Abstract
Background
At present, the relationship between depression and the triglyceride glycemic (TyG) index remains a topic of debate. This study sought to elucidate the relationship between depression and the TyG index to create a predictive model that would help doctors diagnose patients.
Methods
We conducted a cross-sectional study utilizing the National Health and Nutrition Examination Survey (NHANES) dataset, which comprises data from 2009 to 2018. The analysis involved 11,222 adults with a Patient Health Questionnaire-9 (PHQ-9) score of 5 or higher, indicating the presence of depression. As part of the analysis, multiple regression models were used to test whether a linear relationship existed between the TyG index and depression. A threshold effects analysis was used to generate smoothed curves and detect nonlinear correlations. Additionally, the Least Absolute Shrinkage and Selection Operator (LASSO) regression were employed to identify the key risk factors associated with depression. The factors identified were then used to construct the risk prediction nomogram. Finally, Receiver Operating Characteristic (ROC) curves were used to evaluate the discriminative performance of the model.
Results
Multivariable linear regression analysis indicated a strong positive correlation between depression and the TyG index (β: 0.38, 95 % CI: 0.16–0.60, p = 0.0008). A U-shaped relationship with an inflection point was observed at a TyG index of 8.16. The nomogram model, constructed using risk factors identified by LASSO, exhibited a significant predictive value (AUC = 0.888).
Conclusions
The results of this investigation point to a U-shaped association between depression risk and the TyG index among Americans. Those with a TyG index of over 8.16 are significantly more likely to develop depression. These results suggest a possible causal relationship and emphasize the importance of monitoring the TyG index in depression risk assessment.
Keywords: NHANES, Machine learning, TyG index, Depression, PHQ-9, LASSO
1. Background
Depression is a widespread mental disorder that affects an increasing number of individuals [1]. The World Health Organization (WHO) projected that by 2019, roughly 280 million individuals would be affected by depression, with a higher prevalence among women [2]. Depression places a heavy burden on patients and public health expenditures [3]. Major depressive disorder affects 6.7 % of the U.S. population who reported having at least one major depressive episode in 2017 [4]. Given the high recurrence rate of major depression and its comorbidity with other disorders, developing effective prevention strategies is urgently needed.
Numerous studies have established a strong link between depression and chronic physical ailments. In particular, diabetic patients with insulin resistance (IR) are at a higher risk of developing depression. Furthermore, compared to healthy controls, patients with mental disorders may show variable degrees of IR and larger variations in lipid, glucose, and insulin parameters early in the course of the disease [5]. Traditionally, the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) has been used as the gold standard for measuring IR because of its accuracy and predictability. It is effective in predicting cardiovascular disease [[6], [7], [8]]. However, this requires measurement of the patient's fasting insulin level and has limited clinical utility. The triglyceride-glucose (TyG) index is a simple surrogate for IR and is easily obtained from clinical laboratory test results [9]. The TyG index is calculated as fasting triglycerides (mg/dL) ∗ fasting glucose (mg/dL)/2. It was proposed by Mendía et al. in 2008 and has been studied extensively [10]. Recent research has shown that the TyG index is a more reliable and practical diagnostic tool for recognizing IR [[11], [12], [13], [14], [15], [16], [17]]. Studies have shown that the TyG index is associated with heart failure, coronary heart disease, atherosclerosis, carotid atherosclerosis, hypertension and stroke [[18], [19], [20], [21], [22], [23]].
This study investigated the connection between depression and the TyG index among participants in the National Health and Nutrition Examination Survey (NHANES). A U-shaped association between the TyG index and depression was observed in the general American population, which aligns with previous research that has found similar relationships between the TyG index and other ailments, such as obesity and hyperuricemia. In addition, we introduced machine learning techniques such as LASSO regression analysis and histogram modeling to identify key risk factors associated with depression. This approach provides valuable insights for identifying populations at high risk of depression. The NHANES is a comprehensive national survey covering non-institutionalized U.S. citizens nationwide through an unbiased selection process, data processing, and quality control. These rigorous methods ensure the authenticity and reliability of the published results.
2. Methods
2.1. Information in our research
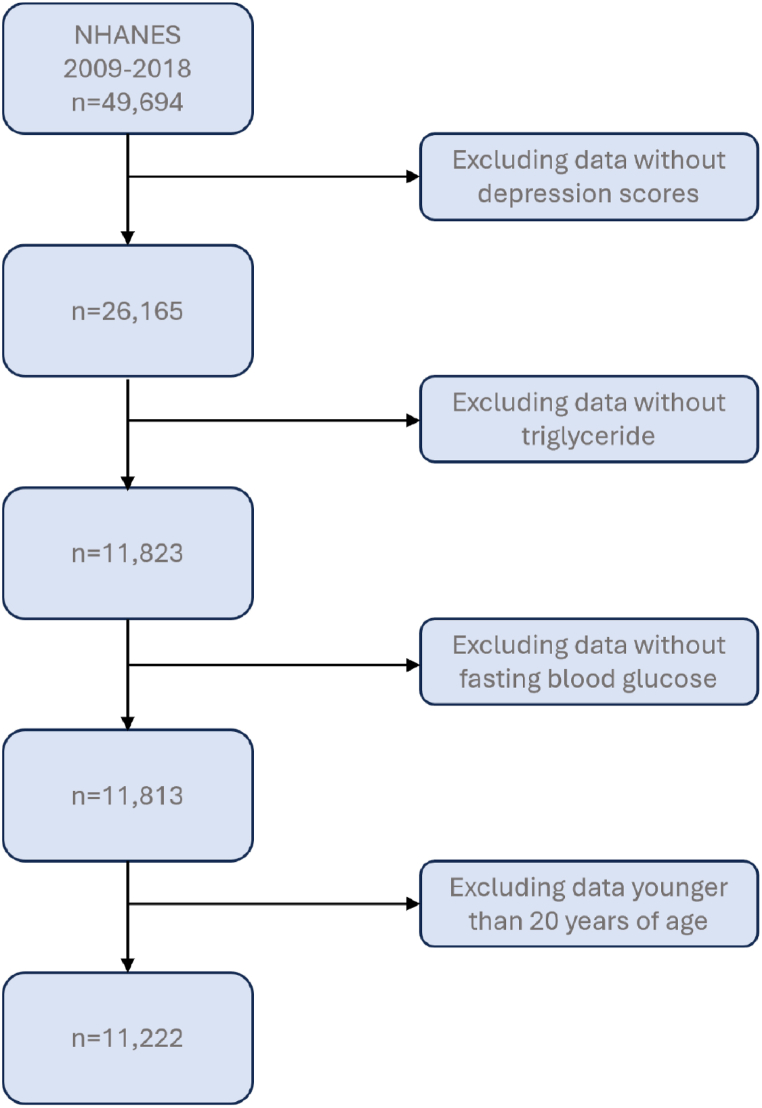
The data used came from the 2009–2018 NHANES, a health survey conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS). Participants who were at least 20 years of age, had complete fasting blood glucose and fasting lipids, were assessed, and received a comprehensive depression rating were selected for this study [24]. For this study, participants had been assessed using the TyG index, and had undergone full depression ratings were selected. Following the exclusion of respondents with missing covariate data, 11,222 individuals were enrolled in the study. A schematic representation of this procedure is provided in Fig. 1. Additional ethical clearance was not needed because an ethics committee had approved all the datasets used.
Fig. 1.
Flowchart of subject selection.
2.2. Variables
2.2.1. TyG index
In this study, the primary variable of importance was the TyG index. By drawing blood in the morning [25] following an overnight period of fasting, data on total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), fasting blood glucose (FBG), and insulin was collected. By drawing blood in the morning 21 following an overnight period of fasting, data on total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), fasting blood glucose (FBG), and insulin was collected. The Laboratory Blood Assay Related Data Researcher dispenses blood into containers for storage and transportation to laboratories throughout the United States for analysis and serum into containers for future research and storage. The purpose of filling cell processing is to dispense washed filling cells to two different analytical laboratories for storage and transportation. Processing includes centrifuging and storing blood collection tubes; dispensing whole blood into storage tubes; centrifuging, separating, and dispensing plasma and serum into storage containers; performing dilutions; washing and lysing whole blood cells; and storing specimens. The TyG index was calculated using the formula TyG = In [TG (mg/dL) × FBG (mg/dL)/2].
2.2.2. Depression
Symptoms of depression were assessed using the ninth edition of the Patient Health Questionnaire (PHQ-9). This questionnaire rates nine depressive symptoms on a scale from 0 (none) to 3 (nearly daily). According to the study criteria, participants were classified as experiencing depression if their total PHQ-9 score exceeded 5 [26,27]. In this assessment, each item is scored on a scale from 0 to 3, with 0 indicating no symptoms and 3 indicating severe symptoms. The total score, which is the sum of all item scores, can range from 0 to 30. A total score exceeding 9 suggests the presence of significant depressive symptoms. The depression variable is categorized into three levels: no depression (values ≤ 5), moderate depression (values > 5 and ≤ 19), and severe depression (values > 19).
2.2.3. Covariate information
Based on the existing literature and drawing from biological rationale, we selected a comprehensive set of covariates known to have confounding effects on depression. Demographic and health-related data were collected through NHANES at-home interviews. This included basic personal information, such as gender, education, marital status, household income to poverty ratio, body mass index (BMI), and waist circumference. Adults who were 20 years old or above were selected for the study. Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other races were among the race categories of the participants enrolled. The following question was used to determine lifestyle habits, “On days when {you/SP} consumed alcoholic beverages in the past 12 months, how many drinks did {you/he/she} consume on average?” This question was used to understand patterns of alcohol consumption.
Data on the participant's clinical parameters, including insulin, TG, FBG, HDL-C, and LDL-C, were also obtained from NHANES laboratory records. To improve the methodological transparency of the study, comprehensive data on variables was obtained from previous research [28,29]. The existence or absence of ten diseases – asthma, arthritis, chronic heart failure, coronary heart disease (CHD), angina pectoris, emphysema, stroke, chronic obstructive pulmonary disease (COPD), cancer, and hypertension – was determined by a “yes” or “no” response. Diabetes was defined as a “yes” or “no” and “borderline.”
2.3. Statistical analysis
2.3.1. Cross-sectional study
R software (4.1.6) was used for statistical analysis. Sample weights from several study periods were taken into account in our analytical technique to accurately estimate health-related data because the NHANES survey uses a variety of intricate selection designs. Using multivariate logistic regression analysis, we were able to derive β-values and 95 % confidence intervals (CIs) between depression and the TyG index.
One of the primary reasons for choosing multivariate logistic regression is its interpretability. Logistic regression provides clear and easily interpretable coefficients that represent the odds ratios associated with each predictor variable. This level of transparency is particularly important in clinical and epidemiological research, where understanding the relationship between covariates and outcomes is critical. In contrast, while methods like GBM, SVM, and CART can provide high predictive accuracy, they are often considered “black box” models, making it more challenging to derive clear, actionable insights from the results. Our dataset is relatively balanced and does not contain large-scale, complex interactions that might necessitate the use of more advanced techniques like GBM or SVM. In preliminary analyses, we found that logistic regression performed comparably to these more complex models in terms of predictive accuracy, without the need for extensive hyperparameter tuning or computational resources. Additionally, logistic regression is robust to overfitting in situations with smaller sample sizes or when the number of predictors is manageable, which was the case in our study.
Logistic regression is widely accepted and understood in the medical community, making it easier to communicate findings to clinicians and policymakers. The ability to produce odds ratios and confidence intervals allows for straightforward translation of results into clinical practice, which is less feasible with more complex models like SVM or GBM. Logistic regression tends to generalize well to new data, particularly when the underlying relationships are linear or approximately linear. The simplicity of the model helps prevent overfitting, which can be a risk with more complex models like GBM or SVM, especially in studies with smaller sample sizes.
A second model (Model 2) considered factors such as gender, age, race, education, marital status, and family the ratio of income to poverty (RIP), while an initial model (Model 1) was left uncorrected. In addition, a third model (Model 3) was designed to take in the inputs of BMI, waist circumference, HDL, LDL, insulin, asthma, arthrolithiasis, and chronic heart failure and was corrected for age, gender, race, marital status, education, family RIP. heart attack, angina pectoris, stroke, COPD, pulmonary emphysema, cancer, diabetes, and hypertension. Smoothed curve fitting was performed post-adjustment to identify inflection points between the TyG index and depression using a threshold effect analysis model. We also conducted subgroup analyses and interaction tests to delve deeper into the dynamics of the relationship between depression and the TyG index.
2.3.2. Machine learning
Using the regression model Least Absolute Shrinkage and Selection Operator (LASSO), we were able to determine the most important depression predictors while controlling for covariation among covariates. LASSO is a type of regression analysis that enhances both the prediction accuracy and interpretability of statistical models. It does this by adding a penalty to the regression equation that is proportional to the sum of the absolute values of the coefficients. This penalty shrinks some coefficients to zero, effectively performing variable selection and regularization simultaneously. This makes LASSO particularly useful in models with many predictors, where it helps to avoid overfitting and improve model generalization [30]. For model evaluation and parameter optimization, we utilized a cross-validation method, systematically dividing the dataset into ten subsets. Each subset sequentially served as a validation set against the model trained on the remaining nine subsets, facilitating robust evaluation and optimal parameter determination. During the cross-validation, we plotted a curve against various lambda values to assess model performance across these settings. The term “minimum deviation” refers to the lambda value corresponding to the lowest deviation observed, indicating the best fit to the data. To enhance model stability and prevent overfitting, we opted for a lambda value slightly higher than the minimum, typically increased by one standard deviation. This adjustment aims to improve the generalizability of the model to new datasets. Furthermore, we developed a risk prediction model incorporating several key variables related to depression. The discriminative ability of this model to predict depression risk was validated through Receiver Operating Characteristic (ROC) curves, providing a measure of the model's efficacy in distinguishing between cases with and without depression.
3. Results
3.1. Cross-sectional research design
3.1.1. Participant characteristics
The fundamental traits of the 11,222 study participants are displayed in Table 1. Several significant indications, including age, gender, BMI, and laboratory test results, are included in these features. These data helped researchers better grasp the diversity and representativeness of the study sample and served as the foundation for additional analysis. Of them, 2808 (25.02 %) had a diagnosis of depression. The proportion of female, Mexican American, non-Hispanic Black participants with underlying conditions (e.g., asthma, arthrolithiasis, chronic heart failure, CHD, angina pectoris, stroke, COPD, pulmonary emphysema, diabetes, and hypertension) demonstrated an increase of statistical significance in the group suffering depression as opposed to the group with no depression (P < 0.05). Furthermore, individuals with depression exhibited an elevated BMI, insulin, TyG index, and waist circumference, although their HDL was notably lower (P < 0.05) in comparison to the non-depressed group. Married individuals and those with greater educational attainment had lower rates of depression (P < 0.05).
Table 1.
Characteristics of the study population.
| Characteristic | Non-depression (n = 8414) | Depression (n = 2808) | P-value |
|---|---|---|---|
| Age | 48.27 ± 16.92 | 48.28 ± 16.69 | 0.9950 |
| Gender (%) | <0.0001 | ||
| Male | 51.75 | 39.95 | |
| Female | 48.25 | 60.05 | |
| Family RIP | 58.33 | 45.65 | <0.0001 |
| Marital Status (%) | <0.0001 | ||
| Married | 58.33 | 45.65 | |
| Widowed | 5.09 | 6.82 | |
| Divorced | 9.28 | 15.33 | |
| Separated | 1.68 | 3.52 | |
| Never married | 17.16 | 19.39 | |
| Living with partner | 8.47 | 9.29 | |
| Race (%) | 0.0065 | ||
| Mexican American | 8.47 | 8.75 | |
| Other Hispanic | 5.84 | 6.76 | |
| Non-Hispanic White | 67.92 | 65.95 | |
| Non-Hispanic Black | 9.75 | 11.62 | |
| Other Races | 8.03 | 6.92 | |
| Education (%) | <0.0001 | ||
| Less Than 9th Grade | 4.39 | 6.52 | |
| 9-11th Grade (Includes 12th grade with no diploma) | 9.11 | 13.33 | |
| High School Grad/GED or Equivalent | 21.72 | 26.05 | |
| Some College or AA degree | 31.05 | 33.58 | |
| College Graduate or above | 33.74 | 20.52 | |
| Alcohol | 4.45 ± 25.17 | 4.24 ± 20.09 | 0.7568 |
| BMI | 29.37 ± 6.93 | 31.77 ± 6.74 | <0.0001 |
| Waist circumference | 100.48 ± 16.02 | 107.55 ± 15.21 | <0.0001 |
| Asthma (%) | 0.9403 | ||
| Yes | 13.59 | 20.15 | |
| No | 86.41 | 79.85 | |
| Arthrolithiasis (%) | <0.0001 | ||
| Yes | 3.84 | 5.31 | |
| No | 96.16 | 94.69 | |
| Chronic heart failure (%) | <0.0001 | ||
| Yes | 2.01 | 4.70 | |
| No | 97.99 | 95.30 | |
| CHD (%) | 0.0007 | ||
| Yes | 3.32 | 4.73 | |
| No | 96.68 | 95.27 | |
| Angina pectoris (%) | <0.0001 | ||
| Yes | 1.79 | 4.14 | |
| No | 98.21 | 95.86 | |
| Stroke (%) | 0.0047 | ||
| Yes | 2.38 | 4.56 | |
| No | 97.62 | 95.44 | |
| COPD (%) | <0.0001 | ||
| Yes | 4.40 | 10.94 | |
| No | 95.60 | 89.06 | |
| Cancer (%) | 0.1887 | ||
| Yes | 10.16 | 11.05 | |
| No | 89.84 | 88.95 | |
| Pulmonary emphysema (%) | 0.0073 | ||
| Yes | 1.49 | 3.09 | |
| No | 98.51 | 96.91 | |
| Diabetes (%) | <0.0001 | ||
| Yes | 9.22 | 14.11 | |
| No | 88.51 | 82.87 | |
| Borderline | 2.26 | 3.02 | |
| Hypertensive (%) | <0.0001 | ||
| Yes | 31.95 | 40.12 | |
| No | 68.05 | 59.88 | |
| LDL-C | 2.93 ± 0.89 | 2.96 ± 0.97 | 0.2366 |
| HDL-C | 1.42 ± 0.43 | 1.37 ± 0.41 | <0.0001 |
| Insulin | 77.47 ± 93.78 | 89.87 ± 114.11 | <0.0001 |
| TyG index | 8.55 ± 0.65 | 8.67 ± 0.71 | <0.0001 |
Mean + SD for continuous variables: the P value was calculated by the weighted linear regression model; (%) for categorical variables: the P value was calculated by the weighted chi-square test.
Abbreviations: RIP, ratio of family income to poverty; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TyG index, triglyceride glucose index; COPD, chronic obstructive pulmonary disease; CHD, coronary heart disease.
3.1.2. Characteristics of the participants according to the quartiles of the TyG index
A summary of the fundamental traits of the study population, based on the TyG index tertiles, is provided in Table 2, including age, sex, family RIP, marital status, race, education, BMI, waist circumference, arthritis, chronic heart failure, CHD, angina pectoris, stroke, diabetes, hypertension, COPD, cancer, emphysema, insulin, HDL, LDL, and depression. Statistically significant differences were observed between the TyG index tertiles, while no statistically significant differences were observed for alcohol consumption and asthma.
Table 2.
Characteristics of the participants according to the tertiles of the TyG index.
| Characteristic | Tertiles of TyG index |
|||
|---|---|---|---|---|
| T1 | T2 | T3 | p-value | |
| Age | 44.01 ± 17.08 | 49.03 ± 17.05 | 52.10 ± 15.36 | <0.0001 |
| Gender (%) | <0.0001 | |||
| Male | 41.51 | 50.17 | 55.81 | |
| Female | 58.49 | 49.83 | 44.19 | |
| Family RIP | 3.07 ± 1.65 | 2.98 ± 1.66 | 2.86 ± 1.62 | <0.0001 |
| Marital Status (%) | <0.0001 | |||
| Married | 51.70 | 55.08 | 59.55 | |
| Widowed | 3.80 | 5.91 | 6.91 | |
| Divorced | 9.11 | 11.25 | 11.85 | |
| Separated | 1.82 | 2.42 | 2.11 | |
| Never married | 23.53 | 16.79 | 12.27 | |
| Living with partner | 10.02 | 8.55 | 7.30 | |
| Race (%) | <0.0001 | |||
| Mexican American | 6.35 | 9.00 | 10.43 | |
| Other Hispanic | 5.46 | 6.26 | 6.48 | |
| Non-Hispanic White | 65.05 | 68.26 | 69.21 | |
| Non-Hispanic Black | 15.15 | 9.02 | 6.04 | |
| Other Races | 7.99 | 7.47 | 7.84 | |
| Education (%) | <0.0001 | |||
| Less Than 9th Grade | 3.28 | 4.88 | 6.65 | |
| 9-11th Grade (Includes 12th grade with no diploma) | 8.07 | 10.25 | 12.15 | |
| High School Grad/GED or Equivalent | 19.92 | 23.73 | 24.76 | |
| Some College or AA degree | 31.91 | 31.11 | 31.91 | |
| College Graduate or above | 36.83 | 30.04 | 24.54 | |
| Alcohol | 5.12 ± 22.80 | 3.56 ± 6.78 | 4.45 ± 34.23 | 0.0795 |
| BMI | 26.82 ± 6.50 | 29.37 ± 6.93 | 31.77 ± 6.74 | <0.0001 |
| Waist circumference | 92.61 ± 15.40 | 100.48 ± 16.02 | 107.55 ± 15.21 | <0.0001 |
| Asthma (%) | 0.9403 | |||
| Yes | 15.00 | 15.29 | 15.13 | |
| No | 85.00 | 84.71 | 84.87 | |
| Arthrolithiasis (%) | <0.0001 | |||
| Yes | 2.35 | 3.41 | 6.97 | |
| No | 97.65 | 96.59 | 93.03 | |
| Chronic heart failure (%) | <0.0001 | |||
| Yes | 1.65 | 2.02 | 4.35 | |
| No | 98.35 | 97.98 | 95.65 | |
| CHD (%) | <0.0001 | |||
| Yes | 2.46 | 3.21 | 5.40 | |
| No | 97.54 | 96.79 | 94.60 | |
| Angina pectoris (%) | <0.0001 | |||
| Yes | 1.26 | 2.13 | 3.74 | |
| No | 98.74 | 97.87 | 96.26 | |
| Stroke (%) | 0.0047 | |||
| Yes | 2.19 | 3.26 | 3.29 | |
| No | 97.81 | 96.74 | 96.71 | |
| COPD (%) | <0.0001 | |||
| Yes | 3.67 | 6.40 | 7.91 | |
| No | 96.33 | 93.60 | 92.09 | |
| Cancer (%) | <0.0001 | |||
| Yes | 8.50 | 10.57 | 12.18 | |
| No | 91.50 | 89.43 | 87.82 | |
| Pulmonary emphysema (%) | 0.0073 | |||
| Yes | 1.32 | 2.05 | 2.25 | |
| No | 98.68 | 97.95 | 97.75 | |
| Diabetes (%) | <0.0001 | |||
| Yes | 2.86 | 6.87 | 22.13 | |
| No | 95.67 | 90.44 | 74.64 | |
| Borderline | 1.47 | 2.69 | 3.23 | |
| Hypertensive (%) | <0.0001 | |||
| Yes | 22.57 | 33.51 | 46.45 | |
| No | 77.43 | 66.49 | 53.55 | |
| LDL-C | 2.65 ± 0.77 | 3.05 ± 0.87 | 3.13 ± 1.02 | <0.0001 |
| HDL-C | 1.63 ± 0.46 | 1.41 ± 0.37 | 1.16 ± 0.31 | <0.0001 |
| Insulin | 51.84 ± 48.13 | 75.31 ± 76.50 | 116.41 ± 140.72 | <0.0001 |
| Depression | 2.77 ± 3.91 | 2.99 ± 4.08 | 3.62 ± 4.74 | <0.0001 |
Mean + SD for continuous variables: the P value was calculated by the weighted linear regression model; (%) for categorical variables: the P value was calculated by the weighted chi-square test.
Abbreviations: RIP, ratio of family income to poverty; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TyG index, triglyceride glucose index; COPD, chronic obstructive pulmonary disease; CHD, coronary heart disease.
3.1.3. Relationship between the TyG index and depression
The results in Table 3 demonstrate a statistically significant association between the TyG index and depression among Americans over the age of 20. The TyG index, which is a marker of insulin resistance, was examined in three models: a crude model with no covariate adjustments (Model 1), a minimally adjusted model (Model 2), and a fully adjusted model accounting for a wide range of potential confounders (Model 3).
Table 3.
|Association Between TyG index and depression.
| β (95%CI), p-value |
|||
|---|---|---|---|
| Crude model (Model 1) [1] | Minimally adjusted model (Model 2) [2] | Fully adjusted model (Model 3) [3] | |
| TyG index | 0.54 (0.42, 0.66) | 0.58 (0.45, 0.72) | 0.38 (0.16, 0.60) |
| <0.0001 | <0.0001 | 0.0008 | |
| Tertiles of TyG index | |||
| T1 | Ref. (1.00) | Ref. (1.00) | Ref. (1.00) |
| T2 | 0.24 (0.04, 0.45) 0.0211 | 0.29 (0.08, 0.50) 0.0078 |
0.01 (−0.28, 0.29) 0.9679 |
| T3 | 0.86 (0.65, 1.07) <0.0001 | 0.86 (0.64, 1.08) <0.0001 | 0.39 (0.05, 0.72) 0.0228 |
1Model 1: no covariates were adjusted.
2Model 2: adjusted for gender, age, race, education, marital status, and family RIP.
3Model 3: adjusted for gender, age, race, education, marital status, family RIP., alcohol, body mass index, waist circumference, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), insulin, asthma, arthrolithiasis, chronic heart failure, CHD, angina pectoris, pulmonary emphysema, stroke, COPD, cancer, diabetes and hypertensive.
In Model 3, after adjusting for all relevant covariates, the TyG index remained significantly negatively associated with depression (β: 0.38, 95 % CI: 0.16–0.60, p = 0.0008). This suggests that higher levels of insulin resistance, as indicated by the TyG index, are associated with lower levels of depression, even when controlling for factors such as demographic variables, lifestyle factors, and comorbid conditions.
To further explore this relationship, we divided the TyG index into tertiles. The results show an interesting trend:In the second tertile (T2) compared to the first tertile (T1), the association with depression was significant in Models 1 and 2 (β: 0.24, 95 % CI: 0.04–0.45, p = 0.0211 in Model 1; β: 0.29, 95 % CI: 0.08–0.50, p = 0.0078 in Model 2). However, in the fully adjusted Model 3, this association was no longer significant (β: 0.01, 95 % CI: 0.28–0.29, p = 0.9679), indicating that the relationship in this middle range of the TyG index may be largely explained by other covariates.In the third tertile (T3), which represents the highest levels of insulin resistance, the TyG index consistently showed a strong positive association with depression across all models (β: 0.86, 95 % CI: 0.65–1.07, p < 0.0001 in Model 1; β: 0.86, 95 % CI: 0.64–1.08, p < 0.0001 in Model 2; β: 0.39, 95 % CI: 0.05–0.72, p = 0.0228 in Model 3). This suggests that individuals with the highest TyG index values are at an increased risk of depression, and this association persists even after adjusting for a wide range of potential confounders.
3.1.4. Subgroup analyses
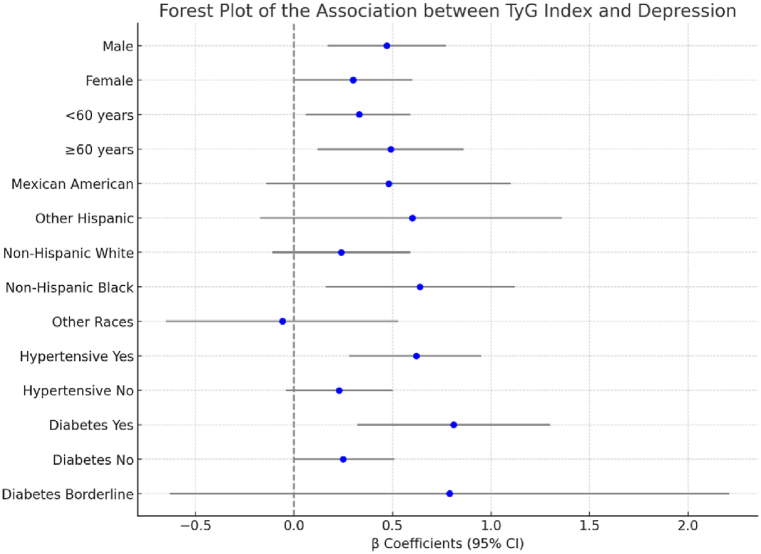
During subgroup analyses conducted by gender, age, race, hypertension, and diabetes in both the male and female populations, a strong association was observed between the TyG index and depression levels. A 100-unit increase in the TyG index was associated with a 0.47-point increase in overall depression scores, with a 0.64-point increase specifically among non-Hispanic Black participants. No significant association was observed in other racial groups. This suggests that while higher TyG index values are generally associated with higher depression scores, the effect is particularly pronounced in the non-Hispanic Black population. The TyG index was significantly and positively associated with depression scores in participants with hypertension, as well as in all populations with or without diabetes. In addition, as illustrated in Fig. 2, no interaction effect was observed between the TyG index and potential mediators (Supplementary Table 1).
Fig. 2.
Forest plot of the Association between TyG index and Depression.
3.1.5. Nonlinear correlation analysis
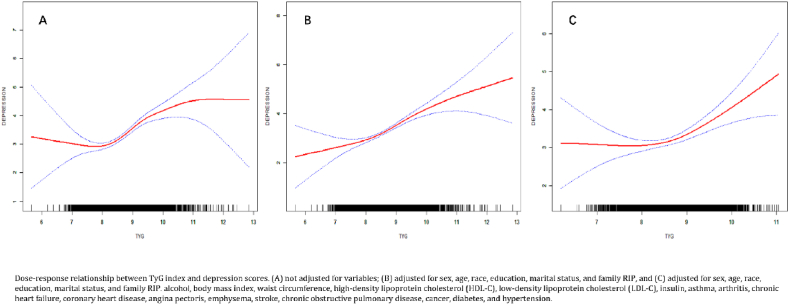
Fig. 3 presents the U-shaped nonlinear connection between the TyG index and depression scores, as determined by nonlinear regression analysis. To investigate this relationship further, we conducted a threshold effect analysis, which is summarized in Table 4. This analysis revealed different effects of the TyG index on depression depending on specific threshold values.
Fig. 3.
Dose-response relationship between the TyG index and depression.
Table 4.
Threshold effects of the TyG index on depression were analysed using piecewise linear regression.
| Outcome | TyG Index |
||
|---|---|---|---|
| β(95%CI) | P value | P for log-likelihood ratio test | |
| Model 1 | |||
| <8.12 | −0.17 (−0.64, 0.30) | 0.4711 | 0.002 |
| >8.12 | 0.70 (0.54, 0.86) | <0.0001 | |
| Model 2 | |||
| <8.73 | −0.03 (−0.38, 0.32) | 0.8810 | 0.003 |
| >8.73 | 0.86 (0.47, 1.24) | <0.0001 | |
| Model 3 | |||
| <8.16 | 0.13 (−0.33, 0.58) | 0.5765 | 0.040 |
| >8.16 | 0.69 (0.52, 0.86) | <0.0001 | |
Model 1: no covariates were adjusted.
Model 2: adjusted for gender, age, race, education, marital status, and family RIP.
Model 3: adjusted for gender, age, race, education, marital status, family RIP., alcohol, body mass index, waist circumference, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), insulin, asthma, arthrolithiasis, chronic heart failure, CHD, angina pectoris, pulmonary emphysema, stroke, COPD, cancer, diabetes and hypertensive.
In the Model 1, below the threshold of 8.12, the TyG index had no significant effect on depression (β: 0.17, 95 % CI: 0.64 - 0.30, p = 0.4711). However, when the TyG index was greater than 8.12, a significant positive effect on depression was observed (β: 0.70, 95 % CI: 0.54–0.86, p < 0.0001) (Fig. 3A).
After adjusting for Gender, Age, Race, Education, Marital Status, and Family RIP, similar to Model 1, no significant effect was observed for TyG index values below 8.73 (β: 0.03, 95 % CI: 0.38 - 0.32, P = 0.8810). For values above 8.73, the TyG index had a significant positive impact on depression scores (β: 0.86, 95 % CI: 0.47–1.24, P < 0.0001) (Fig. 3B). After adjusting for additional covariates like alcohol consumption, BMI, and various health conditions, the TyG index showed a significant positive effect on depression scores only when above the threshold of 8.16 (β: 0.69, 95 % CI: 0.52–0.86, p < 0.0001) (Fig. 3C).
3.2. Machine learning
3.2.1. Identify key risk factors associated with depression
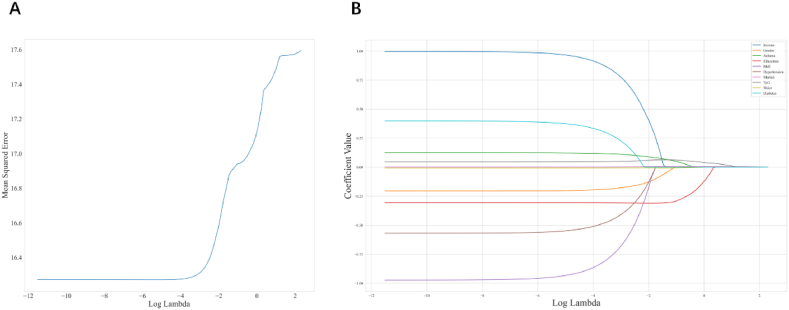
In this study, a risk prediction model was constructed using LASSO regularized regression, which combined 28 covariates to identify the factors most strongly associated with depression (Fig. 4). LASSO regression facilitates variable selection by applying L1 penalties (absolute value penalties) to ordinary least squares regressions, which reduces some coefficients to zero. This approach mitigates overfitting, enhances the generalizability of the model, and performs feature selection, thus improving the interpretability and performance of the model, particularly when dealing with large numbers of correlated features. Coefficient shrinking is accomplished using LASSO regularization by minimizing the L1 term and the loss function, thereby encouraging the reduction of selected coefficients to zero and effectively omitting the corresponding features. In the present study, the preliminary dataset was comprised of 28 variables, including but not limited to RIP, gender, asthma, education, BMI, hypertension, marital status, TyG, waist circumference, diabetes, insulin, emphysema, arthritis, heart failure, age, bronchitis, HDL, ethnicity, alcohol consumption, cancer, triglycerides, stroke, LDL, heart disease, smoking status, angina, and fasting glucose.
Fig. 4.
The LASSO penalized regression analysis for identifying key depressoin-related risk factors.(A) A 10-fold cross-validation of the LASSO regression model.LASSO, least absolute shrinkage and selection operator (B) The coefficient shrinkage process of all 28 covariates,we represent the changes in coefficients of different features under various levels of shrinkage by drawing lines of different colors.
3.2.2. Predictive performance of the model for depression
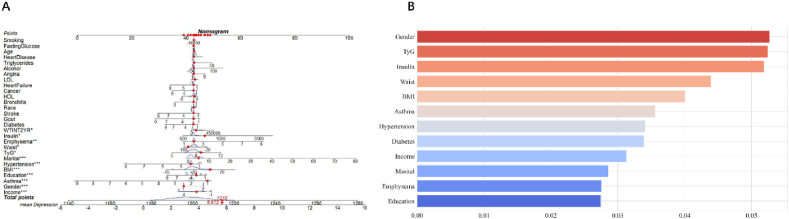
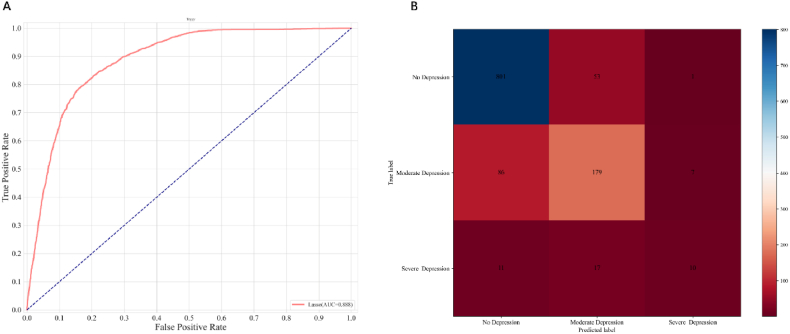
“A nomogram was developed based on twelve statistically significant predictive variables: emphysema, insulin, diabetes, waist circumference, TyG index, marital status, hypertension, BMI, educational level, asthma, gender, and RIP (Fig. 6A). Utilizing these variables and informed by prior research, the depression variable was categorized into three levels: no depression (values ≤ 5), moderate depression (values > 5 and ≤ 19), and severe depression (values > 19). This classification facilitated the construction of a tri-classification model (Fig. 5B). The final Lasso regression model achieved a receiver operating characteristic (ROC) area under the curve (AUC) of 88.8 % and demonstrated an accuracy of 84.98 % on the test data, thereby providing substantial evidence of its prognostic capability for depression (Fig. 5A). Additionally, histograms of the predictor variables were generated and ranked according to their contribution to the model. This analysis identified the twelve covariates most strongly associated with depression, establishing them as the most prominent risk factors (Fig. 6B).”
Fig. 6.
Development and validation of a risk prediction model for depression.(A) Nomogram model based on 12 major relevant risk factors identified by LASSO regression analysis.(B) Importance of 12 covariate characteristics.LASSO, least absolute shrinkage and selection operator.∗P value < 0.05,∗∗P value < 0.01,∗∗∗p value < 0.001.
Fig. 5.
Development and validation of a risk prediction model for depression.(A) ROC curves are used to assess the depression prediction ability of the nomogram model.(B) Confusion matrix result for the trained model.LASSO, least absolute shrinkage and selection operator; ROC,receiver operating characxteristic.∗P value < 0.05,∗∗P value < 0.01,∗∗∗p value < 0.001.
4. Discussion
A total of 11,222 individuals took part in this study, making it the largest sample size for a study on depression to date. As a result, a U-shaped relationship was observed between the TyG index and the cohort's depressed participants, with a notable turning point at a TyG index of 8.16. Furthemore, a strong correlation was also observed between raised TyG index values and a higher risk of depression within a particular range. This suggests that lowering the prevalence of depression could be achieved by maintaining the TyG index within a particular range. These findings underscore the complex interplay between metabolic dysregulation and mental health, providing valuable insights that could inform targeted therapeutic interventions, dietary modifications, and preventative strategies aimed at reducing the risk of depression. Particularly for individuals with diabetes, awareness of the heightened risk of depression associated with increased TyG index values is crucial. Additionally, this study pinpointed the 12 risk factors most strongly associated with depression. As a result, we were able to develop and validate a nomogram model that effectively predicted depression risk, offering a practical tool for healthcare providers to identify and manage individuals at heightened risk of depression.
In recent decades, the prevalence of diabetes mellitus has been increasing year on year, becoming an important issue in the field of global public health [31]. Typical manifestations of diabetes are IR. In this study, the TyG index, which is an indicator of IR, was found to be positively correlated with depression, a correlation that persisted even after accounting for relevant confounders. IR was also found to be associated with depression, even in non-diabetic individuals. Previously, a cross-sectional study from Korea found that IR was correlated with a higher risk of depression, with a 4 % higher prevalence in non-diabetic individuals [32]. Discussions on the role played by lipids in the development of depression are widespread in the literature [33,34]. An elevated TyG index suggests not only insulin resistance but also ill health resulting from metabolic syndromes and lipid metabolism issues [35,36]. Numerous biological functions, including energy storage, cell membrane creation, and molecular signaling, depend on lipids. Phosphatidylcholine was discovered to be a female-specific biomarker by Jiang et al. utilizing UHPLC-Q-TOF-MS when investigating plasma metabolite biomarkers in youthful MDD patients [37]. Depression can trigger a series of inflammatory changes, including the activation of mitogen-activated protein kinases (MAPKs). This activation can lead to the inhibition of extracellular signal-regulated kinase (ERK) by MAPK phosphatase (MKP), ultimately resulting in depression-like behaviors [38]. Another study also found that lower cholesterol levels due to a loss of appetite and weight loss led to a decrease in serotonin, which increased depression-like behaviors [39]. In the present study, a U-shaped relationship was observed between the TyG index and depression, with an inflection point around 8.16. This finding sheds light on the contradictory findings of previous research, wherein a TyG index above 8.16 increases the likelihood of depression, highlighting the need to maintain the index within a particular concentration range. The brain's dependence on glucose and high energy levels for sophisticated information processing could account for this phenomenon. The brain uses a lot of energy, particularly while doing complicated tasks, which raises the need for glucose. Elevated amounts of energy guarantee optimal brain function, facilitating cognitive processes and information-processing capacities. Thus, modifications in energy metabolism may have a direct effect on the brain's capacity for processing information and general functioning. However, long-term elevated blood sugar and fat levels can damage brain tissue by mechanisms including microangiopathy, increased production of reactive oxygen species (ROS), and advanced glycosylation end products, all of which can result in depression [40]. In another study, Lee et al. found that each standard deviation increase in genetically predicted triglycerides was associated with an 18 % increase in the odds of developing depression [41].
In a recent animal study, targeting ROS or p21 in the hippocampus was identified as a promising strategy for alleviating chronic stress-induced anxiety disorders [42]. Mitochondria play a key role in energy metabolism, apoptosis, and oxidative stress by generating ROS through oxidative phosphorylation [43]. The formation of excessive ROS or impaired mitochondrial antioxidant defenses can lead to damage in mitochondrial DNA, proteins, and lipids, which impairs mitochondrial function and triggers cell death through the release of pro-apoptotic factors from the mitochondrial membrane compartment, leading to neuroinflammation and subsequently depression [44,45]. Since glucose produces ATP, maintaining a constant level of glucose in the blood ensures that brain neurons' energy needs are met, supporting the physiological functions of the brain [46]. Exceeding the inflection point, an elevated TyG index heightens the risk of depression. Sustained high blood glucose levels can result in detrimental effects, including mitochondrial damage and the inhibition of ATP production associated with irregular oxidative phosphorylation. According to our findings, the TyG index and depression among U.S. citizens exhibit a U-shaped association, wherein the risk of depression was remarkably strongly and significantly correlated with a TyG score greater than 8.16. This highlights the unfavorable correlation between IR and depression.
5. Strengths and limitations
The large number of participants in this study allowed for subgroup analyses to improve the validity and generalizability of the results. Such a large sample size also facilitated a more comprehensive investigation. Additionally, by using LASSO regression methods and creating a nomogram model, we determined the top risk variables for depression, thereby enabling accurate depression prediction. These findings provide insights valuable for the assessment and identification of individuals at high risk of depression in clinical practice.
This study has some limitations. Although the data was collected from a large and nationally representative sample, research on populations in other countries and regions is necessary. Furthermore, although every effort was made to account for confounding variables, external influences may still exist that could bias the results of this study. In addition, as the study used questionnaires to obtain data from individuals with chronic diseases, bias may exist in the data collection process since some individuals may find it difficult to participate in the interviews. Finally, the use of cross-sectional data to construct our nomogram model limits the predictive power of our findings. Therefore, a degree of caution is required when interpreting these results. The relationship between depression and the TyG index will need to be explored further through prospective studies.
6. Conclusions
In this study, a U-shaped relationship was observed between depression and the TyG index in the U.S. population. Specifically, a TyG index greater than 8.16 was found to be highly correlated with the likelihood of developing depression. In addition, several key risk factors for depression were also identified, from which a nomogram model was created to predict depression in the U.S. population as a whole. These findings suggest that the TyG index represents a predictive tool for depression in the general American population, particularly in individuals with metabolic conditions such as hyperglycemia and hyperlipidemia.
However, due to the inherent limitations of cross-sectional studies, further research is needed to validate these findings in longitudinal studies and to explore the causal relationships between the TyG index and depression. Future research should also investigate the underlying biological mechanisms that link metabolic dysfunction to depression, potentially involving inflammatory pathways or insulin resistance. Moreover, expanding the scope of this research to include diverse populations and considering other potential confounding factors would provide a more comprehensive understanding of how the TyG index can be utilized in clinical practice for depression screening and prevention. Finally, intervention studies could be conducted to determine whether modifying the TyG index through lifestyle changes or medication can effectively reduce the risk of depression.
7. Declarations
The writers employed chatGPT during the writing process to enhance language and readability. The authors took full responsibility for the publication's content after utilizing this tool to evaluate and edit the text as necessary.
Ethics approval and consent to participate
Research Ethics Review Board, and informed consent from all participants was documented. NHANES was approved by the National Center for Health Statistics.
Funding
Shanghai Putuo District Health System Clinical Characteristic Special Disease Construction Project (No. 2023tszb04)
Data availability
The information used in NHANES (NHANES Questionnaires, Datasets, and Related Documentation (cdc.gov)) is freely accessible and supports the study's conclusions.
CRediT authorship contribution statement
Chao Ding: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization. Zhiyu Kong: Writing – review & editing. Jiwei Cheng: Writing – review & editing, Funding acquisition. Rong Huang: Writing – review & editing, Supervision, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e38615.
Abbreviations
- TyG
Triglyceride-Glucose
- NHANES
National Health and Nutrition Examination Survey
- LASSO
Least Absolute Shrinkage and Selection Operator
- AUC
area under the curve
- RIP
income to poverty
- ROC
Receiver Operating Characteristic
- IR
insulin resistance
- HOMA-IR
Homeostasis Model Assessment of Insulin Resistance
- CHD
coronary heart disease
- NCHS
National Center for Health Statistics
- CDC
Centers for Disease Control and Prevention
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- TG
triglycerides
- FBG
fasting blood glucose)
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- MKP
MAPK phosphatase
- ERK
signal-regulated kinase
- ROC
reactive oxygen species
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Herrman H., Patel V., Kieling C., Berk M., Buchweitz C., Cuijpers P., Furukawa T.A., Kessler R.C., Kohrt B.A., Maj M., et al. Time for united action on depression: a lancet–world psychiatric association commission. Lancet. 2022;399:957–1022. doi: 10.1016/S0140-6736(21)02141-3. [DOI] [PubMed] [Google Scholar]
- 2.Chisholm D., Sweeny K., Sheehan P., Rasmussen B., Smit F., Cuijpers P., Saxena S. Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatr. 2016;3:415–424. doi: 10.1016/S2215-0366(16)30024-4. [DOI] [PubMed] [Google Scholar]
- 3.Scherrer J.F., Salas J., Copeland L.A., Stock E.M., Schneider F.D., Sullivan M., Bucholz K.K., Burroughs T., Lustman P.J. Increased risk of depression recurrence after initiation of prescription opioids in noncancer pain patients. J. Pain. 2016;17:473–482. doi: 10.1016/j.jpain.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Q., Wang D., Chen S., Tang L., Ma C. Association of METS-IR index with depressive symptoms in US adults: a cross-sectional study. J. Affect. Disord. 2024;355:355–362. doi: 10.1016/j.jad.2024.03.129. [DOI] [PubMed] [Google Scholar]
- 5.Pillinger T., Beck K., Stubbs B., Howes O.D. Cholesterol and triglyceride levels in first-episode psychosis: systematic review and meta-analysis. Br. J. Psychiatr. : J. Ment. Sci. 2017;211:339–349. doi: 10.1192/bjp.bp.117.200907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strisciuglio T., Izzo R., Barbato E., Di Gioia G., Colaiori I., Fiordelisi A., Morisco C., Bartunek J., Franco D., Ammirati G., et al. Insulin resistance predicts severity of coronary atherosclerotic disease in non-diabetic patients. J. Clin. Med. 2020;9 doi: 10.3390/jcm9072144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai X., Zhang Y., Li M., Wu J.H., Mai L., Li J., Yang Y., Hu Y., Huang Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. Br. Med. J. 2020;370 doi: 10.1136/bmj.m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith G.I., Mittendorfer B., Klein S. Metabolically healthy obesity: facts and fantasies. J. Clin. Invest. 2019;129:3978–3989. doi: 10.1172/jci129186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y., Yang Y., Zhang J., Liu S., Zhuang W. The change of triglyceride-glucose index may predict incidence of stroke in the general population over 45 years old. Cardiovasc. Diabetol. 2023;22:132. doi: 10.1186/s12933-023-01870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simental-Mendía L.E., Rodríguez-Morán M., Guerrero-Romero F.J.M.s., disorders r. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 11.Byers A.L., Yaffe K. Depression and risk of developing dementia. Nat. Rev. Neurol. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y.Y., Zheng R., Cai J.J., Qian S.Z. The association between triglyceride glucose index and depression: data from NHANES 2005-2018. BMC Psychiatr. 2021;21:267. doi: 10.1186/s12888-021-03275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao C., Liu Q., Yang L., Zheng X., Lan P., Koyanagi A., Vancampfort D., Soysal P., Veronese N., Stubbs B., et al. Handgrip strength is associated with suicidal thoughts in men: cross-sectional analyses from NHANES. Scand. J. Med. Sci. Sports. 2020;30:92–99. doi: 10.1111/sms.13559. [DOI] [PubMed] [Google Scholar]
- 14.Behnoush A.H., Mousavi A., Ghondaghsaz E., Shojaei S., Cannavo A., Khalaji A. The importance of assessing the triglyceride-glucose index (TyG) in patients with depression: a systematic review. Neurosci. Biobehav. Rev. 2024;159 doi: 10.1016/j.neubiorev.2024.105582. [DOI] [PubMed] [Google Scholar]
- 15.Park K., Ahn C.W., Lee S.B., Kang S., Nam J.S., Lee B.K., Kim J.H., Park J.S.J.D.c. vol. 42. 2019. pp. 1569–1573. (Elevated TyG Index Predicts Progression of Coronary Artery Calcification). [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee S., Peters S.A., Woodward M., Mejia Arango S., Batty G.D., Beckett N., Beiser A., Borenstein A.R., Crane P.K., Haan M., et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39:300–307. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wimberley T., Horsdal H.T., Brikell I., Laursen T.M., Astrup A., Fanelli G., Bralten J., Poelmans G., Gils V.V., Jansen W.J., et al. Temporally ordered associations between type 2 diabetes and brain disorders - a Danish register-based cohort study. BMC Psychiatr. 2022;22:573. doi: 10.1186/s12888-022-04163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y., Gu Y., Zhang B. Associations of triglyceride-glucose (TyG) index with chest pain incidence and mortality among the U.S. population. Cardiovasc. Diabetol. 2024;23:111. doi: 10.1186/s12933-024-02209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S., Wang Q., Yan X. Association between triglyceride-glucose index and hypertension: a cohort study based on the China Health and Nutrition Survey (2009-2015) BMC Cardiovasc. Disord. 2024;24:168. doi: 10.1186/s12872-024-03747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X., Lin X., Cai Y., Zhang X., Meng H., Chen W., Yu P., Chen X. Association of the triglyceride-glucose index with severity of coronary stenosis and in-hospital mortality in patients with acute ST elevation myocardial infarction after percutaneous coronary intervention: a multicentre retrospective analysis cohort study. BMJ Open. 2024;14 doi: 10.1136/bmjopen-2023-081727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Y., Cen K., Dan B., Zou L., Zhang L., Zhang R., Li H., Cai Q., Aiziretiaili N., Liu Z., Liu Y. Association between triglyceride-glucose index and intracranial/extracranial atherosclerotic stenosis: findings from a retrospective study. Cardiovasc. Diabetol. 2024;23:95. doi: 10.1186/s12933-024-02187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu L., Bao H., Huang X., Zhou W., Wang T., Zhu L., Liu X., Li M., Cheng X. Relationship between the triglyceride glucose index and the risk of first stroke in elderly hypertensive patients. Int. J. Gen. Med. 2022;15:1271–1279. doi: 10.2147/ijgm.S350474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S., Xu L., Wu M., Chen S., Wang Y., Tian Y. Association between triglyceride-glucose index and risk of arterial stiffness: a cohort study. Cardiovasc. Diabetol. 2021;20:146. doi: 10.1186/s12933-021-01342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barak-Corren Y., Castro V.M., Javitt S., Hoffnagle A.G., Dai Y., Perlis R.H., Nock M.K., Smoller J.W., Reis B.Y. Predicting suicidal behavior from longitudinal electronic health records. Am. J. Psychiatr. 2017;174:154–162. doi: 10.1176/appi.ajp.2016.16010077. [DOI] [PubMed] [Google Scholar]
- 25.MEC_Laboratory_Procedures_Manual.pdf. 2017. [Google Scholar]
- 26.McIntyre R.S., Lee Y., Rong C., Rosenblat J.D., Brietzke E., Pan Z., Park C., Subramaniapillai M., Ragguett R.-M., Mansur R.B., et al. Ecological momentary assessment of depressive symptoms using the mind.me application: convergence with the Patient Health Questionnaire-9 (PHQ-9) J. Psychiatr. Res. 2021;135:311–317. doi: 10.1016/j.jpsychires.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Liu H., Nie X., Lu N., Yan S., Wang X., Zhao Y. L-shaped association of triglyceride glucose index and sensorineural hearing loss: results from a cross-sectional study and Mendelian randomization analysis. Front. Endocrinol. 2024;15 doi: 10.3389/fendo.2024.1339731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei X., Xu Z., Chen W. Association of oxidative balance score with sleep quality: NHANES 2007–2014. J. Affect. Disord. 2023;339:435–442. doi: 10.1016/j.jad.2023.07.040. [DOI] [PubMed] [Google Scholar]
- 30.Rajaratnam B., Roberts S., Sparks D., Dalal O. Lasso regression: estimation and shrinkage via the limit of gibbs sampling. J. Roy. Stat. Soc. B Stat. Methodol. 2015;78:153–174. 10.1111/rssb.12106 %J Journal of the Royal Statistical Society Series B: Statistical Methodology. [Google Scholar]
- 31.Guariguata L., Whiting D.R., Hambleton I., Beagley J., Linnenkamp U., Shaw J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.-H., Park S.K., Ryoo J.-H., Oh C.-M., Mansur R.B., Alfonsi J.E., Cha D.S., Lee Y., McIntyre R.S., Jung J.Y.J.J.o.a.d. vol. 208. 2017. pp. 553–559. (The Association between Insulin Resistance and Depression in the Korean General Population). [DOI] [PubMed] [Google Scholar]
- 33.Schulte E.C., Schulze T.G. How do lipids fit into the picture of severe mental health disorders? A missing pathophysiologic link or just a consequence of behavior and treatment. Eur. Neuropsychopharmacol : the journal of the European College of Neuropsychopharmacology. 2024;83:27–29. doi: 10.1016/j.euroneuro.2024.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Mehdi S.M.A., Costa A.P., Svob C., Pan L., Dartora W.J., Talati A., Gameroff M.J., Wickramaratne P.J., Weissman M.M., McIntire L.B.J. Depression and cognition are associated with lipid dysregulation in both a multigenerational study of depression and the National Health and Nutrition Examination Survey. Transl. Psychiatry. 2024;14:142. doi: 10.1038/s41398-024-02847-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bornfeldt K.E., Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metabol. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu B., Wang J., Chen K., Yan W., Wang A., Wang W., Gao Z., Tang X., Yan L., Wan Q., et al. A high triglyceride glucose index is more closely associated with hypertension than lipid or glycemic parameters in elderly individuals: a cross-sectional survey from the Reaction Study. Cardiovasc. Diabetol. 2020;19:112. doi: 10.1186/s12933-020-01077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Y., Qin M., Teng T., Li X., Yu Y., Wang J., Wu H., He Y., Zhou X., Xie P. Identification of sex-specific plasma biomarkers using metabolomics for major depressive disorder in children and adolescents. Front. Psychiatr. 2022;13 doi: 10.3389/fpsyt.2022.929207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu X., Ke S., Wang Q., Zhuang T., Xia C., Xu Y., Yang L., Zhou M.J.B., Pharmacotherapy . vol. 141. 2021. (Energy Metabolism in Major Depressive Disorder: Recent Advances from Omics Technologies and Imaging). [DOI] [PubMed] [Google Scholar]
- 39.Sun S., Yang S., Mao Y., Jia X., Zhang Z. Reduced cholesterol is associated with the depressive-like behavior in rats through modulation of the brain 5-HT1A receptor. Lipids Health Dis. 2015;14:22. doi: 10.1186/s12944-015-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y.M., Qi Y.B., Gao Y.N., Chen W.G., Zhou T., Zang Y., Li J. Astrocyte metabolism and signaling pathways in the CNS. Front. Neurosci. 2023;17 doi: 10.3389/fnins.2023.1217451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khandaker G.M., Zuber V., Rees J.M.B., Carvalho L., Mason A.M., Foley C.N., Gkatzionis A., Jones P.B., Burgess S. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol. Psychiatr. 2020;25:1477–1486. doi: 10.1038/s41380-019-0395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang G., Cao L., Li S., Zhang M., Li Y., Duan J., Li Y., Hu Z., Wu J., Li T., et al. Corticosterone impairs hippocampal neurogenesis and behaviors through p21-mediated ROS accumulation. Biomolecules. 2024;14 doi: 10.3390/biom14030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan W.J., Song L.J.H.r. vol. 434. 2023. p. 108783. (Role of Mitochondrial Dysfunction and Oxidative Stress in Sensorineural Hearing Loss). 108783. [DOI] [PubMed] [Google Scholar]
- 44.Taanman J.W. The mitochondrial genome: structure, transcription, translation and replication. Biochim. Biophys. Acta. 1999;1410:103–123. doi: 10.1016/s0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 45.Su L., Lu H., Zhang D., Zhu X., Li J., Zong Y., Zhao Y., He Z., Chen W., Du R. Total paeony glycoside relieves neuroinflammation to exert antidepressant effect via the interplay between NLRP3 inflammasome, pyroptosis and autophagy. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2024;128 doi: 10.1016/j.phymed.2024.155519. [DOI] [PubMed] [Google Scholar]
- 46.Mergenthaler P., Lindauer U., Dienel G.A., Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587–597. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The information used in NHANES (NHANES Questionnaires, Datasets, and Related Documentation (cdc.gov)) is freely accessible and supports the study's conclusions.