolds M. Evaluation of physiological and morphological traits for improving spring wheat adaptation to terminal heat stress. Plants. 2021;10:455. doi: 10.3390/plants10030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhai H., Jiang C., Zhao Y., Yang S., Li Y., Yan K., Wu S., Luo B., Du Y., Jin H., Liu X., Zhang Y., Lu F., Reynolds M., Ou X., Qiao W., Jiang Z., Peng T., Gao D., Hu W., Wang J., Gao H., Yin G., Zhang K., Li G., Wang D. Wheat heat tolerance is impaired by heightened deletions in the distal end of 4AL chromosomal arm. Plant Biotechnol. J. 2021;19:5. doi: 10.1111/pbi.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farid M., N, Musa Y., Ridwan I., Fuad Ansho M. Effective screening of tropical wheat mutant lines under hydroponically induced drought stress using multivariate analysis approach. Asian J. Plant Sci. 2021;20:172–182. doi: 10.3923/ajps.2021.172.182. [DOI] [Google Scholar]
- 36.Mohi-Ud-din M., Hossain M.A., Rohman M.M., Uddin M.N., Haque M.S., Ahmed J.U., Hossain A., Hassan M.M., Mostofa M.G. Multivariate analysis of morpho-physiological traits reveals differential drought tolerance potential of bread wheat genotypes at the seedling stage. Plants. 2021;10:879. doi: 10.3390/plants10050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhanda S.S., Munjal R. Genetic diversity in bread wheat for heat tolerance. Ekin J. Crop Breed. Genet. 2017;3(2):60–78. [Google Scholar]
- 38.Cozzolino D., Power A., Chapman J. Interpreting and reporting principal component analysis in food science analysis and beyond. Food Anal. Methods. 2019;12:2469–2473. doi: 10.1007/s12161-019-01605-5. [DOI] [Google Scholar]
- 39.Ullah A., Shakeel A., Ahmed H.G.M.-D., Naeem M., Ali M., Shah A.N., Wang L., Jaremko M., Abdelsalam N.R., Ghareeb R.Y., Hasan M.E. Genetic basis and principal component analysis in cotton (Gossypium hirsutum L.) grown under water deficit condition. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.981369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aslam M., Maqbool M.A., Zaman Q.U., Shahid M., Akhtar M.A., Rana A.S. Comparison of different tolerance indices and PCA biplot analysis for assessment of salinity tolerance in lentil (Lens culinaris) genotypes. Int. J. Agric. Biol. 2017;19:470–478. doi: 10.17957/IJAB/15.0308. [DOI] [Google Scholar]
- 41.Sneha G.R., Annayya, Hembrom B.B., Varghese E., Yadav R.K., Abraham G. Screening and selection of Anabaena spp. for desiccation tolerance through physiological parameters and multivariate analysis. J. Appl. Phycol. 2023;35:1273–1284. doi: 10.1007/s10811-023-02942-z. [DOI] [Google Scholar]
- 42.Olivoto T., Nardino M. MGIDI: toward an effective multivariate selection in biological experiments. Bioinformatics. 2021;37:10 1383–1389. doi: 10.1093/bioinformatics/btaa981. [DOI] [PubMed] [Google Scholar]
- 43.Khatun S., Ahmed J.U., Mollah M.M.I., Taewan K. Physiological mechanism of thermotolerance in wheat (Triticum aestivum Lin.) seedlings. Am. J. Plant Sci. 2018;9:2719–2727. doi: 10.4236/ajps.2018.913198. [DOI] [Google Scholar]
- 44.Mickky B.M. Could sodium benzoate enhance broad bean salinity tolerance? II. germination parameters, carbohydrates, proteins, nucleic acids and hydrolytic enzymes. J. Che. Bio. Phy. Sci. 2016;6(2):351–367. [Google Scholar]
- 45.Hellal F.A., El-Shabrawi H.M., Abd El-Hady M., Khatab I.A., El-Sayed S.A.A., Abdelly C. Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars. J. Genet. Eng. Biotechnol. 2018;16:203–212. doi: 10.1016/j.jgeb.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meher P. Shivakrishna, Ashok Reddy K., Manohar Rao D. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018;25:285–289. doi: 10.1016/j.sjbs.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasool B., ur-Rahman Mahmood, Zubair M., Khan M.A., Ramzani P.M.A., Dradrach A., Turan V., Iqbal M., Khan S.A., Tauqeer H.M., Farhad M., Virk Z.A., Iqbal M. Synergetic efficacy of amending Pb-polluted soil with P-loaded jujube (Ziziphus mauritiana) twigs biochar and foliar chitosan application for reducing Pb distribution in moringa leaf extract and improving its anti-cancer potential, Water. Air. Soil Pollut. 2022;233:344. doi: 10.1007/s11270-022-05807-2. [DOI] [Google Scholar]
- 48.Mohi-Ud-Din M., Talukder D., Rohman M., Ahmed J.U., Jagadish S.V.K., Islam T., Hasanuzzaman M. Exogenous application of methyl jasmonate and salicylic acid mitigates drought-induced oxidative damages in French bean (Phaseolus vulgaris L.) Plants. 2021;10:2066. doi: 10.3390/plants10102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan N.A., Asgher M., Per T.S., Masood A., Fatma M., Khan M.I.R. Ethylene potentiates sulfur-mediated reversal of cadmium inhibited photosynthetic responses in mustard. Front. Plant Sci. 2016;7:1628. doi: 10.3389/fpls.2016.01628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sperdouli I., Andreadis S., Moustaka J., Panteris E., Tsaballa A., Moustakas M. Changes in light energy utilization in photosystem II and reactive oxygen species generation in potato leaves by the pinworm Tuta absoluta. Molecules. 2021;26:2984. doi: 10.3390/molecules26102984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porra R.J., W, Thompson, Kriedemann P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta. 1989;975:384–394. [Google Scholar]
- 52.Wahab M.M.S., Akkareddy S., Shanthi P., Latha P. Identification of differentially expressed genes under heat stress conditions in rice (Oryza sativa L.) Mol. Biol. Rep. 2020;47:1935–1948. doi: 10.1007/s11033-020-05291-z. [DOI] [PubMed] [Google Scholar]
- 53.Bates D., Mächler M., Bolker B.M., Walker S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 54.de Mendiburu F. Universidad Nacional Agraria, La Monila; Peru: 2021. Agricolae Tutorial. [Google Scholar]
- 55.Morales C., Giraldo R., Torres M. Boxplot fences in proficiency testing. Accredit. Qual. Assur. 2021;26:193–200. doi: 10.1007/s00769-021-01474-8. [DOI] [Google Scholar]
- 56.Brenner D.M., Dove L.S., Andrae D.A., Covington P.S., Gutman C., Chey W.D. Radar plots: a novel modality for displaying disparate data on the efficacy of eluxadoline for the treatment of irritable bowel syndrome with diarrhea. Neuro Gastroenterol. Motil. 2018;30 doi: 10.1111/nmo.13331. 8. [DOI] [PubMed] [Google Scholar]
- 57.Liu J., Lu Y., Dai Y., Shen Y., Zeng C., Liu X., Yu H., Deng J., Lu W. A comprehensive analysis and validation of cuproptosis-associated genes across cancers: overall survival, the tumor microenvironment, stemness scores, and drug sensitivity. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.939956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mamen A., Braaum L.E., Fredriksen P.M. Presenting health status in children using a radar plot. Sports. 2020;8:53. doi: 10.3390/sports8040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gewers F.L., Ferreira G.R., De Arruda H.F., Filipi N., Comin C.H., Amancio D.R., Costa L.F. Principal component analysis: a natural approach to data exploration. ACM Comput. Surv. 2021;54(4):1–34. doi: 10.1145/3447755. [DOI] [Google Scholar]
- 60.Bouras H., Choukr-Allah R., Mosseddaq F., Bouaziz A., Devkota K.P., El Mouttaqi A., Bouazzama B., Hirich A. Does phosphorus fertilization increase biomass production and salinity tolerance of blue panicum (Panicum antidotale Retz.) in the salt-affected soils of arid regions? Agronomy. 2022;12:791. doi: 10.3390/agronomy12040791. [DOI] [Google Scholar]
- 61.Wang L., Tang X., Zhang J., Guan D. Correlation analysis for exploring multivariate data sets. IEEE Access. 2018;6:44235–44243. doi: 10.1109/ACCESS.2018.2864685. [DOI] [Google Scholar]
- 62.Olivoto T., Lúcio A.D. metan: an R package for multi‐environment trial analysis. Methods Ecol. Evol. 2020;11:783–789. doi: 10.1111/2041-210X.13384. [DOI] [Google Scholar]
- 63.Kassambara A. Practical guide to principal component methods in R: PCA, M(CA), FAMD, MFA, HCPC, factoextra. 2017;2:STHDA. [Google Scholar]
- 64.Hageman J.A., Malosetti M., van Eeuwijk F.A. Two-mode clustering of genotype by trait and genotype by environment data. Euphytica. 2012;183:349–359. doi: 10.1007/s10681-010-0236-6. [DOI] [Google Scholar]
- 65.Walasek-Janusz M., Grzegorczyk A., Zalewski D., Malm A., Gajcy S., Gruszecki R. Variation in the antimicrobial activity of essential oils from cultivars of Lavandula angustifolia and L. × intermedia. Agronomy. 2022;12:2955. doi: 10.3390/agronomy12122955. [DOI] [Google Scholar]
- 66.Tharwat A., Gaber T., Ibrahim A., Hassanien A.E. Linear discriminant analysis: a detailed tutorial. AI Commun. 2017;30:2 169–190. doi: 10.3233/AIC-170729. [DOI] [Google Scholar]
- 67.Gomes L., Nobre T., Sousa A., Rei F., Guiomar N. Hyperspectral reflectance as a basis to discriminate olive varieties—a tool for sustainable crop management. Sustainability. 2020;12:3059. doi: 10.3390/su12073059. [DOI] [Google Scholar]
- 68.Lahav A., Talmon R. Mahalanobis distance informed by clustering. Inf. Inference A J. IMA. 2019;8:2 377–406. doi: 10.1093/imaiai/iay011. [DOI] [Google Scholar]
- 69.Ambachew D., Asfaw A., Blair M.W. Phenotypic variability for root traits in andean common beans grown with and without aluminum stress conditions. Agronomy. 2023;13:619. doi: 10.3390/agronomy13030619. [DOI] [Google Scholar]
- 70.Sun Y., Wang X., Zhang C., Zuo M. Multiple regression : methodology and applications, Highlights. Sci. Eng. Technol. 2023;49:542–548. doi: 10.54097/hset.v49i.8611. [DOI] [Google Scholar]
- 71.Kassambara A. STHDA; 2017. Machine Learning Essentials: Practical Guide in R. [Google Scholar]
- 72.Ali R., Gul H., Hamayun M., Rauf M., Iqbal A., Shah M., Hussain A., Bibi H., Lee I.J. Aspergillus awamori ameliorates the physicochemical characteristics and mineral profile of mung bean under salt stress. Chem. Biol. Technol. Agric. 2021;8:9. doi: 10.1186/s40538-021-00208-9. [DOI] [Google Scholar]
- 73.Maulana F., Ayalew H., Anderson J.D., Kumssa T.T., Huang W., Ma X.-F. Genome-wide association mapping of seedling heat tolerance in winter wheat. Front. Plant Sci. 2018;9:1272. doi: 10.3389/fpls.2018.01272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bahrami F., Arzani A., Karimi V. Evaluation of yield-based drought tolerance indices for screening safflower genotypes. Agron. J. 2014;106(4):1219–1224. doi: 10.2134/agronj13.0387. [DOI] [Google Scholar]
- 75.Sharma S., Singh V., Tanwar H., Mor V.S., Kumar M., Punia R.C., Dalal M.S., Khan M., Sangwan S., Bhuker A., Dagar C.S., Yashveer S., Singh J. Impact of high temperature on germination, seedling growth and enzymatic activity of wheat. Agriculture. 2022;12:1500. doi: 10.3390/agriculture12091500. [DOI] [Google Scholar]
- 76.Ahmed H.G.M.D., Sajjad M., Li M., Azmat M.A., Rizwan M., Maqsood R.H., Khan S.H. Selection criteria for drought-tolerant bread wheat genotypes at seedling stage. Sustainability. 2019;11:2584. doi: 10.3390/su11092584. [DOI] [Google Scholar]
- 77.Ghosh S., Shahed M.A., Robin A.H.K. Polyethylene glycol induced osmotic stress affects germination and seedling establishment of wheat genotypes. Plant Breed. Biotechnol. 2020;8(2):174∼185. doi: 10.9787/PBB.2020.8.2.174. [DOI] [Google Scholar]
- 78.Uhlarik A., Ćeran M., Živanov D., Grumeza R., Skøt L., Sizer-Coverdale E., Lloyd D. Phenotypic and genotypic characterization and correlation analysis of pea (Pisum sativum L.) diversity panel. Plants. 2022;11:1321. doi: 10.3390/plants11101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vargas Y., Mayor-Duran V.M., Buendia H.F., Ruiz-Guzman H., Raatz B. Physiological and genetic characterization of heat stress effects in a common bean RIL population. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249859. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chachar Z., Chachar N.A., Chachar Q.I., Mujtaba S., Chachar G., Chachar S. Identification of drought tolerant wheat genotypes under water deficit condiitons. Int. J. Res. -Granthaalayah. 2016;4(2):206–214. doi: 10.29121/granthaalayah.v4.i2.2016.2830. [DOI] [Google Scholar]
- 81.Ahammed G.J., Guang Y., Yang Y., Chen J. Mechanisms of elevated CO2-induced thermotolerance in plants: the role of phytohormones. Plant Cell Rep. 2021;40:2273–2286. doi: 10.1007/s00299-021-02751-z. [DOI] [PubMed] [Google Scholar]
- 82.Goswami A.K., Maurya N.K., Goswami S., Bardhan K., Singh S.K., Prakash J., Pradhan S., Kumar A., Chinnusamy V., Kumar P., Sharma R.M., Sharma S., Bisht D.S., Kumar C. Physio-biochemical and molecular stress regulators and their crosstalk for low-temperature stress responses in fruit crops: a review. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.1022167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao J., Lu Z., Wang L., Jin B. Plant responses to heat stress: physiology, transcription, noncoding RNAs, and epigenetics. Int. J. Mol. Sci. 2020;22:117. doi: 10.3390/ijms22010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Yang-Er, Zhang C.-M., Su Y.-Q., Ma J., Zhang Z.-W., Yuan M., Zhang H.-Y., Yuan S. Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. Environ. Exp. Bot. 2017;135:45–55. doi: 10.1016/j.envexpbot.2016.12.001. [DOI] [Google Scholar]
- 85.Li J.-Y., Yang C., Tian Y.-Y., Liu J.-X. Regulation of chloroplast development and function at adverse temperatures in plants. Plant Cell Physiol. 2022;63(5):580–591. doi: 10.1093/pcp/pcac022. [DOI] [PubMed] [Google Scholar]
- 86.Song Y., Feng L., Alyafei M.A.M., Jaleel A., Ren M. Function of chloroplasts in plant stress responses. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222413464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bhattarai S., Harvey J., Djidonou D., Leskovar D. Exploring morpho-physiological variation for heat stress tolerance in tomato. Plants. 2021;10:347. doi: 10.3390/plants10020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun T., Rao S., Zhou X., Li L. Plant carotenoids: recent advances and future perspectives. Mol. Hortic. 2022;2:3. doi: 10.1186/s43897-022-00023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tamindžić G., Ignjatov M., Miljaković D., Červenski J., Milošević D., Nikolić Z., Vasiljević S. Seed priming treatments to improve heat stress tolerance of garden pea (Pisum sativum L.) Agric. For. 2023;13:439. doi: 10.3390/agriculture13020439. [DOI] [Google Scholar]
- 90.Ruban A.V. Nonphotochemical chlorophyll fluorescence quenching: mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016;170:1903–1916. doi: 10.1104/pp.15.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.El-Mohsen A.A.A., El-Shafi M.A.A., Gheith E.M.S., Suleiman H.S. Using different statistical procedures for evaluating drought tolerance indices of bread wheat genotypes. Adv. Agric. Biol. 2015;4(1):19–30. doi: 10.15192/PSCP.AAB.2015.4.1.1930. [DOI] [Google Scholar]
- 92.Grzesiak S., Hordyńska N., Szczyrek P., Grzesiak M.T., Noga A., Szechyńska-Hebda M. Variation among wheat (Triticum aestivum L.) genotypes in response to the drought stress: I–selection approaches. J. Plant Interact. 2019;14(1):30–44. doi: 10.1080/17429145.2018.1550817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rupji M., Dwivedi B., Kowalski J. NOJAH: NOt just another heatmap for genome-wide cluster analysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0204542. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Olivoto T., Diel M.I., Schmidt D., Lúcio A.D. MGIDI: a powerful tool to analyze plant multivariate data. Plant Methods. 2022;18:121. doi: 10.1186/s13007-022-00952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Filho J.S.S., Olivoto T., Campos M. de S., de Oliveira E.J. Multi-trait selection in multi-environments for performance and stability in cassava genotypes. Front. Plant Sci. 2023;14 doi: 10.3389/fpls.2023.1282221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palaniyappan S., Ganesan K.N., Manivannan N., Ravichandran V., Senthil N. Multi trait genotype- ideotype distance index - a tool for identification of elite parental inbreds for developing heterotic hybrids of fodder maize (Zea mays L.), Electron. J. Plant Breed. 2023;14(3):841–849. doi: 10.37992/2023.1403.098. [DOI] [Google Scholar]
- 97.Meier C., Meira D., Marchioro V.S., Olivoto T., Klein L.A., de Souza V.Q. Selection gain and interrelations between agronomic traits in wheat F5 genotypes. Rev. Ceres Viçosa. 2019;66(4):271–278. doi: 10.1590/0034-737x201966040005. [DOI] [Google Scholar]
- 98.Cai F., Mi N., Ming H., Zhang Y., Zhang H., Zhang S., Zhao X., Zhang B. Responses of dry matter accumulation and partitioning to drought and subsequent rewatering at different growth stages of maize in Northeast China. Front. Plant Sci. 2023;14 doi: 10.3389/fpls.2023.1110727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamamoto Y. Quality control of photosystem II: the mechanisms for avoidance and tolerance of light and heat stresses are closely linked to membrane fluidity of the thylakoids. Front. Plant Sci. 2016;7:1136. doi: 10.3389/fpls.2016.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.