Abstract
Background
Giant coronary artery aneurysms (GCAA), are rare findings often discovered incidentally in adults. GCAAs are defined by a significant enlargement of coronary arteries, posing a heightened risk of myocardial infarction and thrombosis.
Case presentation
A 52-year-old male known case of ischemic heart disease (IHD), presented with chest pain and signs of anterior ST-elevation MI (STEMI). He had a history of MI six years ago and was on a medical regimen. Coronary angiography revealed a huge aneurysm (4.8∗8.2mm) in the left anterior descending artery (LAD) and ectasia in other arteries. Surgical management via coronary artery bypass graft (CABG) was pursued, successfully addressing the aneurysm.
Conclusion
Recent advancements have improved our understanding and imaging capabilities for coronary artery aneurysms (CAAs). Treatment options include medical therapy, percutaneous coronary intervention, or surgery, with decisions tailored to individual cases. Standardized treatment protocols await clarification through further research, including randomized controlled trials.
Keywords: Coronary artery aneurysm, Giant aneurysm, Myocardial infarction, Cardiovascular surgery, Case report
1. Introduction
Coronary artery aneurysms (CAA) are infrequently detected in adults and are commonly diagnosed as an unexpected discovery [1]. Giant coronary artery aneurysm is described as a coronary enlargement that is four times larger than the normal diameter [2]. A giant coronary artery aneurysm (GCAA) is typically defined as a rare condition, having a diameter greater than 20mm with a prevalence of 0.02 % [3,4]. Most CAAs are caused by atherosclerosis or other potential causes including vasculitis and connective tissue diseases, certain infectious, and trauma [[5], [6], [7]]. GCAAs have the highest likelihood of myocardial infarction and thrombosis, with a minor chance of rupture [8]. Further research is required to determine the best approach for managing GCAA whether through medicinal, interventional, or surgical means [3]. Here, we present the case of a patient who had a history of cardiovascular diseases and came here with cardiac symptoms, which finally was diagnosed as GCAA. This manuscript was prepared according to the SCARE 2023 guidelines (Supplemental digital content 1 [SDC 1]) [9].
2. Case presentation
A 52-year-old man, a known case of ischemic heart disease (IHD), was referred to our cardiology service with a chief complaint of chest pain that radiated to his left shoulder. The patient was well until a few hours prior to admission (PTA) when he developed left shoulder pain, cold sweating, nausea, and vomiting. Upon admission, serological examinations for vasculitis or other inflammatory conditions yielded negative results, and there was no documented history of trauma or surgical interventions.
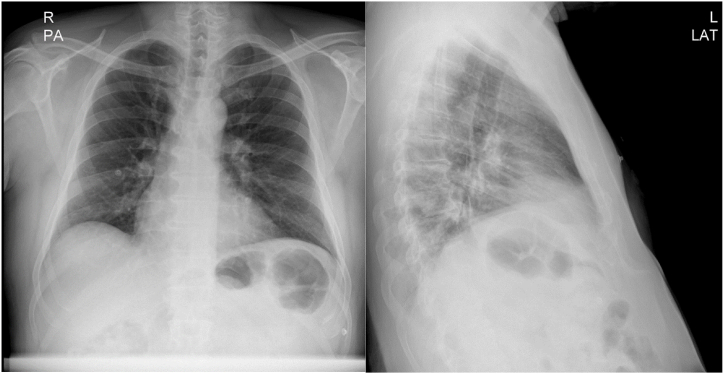
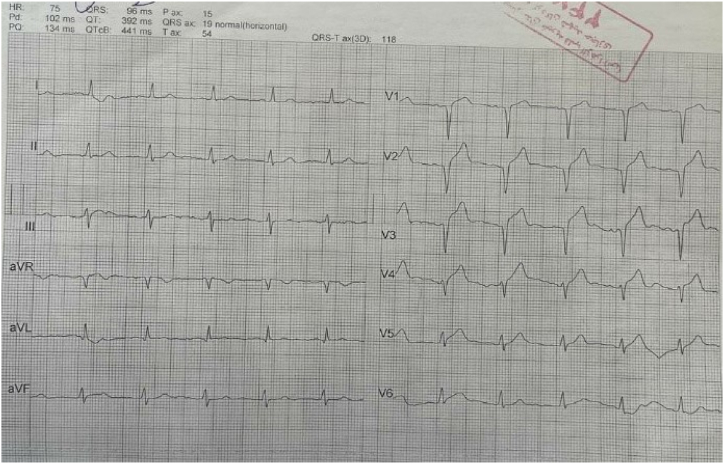
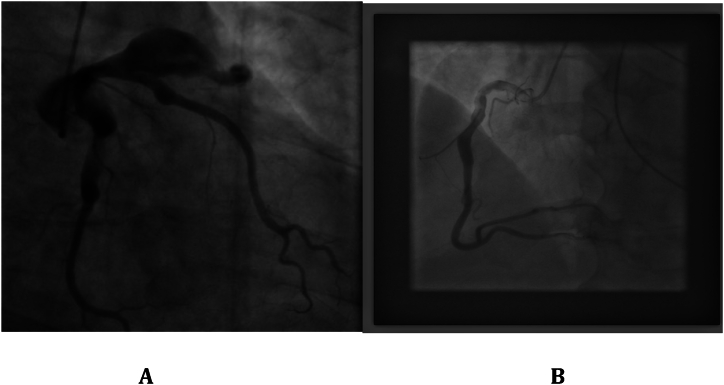
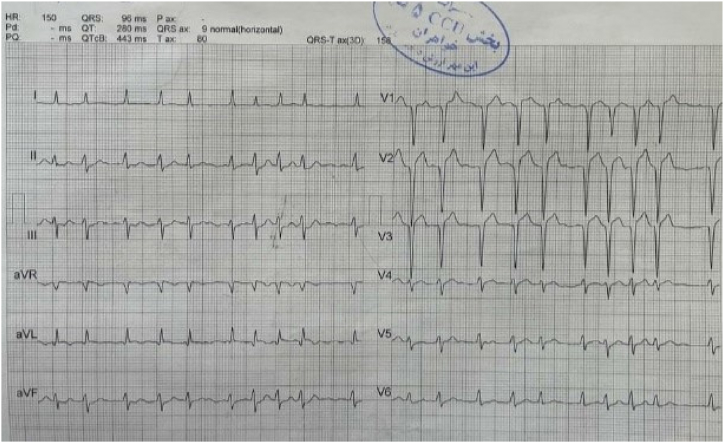
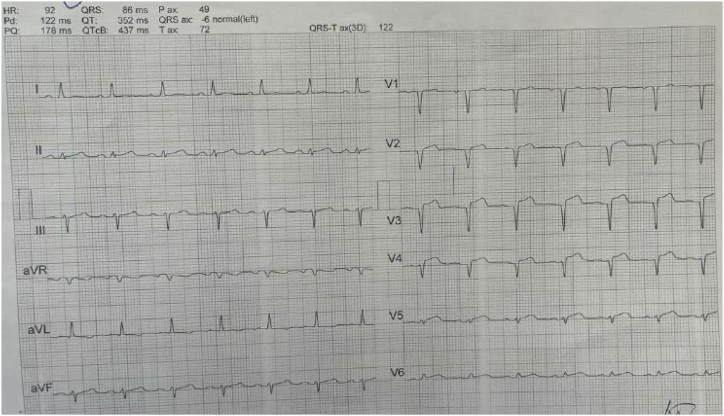
The patient had an episode of myocardial infarction (MI) six years ago and also an aneurysm in his angiography. Since then, medical therapy has started for him, including Acetylsalicylic acid (80 mg, QD), Clopidogrel (75mg, QD), Bisoprolol (5mg, QD), Rosuvastatin (20mg, QD), and Pantoprazole (40mg, QD). On physical examination, neither pulmonary stasis nor peripheral edema was observed. Also, the heart physical examination was normal with normal S1 and S2 sounds. His blood pressure was 120/75, heart rate was 73 beats/min, respiratory rate was 18 breaths/min, body temperature was 36.5 °C, O2 saturation was 95 % and Glasgow coma scale (GCS) was 15 upon admission. Chest X-ray revealed clear lung fields, normal heart size, and no evidence of any abnormalities or pathologies (Fig. 1). Electrocardiogram (ECG) showed anterior ST-elevation MI (STEMI) and cardiac biomarkers, like highly sensitive troponin (12.1 ng/ml), were increased (Fig. 2). The patient was admitted with an anterior MI diagnosis and received two doses of recombinant tissue plasminogen activators (rtPA) in the emergency room (ER), which responded, and the chest pain subsided. TTE revealed severe LV systolic dysfunction with an ejection fraction (EF) of 30 %, akinetic apicoseptal, and hypokinetic septal. According to the ESC (European Society of Cardiology) guidelines, the patient was considered a candidate for an early routine coronary angiogram 3–24 hours after successful thrombolytic therapy [10]. The coronary angiography showed a severe large aneurysm (4.8∗8.2mm) in left anterior descending artery (LAD) [Fig. 3, A]; ectasia and slow flow in the left circumflex (LCX); small diagonal branches; and ectasia and small flow in the right coronary artery (RCA) [Fig. 3, B]. Further ECG findings indicated atrial fibrillation with rapid ventricular response (Fig. 4).
Fig. 1.
Chest X-ray of the patient.
Fig. 2.
Upon admission ECG indicating anterior STEMI.
Fig. 3.
Coronary angiography indicating giant coronary aneurysm: A) Aneurysm in LAD; B) Ectesia in RCA.
Fig. 4.
ECG indicating atrial fibrillation with rapid ventricular response.
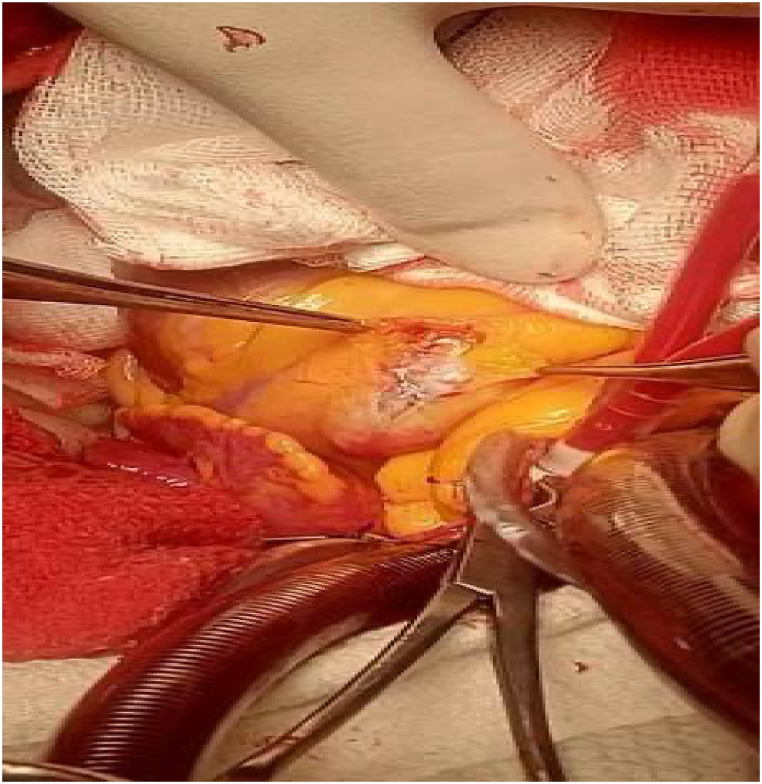
Among various therapeutic modalities, the decision was made to proceed with CABG due to the presence of giant CAAs and the patient's prior history of MI and aneurysm. Under general anesthesia, the left internal mammary artery (LIMA) was harvested through median sternotomy and cardiopulmonary bypass (CBP) started. The aneurysm of LAD was opened, clots were removed, and aneurysm overswened. Finally, the LAD was bypassed with the use of LIMA, the heart was reactivated, and CBP was stopped (Fig. 5, Supplemental digital content 2 [SDC 2]). The patient had an uneventful post-op course with sinus rhythm ECG and he was discharged from the hospital (Fig. 6). In response to the failure of dual antiplatelet therapy (DAPT), we adjusted the therapeutic regimen to a combination of antiplatelet (Acetylsalicylic acid) and anticoagulant (Rivaroxaban) therapy following CABG. This change was implemented to enhance the efficacy of post-operative management and address the limitations observed with DAPT in this patient.
Fig. 5.
Coronary artery aneurysm during coronary artery bypass grafting.
Fig. 6.
ECG indicating sinus ryhtm
3. Discussion
The rise in the utilization of modern coronary imaging techniques combined with the growing number of referrals for coronary angiography in ACS cases has led to a higher occurrence of uncommon angiographic discoveries like CAA [11]. The proximal coronary segments and the right coronary artery are frequently affected.
The true incidence of giant CAAs might be underestimated, as the thrombi can conceal the artery's actual diameter on an angiogram. Coronary artery aneurysms are most commonly found in Asian and Hispanic patients and are least common in individuals of African American ethnicity [12,13]. The clinical manifestations of giant coronary artery aneurysms can range from asymptomatic to dyspnea, angina, and acute coronary syndrome [14]. These aneurysms are predominantly symptomatic and may resemble a mediastinal or cardiac tumor [15]. The pathophysiology of coronary artery aneurysms remains incompletely elucidated. Nonetheless, atherosclerosis is the primary underlying cause in adults, whereas Kawasaki disease is the principal predisposing factor in pediatric cases [16]. Our case involved a history of IHD that was previously treated with PCI, both of which are important risk factors for new cardiovascular events such as CAA. There have been only a small number of reported cases, and because it is rare, the clinical symptoms and the most effective treatment strategies are currently being debated in the medical literature. Up to one-third of patients with coronary artery aneurysms may experience myocardial infarction, angina pectoris, congestive heart failure, or cardiac death [2]. The primary complications are local thrombus formation, CAA rupture causing cardiac tamponade, and myocardial infarction due to subsequent distal embolization. According to a comprehensive study involving multiple medical centers, the death rate and occurrence of major adverse cardiac events (MACE) related to CAAs were 15.3 % and 31 %, respectively [17].
Coronary angiography is considered the best method for diagnosing CAAs. It offers details about the structure and any related coronary artery disease. However, it may not accurately measure aneurysm size when there are intraluminal thrombi. Multislice computed tomography coronary angiography has nearly perfect sensitivity and can reveal intricate details of the structure and identify intramural thrombi. Additionally, a three-dimensional reconstruction helps in understanding how the aneurysms are positioned in relation to nearby structures [18]. The echocardiogram is highly sensitive and specific in assessing the proximal left main coronary artery and the right coronary artery [19].
The management of giant CAAs involves various approaches, like medical therapy, percutaneous intervention, and surgery. Medical treatment typically includes antiplatelet therapy, with aspirin being the most frequently recommended medication. Percutaneous interventions, such as angioplasty using covered stents and coil embolization, are potential alternatives [[20], [21], [22]]. Surgical correction, commonly considered the preferred approach for giant CAAs, may include aneurysm ligation, reconstruction, coronary artery bypass, or direct repair [23]. The specific development of large CAAs is not fully understood, and conservative treatment with antiplatelet and anticoagulant medications is sometimes used. Without established guidelines, the treatment for CAA varies based on factors such as the patient's symptoms (silent or ACS), the size, shape, and location of the aneurysm, and the presence of atherosclerosis [11]. Table 1 shows some similar case reports with different treatment modalities for CAAs.
Table 1.
Baseline characteristics of studies with different treatment modalities for CAAs.
| First author | year | Sex and age | Past medical history | Clinical manifestation | Affected vessel | Position of CAA | Aneurysm size | Therapeutic method |
|---|---|---|---|---|---|---|---|---|
| Melissa G.Y. Lee [1] | 2023 | 65-year-old man | hypertension and hypercholesterolemia | atypical chest pain | all 3 coronary arteries particularly LAD RCA |
Proximal segment | LAD: 16 × 13 mm RCA: 17 mm |
long-term oral anticoagulation |
| Hongli Gao and Hongwei Li∗ [4] | 2023 | 29-year-old female | Kawasaki disease | intermittent retrosternal compression pain |
LAD RCA |
Mid part LAD middle segment of (RCA) | LAD:8.8 ± 1.0mm and longitudinal diameter of 24.5 ± 2.6mm. RCA:5.9 ± 0.8mm and longitudinal diameter of 10.5 ± 1.2 mm. |
coronary artery bypass grafting |
| Wouter Holvoet [5] | 2023 | 56-year-old female | hypertension and hypercholesterolaemia | NSTEMI and a history of spontaneous coronary artery dissection of LAD | LMCA | shaft of the LMCA | length 15 mm, and the maximal diameter was 8.5 mm. | PCI |
| Islam Abudayyeh [24] | 2023 | 51-year-old man | Prior Operation for 4-Valve Endocarditis | acute-onset chest pain | LMCA | DISTAL PART LAD | 3-cm aneurysm | surgical intervention |
| Noeul Kang [25] | 2023 | 61-year-old male | idiopathic hypereosinophilic syndrome | chest discomfort | RCA | mid-RCA | 5.0 × 8.2 cm | RCA aneurysmectomy and coronary artery bypass were performed |
| Oghenesuvwe Eboh [26] | 2023 | 39-year-old female | hypertension and stroke | acute chest pain | RCA LAD LMA |
Distal LAD and RCA | LMA measuring 5.5 x 4.1 cm And medium sized RCA + LAD |
anticoagulation |
| Syed M. Ishaq [27] | 2024 | 45-year-old male | 1-severe pulmonary hypertension, 2-obstructive sleep apnea, 3-schizophrenia, 4-polycythemia, 5-right upper extremity deep venous thrombosis | substernal chest pain + nausea, vomiting and diaphoresis |
LAD LCX RCA |
Proximal LAD and LCX proximal and mid-distribution of the RCA | 1 mm in the proximal LAD and 14 mm in the proximal LCX 24 × 14 mm in RCA |
cardiothoracic surgery |
| Pala [28] | 2024 | 72-year-old male | hypertension, hypercholesterolemia, and abdominal aortic aneurysm | chest pain | all 3 coronary arteries | Proximal RCA | 1. Two CAAs in RCA (diameter max 12 mm and diameter max 33 mm, respectively). 2. Circumflex artery(diameter max 20.5 mm) 3. Left main descendent artery (diameter max 8 mm) |
coronary artery bypass grafting |
4. Limitations
This study is limited by its reliance on conventional coronary angiography as the sole imaging modality. While angiography is effective for diagnosing coronary artery aneurysms, it may not provide detailed insights into atherosclerotic or thrombotic characteristics, which advanced imaging techniques such as Optical Coherence Tomography (OCT) or Intravascular Ultrasound (IVUS) could offer. Furthermore, the study does not include long-term follow-up data to assess the durability and late outcomes of the interventions performed. Unmeasured confounding factors, such as the patient's lifestyle and adherence to treatment, could also influence the results and were not fully accounted for in the study. A significant limitation also involves the uncertainty surrounding the optimal antithrombotic therapy for patients with GCAAs following CABG. While DAPT with Acetylsalicylic acid and Clopidogrel is commonly used to reduce the risk of thrombotic events, there is ongoing debate regarding the potential benefit of adding anticoagulant therapy, such as warfarin or direct oral anticoagulants (DOACs). The choice between continuing DAPT alone or combining it with anticoagulation depends on the individual patient's risk profile and the specific characteristics of the aneurysm, which introduces variability and uncertainty in treatment strategies. The lack of consensus on the most effective antithrombotic regimen further complicates the management of such cases and limits the ability to provide definitive recommendations based on this study alone.
5. Conclusion
In recent years, our understanding of the causes and treatments for CAAs has improved. Advances in technology have enhanced both invasive and noninvasive imaging methods. While coronary angiography is still the preferred method for diagnosing CAAs, combining it with other imaging techniques allows for early detection. Treatment options include medication, percutaneous coronary intervention (PCI), or surgery, but there is no consensus on the best approach. Treatment decisions are typically made on a case-by-case basis, taking into account individual factors and the characteristics of the aneurysm. More research is needed through randomized controlled trials to better understand CAAs and establish standardized treatment protocols.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Ethical approval is exempt/waived at our institution for this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
CRediT authorship contribution statement
Mohammad Ghazinour: Investigation, Conceptualization. Arshin Ghaedi: Writing – review & editing, Writing – original draft, Supervision, Methodology. Aida Bazrgar: Writing – original draft, Methodology. Mohammad Montaseri: Data curation, Conceptualization. Mohammad Sasannia: Data curation, Conceptualization. Hamed Bazrafshan drissi: Writing – review & editing, Project administration, Methodology, Investigation, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e38511.
Contributor Information
Mohammad Ghazinour, Email: ghazinour1346@gmail.com.
Arshin Ghaedi, Email: arshin.ghaedi@gmail.com.
Aida Bazrgar, Email: aidabazrgar@gmail.com.
Mohammad Montaseri, Email: montaseri1367@gmail.com.
Mohammad Sasannia, Email: mohammadsasannia@gmail.com.
Hamed Bazrafshan drissi, Email: hamedbazrafshan@yahoo.com, hamedbazrafshan@sums.ac.ir.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Lee M.G., Lefkovits J., Joshi S.B., Pearson M., Better N. Multi-vessel giant coronary artery aneurysms: an unusual cause of chest pain. Radiology Case Reports. 2023;18(3):814–817. doi: 10.1016/j.radcr.2022.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halapas A., Lausberg H., Gehrig T., Friedrich I., Hauptmann K.E. Giant right coronary artery aneurysm in an adult male patient with non-ST myocardial infarction. Hellenic J. Cardiol. 2013;54(1):69–76. [PubMed] [Google Scholar]
- 3.Pham V., Hemptinne Q., Grinda J.M., Duboc D., Varenne O., Picard F. Giant coronary aneurysms, from diagnosis to treatment: a literature review. Arch Cardiovasc Dis. 2020;113(1):59–69. doi: 10.1016/j.acvd.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Gao H., Li H. Case report: giant coronary artery aneurysms with severe stenosis and multiple abdominal artery aneurysms. Front. Med. 2023;10 doi: 10.3389/fmed.2023.1187690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holvoet W., van den Buijs D., Bogaerts E., Willems E., Ameloot K., Dens J. Giant coronary artery aneurysm of the left main treated with a covered stent: a case report. European Heart Journal-Case Reports. 2023;7(1) doi: 10.1093/ehjcr/ytac463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alioglu E., Turk U.O., Engin C., Tengiz I., Tuzun N., Posacioglu H. Left main coronary artery aneurysm in young patient with acute myocardial infarction. J. Cardiovasc. Med. 2009;10(6):494–496. doi: 10.2459/JCM.0b013e3283293349. [DOI] [PubMed] [Google Scholar]
- 7.Kawsara A., Núñez Gil IJ., Alqahtani F., Moreland J., Rihal C.S., Alkhouli M. Management of coronary artery aneurysms. JACC Cardiovasc. Interv. 2018;11(13):1211–1223. doi: 10.1016/j.jcin.2018.02.041. [DOI] [PubMed] [Google Scholar]
- 8.Pinheiro B.B., Fagundes W.V., Gusmão C.A., Lima A.M., Santos L.H., Vieira G.B. Surgical management of a giant left main coronary artery aneurysm. J. Thorac. Cardiovasc. Surg. 2004;128(5):751–752. doi: 10.1016/j.jtcvs.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2023;109(5):1136–1140. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Members A.T.F., Van de Werf F., Bax J., Betriu A., Blomstrom-Lundqvist C., Crea F., et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the task force on the management of ST-segment elevation acute myocardial infarction of the European society of cardiology. Eur. Heart J. 2008;29(23):2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 11.Matta A., Zouari F., Campelo-Parada F., Carrié D. A giant left anterior descending artery (LAD) coronary artery aneurysm treated by covered stent angioplasty: a case report. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.925820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belay E.D., Maddox R.A., Holman R.C., Curns A.T., Ballah K., Schonberger L.B. Kawasaki syndrome and risk factors for coronary artery abnormalities: United States, 1994–2003. Pediatr. Infect. Dis. J. 2006;25(3):245–249. doi: 10.1097/01.inf.0000202068.30956.16. [DOI] [PubMed] [Google Scholar]
- 13.Porcalla A., Sable C., Patel K., Martin G., Singh N. vol. 26. 2005. pp. 775–781. (The Epidemiology of Kawasaki Disease in an Urban Hospital: Does African American Race Protect against Coronary Artery Aneurysms? Pediatric Cardiology). [DOI] [PubMed] [Google Scholar]
- 14.Pham V., De Hemptinne Q., Grinda J.-M., Duboc D., Varenne O., Picard F. Giant coronary aneurysms, from diagnosis to treatment: a literature review. Archives of cardiovascular diseases. 2020;113(1):59–69. doi: 10.1016/j.acvd.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Tirani H.D., Aghajanzadeh M., Pourbahador R., Hassanzadeh R., Ebrahimi H. Giant right coronary artery aneurysm mimicking a mediastinal cyst with compression effects: a case report. Res. Cardiovasc. Med. 2016;5(3) doi: 10.5812/cardiovascmed.32086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eshtehardi P., Cook Sp, Moarof I., Triller H-Jr, Windecker S. Giant coronary artery aneurysm: imaging findings before and after treatment with a polytetrafluoroethylene-covered stent. Circulation: Cardiovascular Interventions. 2008;1(1):85–86. doi: 10.1161/CIRCINTERVENTIONS.107.763656. [DOI] [PubMed] [Google Scholar]
- 17.Núñez-Gil I.J., Cerrato E., Bollati M., Nombela-Franco L., Terol B., Alfonso-Rodríguez E., et al. Coronary artery aneurysms, insights from the international coronary artery aneurysm registry (CAAR) Int. J. Cardiol. 2020;299:49–55. doi: 10.1016/j.ijcard.2019.05.067. [DOI] [PubMed] [Google Scholar]
- 18.Kanamaru H., Sato Y., Takayama T., Ayusawa M., Karasawa K., Sumitomo N., et al. Assessment of coronary artery abnormalities by multislice spiral computed tomography in adolescents and young adults with Kawasaki disease. Am. J. Cardiol. 2005;95(4):522–525. doi: 10.1016/j.amjcard.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 19.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 20.Crawley P.D., Mahlow W.J., Huntsinger D.R., Afiniwala S., Wortham D.C. Giant coronary artery aneurysms: review and update. Tex. Heart Inst. J. 2014;41(6):603–608. doi: 10.14503/THIJ-13-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crawley P.D., Mahlow W.J., Huntsinger D.R., Afiniwala S., Wortham D.C. Giant coronary artery aneurysms: review and update. Tex. Heart Inst. J. 2014;41(6):603–608. doi: 10.14503/THIJ-13-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Della Rosa F., de Nicolas J.M.-M., Bonfils L., Fajadet J. Symptomatic giant coronary artery aneurysm treated with covered stents. Coron. Artery Dis. 2020;31(7):658–659. doi: 10.1097/MCA.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 23.Marla R., Ebel R., Crosby M., Almassi G.H. Multiple giant coronary artery aneurysms. Tex. Heart Inst. J. 2009;36(3):244–246. [PMC free article] [PubMed] [Google Scholar]
- 24.Abudayyeh I., Tankazyan H., Heimes J., Rabkin D.G., Razzouk A.J. Giant aneurysm of left main coronary artery in a patient with prior operation for 4-valve endocarditis. Tex. Heart Inst. J. 2023;50(1) doi: 10.14503/THIJ-21-7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang N., Choi K.H., Kim S.M., Kim D.-K., Sung K., Choi D.-C. Giant coronary artery aneurysm with thrombosis complicated in a patient with idiopathic hypereosinophilic syndrome. Yonsei Med. J. 2023;64(2):148. doi: 10.3349/ymj.2022.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eboh O., Zeltser R., Makaryus A.N. A case of giant left main coronary artery aneurysm. J. Am. Coll. Cardiol. 2023;81(8_Supplement):2428. [Google Scholar]
- 27.Ishaq S.M., Duhan S., Keisham B., Khalid T., Harfouch B. Giant coronary artery aneurysms presenting as posterior myocardial infarction. Cureus. 2024;16(1) doi: 10.7759/cureus.52081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pala B., Tocci G., Bruno N., Barbato E., Gabrielli D. 2024. Giant Coronary Aneurysm and Acute Myocardial Infarction: Clinical Case Report and Literature Review. Clinical Research in Cardiology; pp. 1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.