Abstract
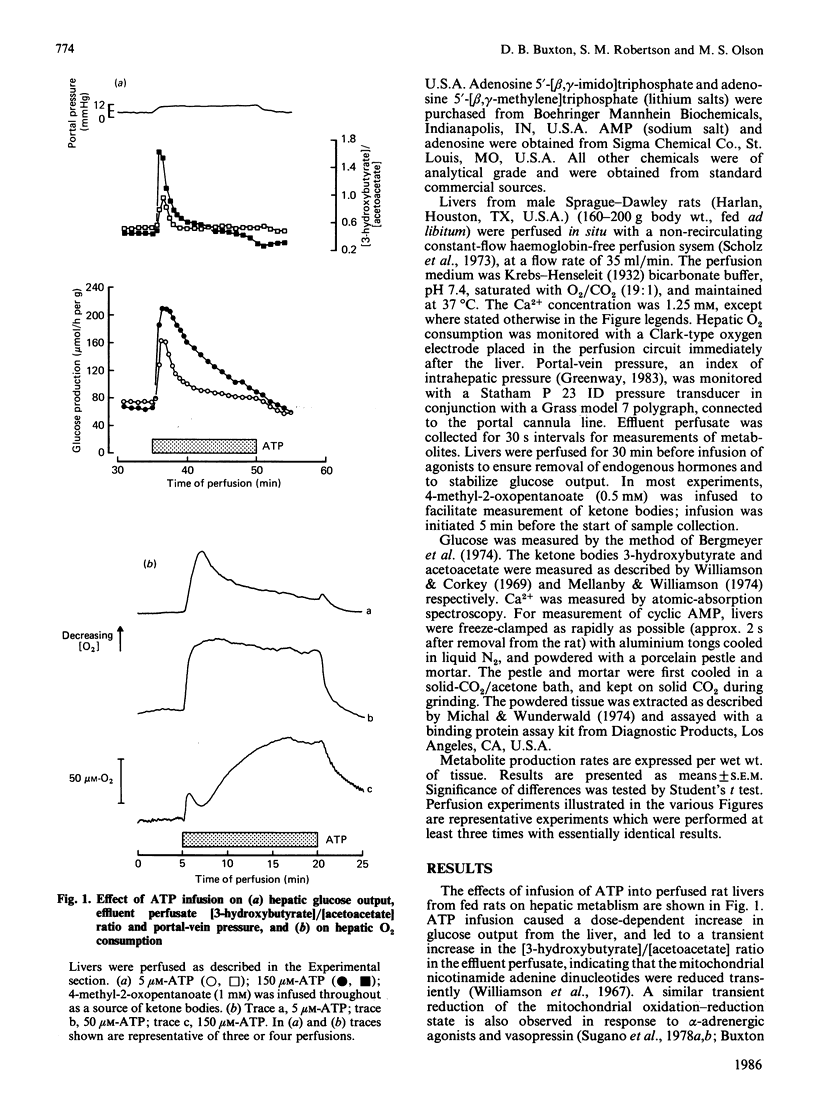
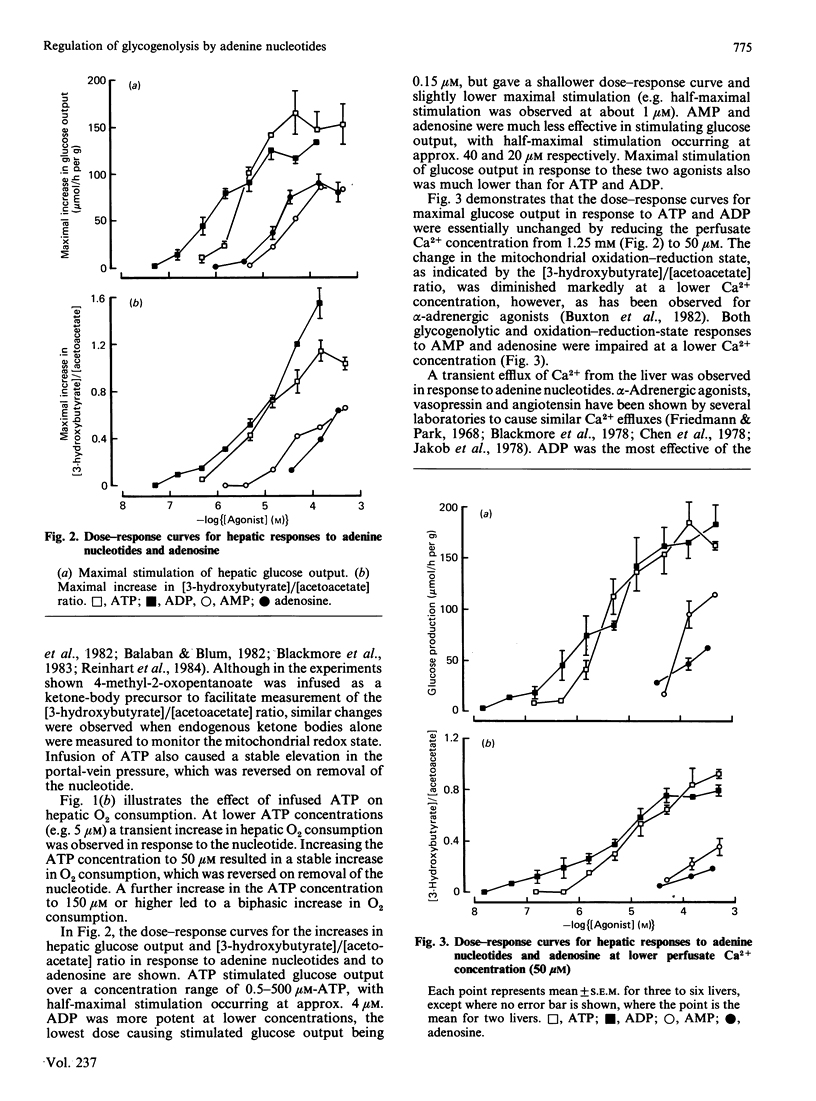
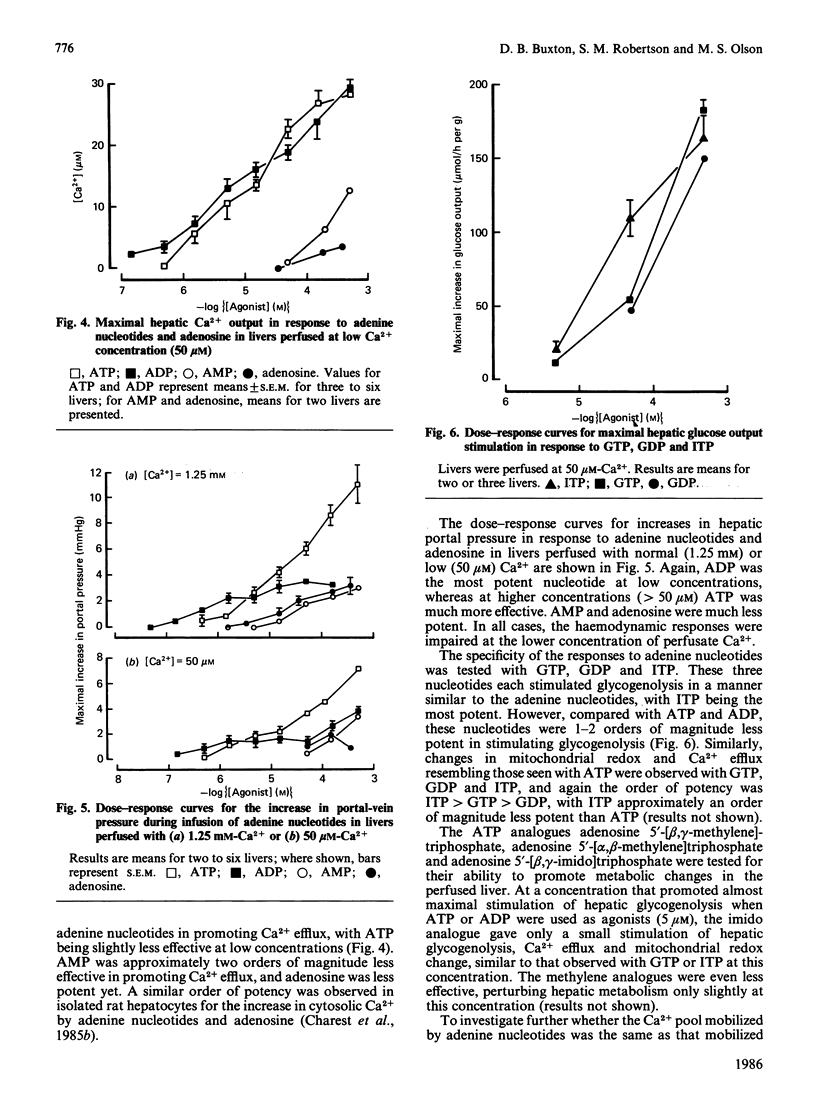
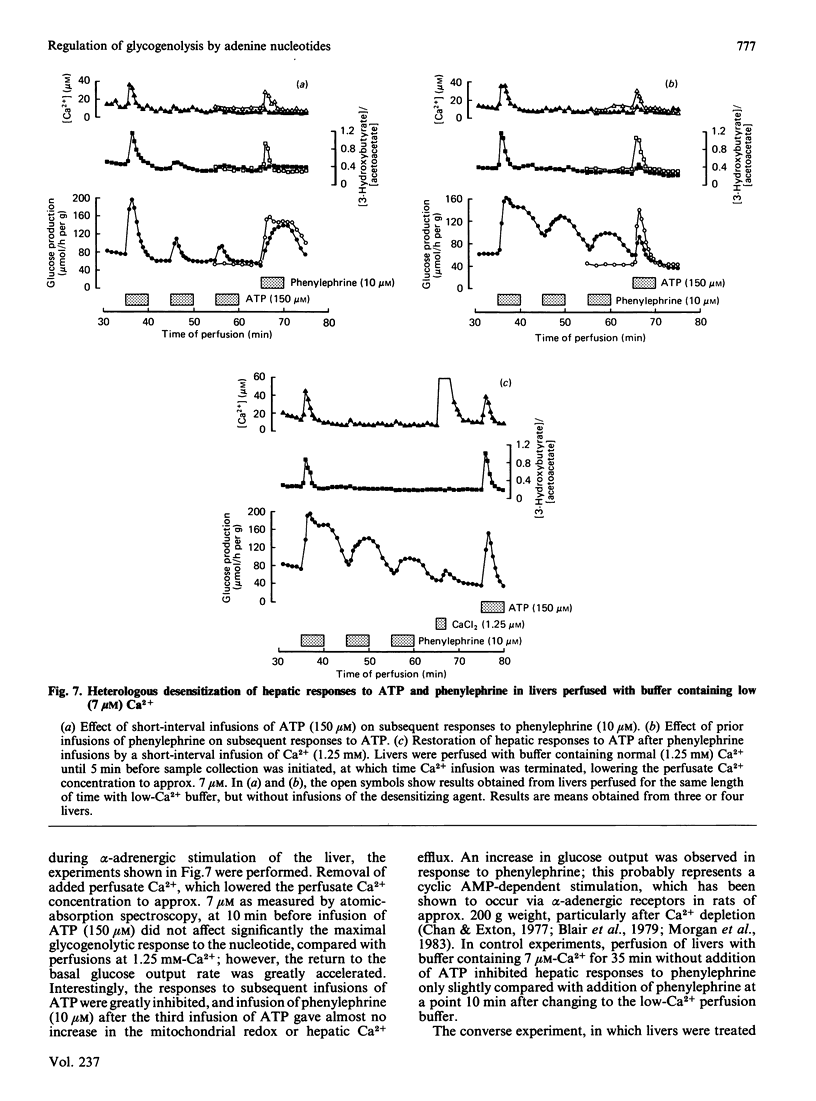
Infusion of adenine nucleotides and adenosine into perfused rat livers resulted in stimulation of hepatic glycogenolysis, transient increases in the effluent perfusate [3-hydroxybutyrate]/[acetoacetate] ratio, and increased portal vein pressure. In livers perfused with buffer containing 50 microM-Ca2+, transient efflux of Ca2+ was seen on stimulation of the liver with adenine nucleotides or adenosine. ADP was the most potent of the nucleotides, stimulating glucose output at concentrations as low as 0.15 microM, with half-maximal stimulation at approx. 1 microM, and ATP was slightly less potent, half-maximal stimulation requiring 4 microM-ATP. AMP and adenosine were much less effective, doses giving half-maximal stimulation being 40 and 20 microM respectively. Non-hydrolysed ATP analogues were much less effective than ATP in promoting changes in hepatic metabolism. ITP, GTP and GDP caused similar changes in hepatic metabolism to ATP, but were 10-20 times less potent than ATP. In livers perfused at low (7 microM) Ca2+, infusion of phenylephrine before ATP desensitized hepatic responses to ATP. Repeated infusions of ATP in such low-Ca2+-perfused livers caused homologous desensitization of ATP responses, and also desensitized subsequent Ca2+-dependent responses to phenylephrine. A short infusion of Ca2+ (1.25 mM) after phenylephrine infusion restored subsequent responses to ATP, indicating that, during perfusion with buffer containing 7 microM-Ca2+, ATP and phenylephrine deplete the same pool of intracellular Ca2+, which can be rapidly replenished in the presence of extracellular Ca2+. Measurement of cyclic AMP in freeze-clamped liver tissue demonstrated that adenosine (150 microM) significantly increased hepatic cyclic AMP, whereas ATP (15 microM) was without effect. It is concluded that ATP and ADP stimulate hepatic glycogenolysis via P2-purinergic receptors, through a Ca2+-dependent mechanism similar to that in alpha-adrenergic stimulation of hepatic tissue. However, adenosine stimulates glycogenolysis via P1-purinoreceptors and/or uptake into the cell, at least partially through a mechanism involving increase in cyclic AMP. Further, the hepatic response to adenine nucleotides may be significant in regulating hepatic glucose output in physiological and pathophysiological states.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartrons R., Van Schaftingen E., Hers H. G. The ability of adenosine to decrease the concentration of fructose 2,6-bisphosphate in isolated hepatocytes. A cyclic AMP-mediated effect. Biochem J. 1984 Feb 15;218(1):157–163. doi: 10.1042/bj2180157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo G., Nicotera P., Orrenius S. Alterations in intracellular calcium compartmentation following inhibition of calcium efflux from isolated hepatocytes. Eur J Biochem. 1984 Oct 1;144(1):19–23. doi: 10.1111/j.1432-1033.1984.tb08425.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Brumley F. T., Marks J. L., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Relationship between alpha-adrenergic stimulation of calcium efflux and activation of phosphorylase in isolated rat liver parenchymal cells. J Biol Chem. 1978 Jul 25;253(14):4851–4858. [PubMed] [Google Scholar]
- Blackmore P. F., Hughes B. P., Charest R., Shuman E. A., 4th, Exton J. H. Time course of alpha1-adrenergic and vasopressin actions on phosphorylase activation, calcium efflux, pyridine nucleotide reduction, and respiration in hepatocytes. J Biol Chem. 1983 Sep 10;258(17):10488–10494. [PubMed] [Google Scholar]
- Blair J. B., James M. E., Foster J. L. Adrenergic control of glucose output and adenosine 3':5'-monophosphate levels in hepatocytes from juvenile and adult rats. J Biol Chem. 1979 Aug 25;254(16):7579–7584. [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of catecholamines, ATP and ionophore A23187 on potassium and calcium movements in isolated hepatocytes. Nature. 1979 Jun 7;279(5713):544–546. doi: 10.1038/279544a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Crowe R., Kennedy C., Török J. Indirect evidence that purinergic modulation of perivascular adrenergic neurotransmission in the portal vein is a physiological process. Br J Pharmacol. 1984 Jun;82(2):359–368. doi: 10.1111/j.1476-5381.1984.tb10770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D., Barron L. L., Olson M. S. The effects of alpha-adrenergic agonists on the regulation of the branched chain alpha-ketoacid oxidation in the perfused rat liver. J Biol Chem. 1982 Dec 10;257(23):14318–14323. [PubMed] [Google Scholar]
- Chan T. M., Exton J. H. alpha-Adrenergic-mediated accumulation of adenosine 3':5' monophosphate in calcium-depleted hepatocytes. J Biol Chem. 1977 Dec 10;252(23):8645–8651. [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors: targets for future drugs. J Med Chem. 1982 Mar;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- DeWitt L. M., Putney J. W., Jr Alpha-adrenergic stimulation of potassium efflux in guinea-pig hepatocytes may involve calcium influx and calcium release. J Physiol. 1984 Jan;346:395–407. doi: 10.1113/jphysiol.1984.sp015030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt L. M., Putney J. W., Jr Stimulation of glycogenolysis in hepatocytes by angiotensin II may involve both calcium release and calcium influx. FEBS Lett. 1983 Aug 22;160(1-2):259–263. doi: 10.1016/0014-5793(83)80978-8. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Shepherd R. E. Adenosine, cyclic AMP metabolism, and glycogenolysis in rat liver cells. J Biol Chem. 1977 Nov 25;252(22):8066–8070. [PubMed] [Google Scholar]
- Friedmann N., Park C. R. Early effects of 3',5'-adenosine monophosphate on the fluxes of calcium end potassium in the perfused liver of normal and adrenalectomized rats. Proc Natl Acad Sci U S A. 1968 Oct;61(2):504–508. doi: 10.1073/pnas.61.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway C. V. Role of splanchnic venous system in overall cardiovascular homeostasis. Fed Proc. 1983 Apr;42(6):1678–1684. [PubMed] [Google Scholar]
- Ingerman-Wojenski C., Smith J. B., Silver M. J. Evaluation of electrical aggregometry: comparison with optical aggregometry, secretion of ATP, and accumulation of radiolabeled platelets. J Lab Clin Med. 1983 Jan;101(1):44–52. [PubMed] [Google Scholar]
- Ismail N. A., Hems D. A. Effects of adenosine on glucose and lipid metabolism and hepatic blood flow. Biochem Pharmacol. 1978 May 1;27(9):1341–1345. doi: 10.1016/0006-2952(78)90117-x. [DOI] [PubMed] [Google Scholar]
- Krell H., Ermisch N., Kasperek S., Pfaff E. On the mechanisms of ATP-induced and succinate-induced redistribution of cations in isolated rat liver cells. Eur J Biochem. 1983 Mar 15;131(2):247–254. doi: 10.1111/j.1432-1033.1983.tb07256.x. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley P., Humphrey P. P. A method for quantitating platelet aggregation and analyzing drug-receptor interactions on platelets in whole blood in vitro. J Pharmacol Methods. 1981 Sep;6(2):153–166. doi: 10.1016/0160-5402(81)90038-3. [DOI] [PubMed] [Google Scholar]
- Morgan N. G., Blackmore P. F., Exton J. H. Age-related changes in the control of hepatic cyclic AMP levels by alpha 1- and beta 2-adrenergic receptors in male rats. J Biol Chem. 1983 Apr 25;258(8):5103–5109. [PubMed] [Google Scholar]
- Pearson J. D., Gordon J. L. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4;281(5730):384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- Prpić V., Blackmore P. F., Exton J. H. Phosphatidylinositol breakdown induced by vasopressin and epinephrine in hepatocytes is calcium-dependent. J Biol Chem. 1982 Oct 10;257(19):11323–11331. [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Calcium ion fluxes induced by the action of alpha-adrenergic agonists in perfused rat liver. Biochem J. 1982 Dec 15;208(3):619–630. doi: 10.1042/bj2080619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The contribution of both extracellular and intracellular calcium to the action of alpha-adrenergic agonists in perfused rat liver. Biochem J. 1984 May 15;220(1):35–42. doi: 10.1042/bj2200035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz R., Hansen W., Thurman R. G. Interaction of mixed-function oxidation with biosynthetic processes. 1. Inhibition of gluconeogenesis by aminopyrine in perfused rat liver. Eur J Biochem. 1973 Sep 21;38(1):64–72. doi: 10.1111/j.1432-1033.1973.tb03034.x. [DOI] [PubMed] [Google Scholar]
- Schütz W., Tuisl E., Kraupp O. Adenosine receptor agonists: binding and adenylate cyclase stimulation in rat liver plasma membranes. Naunyn Schmiedebergs Arch Pharmacol. 1982 Apr;319(1):34–39. doi: 10.1007/BF00491475. [DOI] [PubMed] [Google Scholar]
- Sugano T., Shiota M., Khono H., Shimada M., Oshino N. Effects of calcium ions on the activation of gluconeogenesis by norepinephrine in perfused rat liver. J Biochem. 1980 Feb;87(2):465–472. doi: 10.1093/oxfordjournals.jbchem.a132766. [DOI] [PubMed] [Google Scholar]
- Sugano T., Shiota M., Tanaka T., Miyamae Y., Shimada M., Oshino N. Intracellular redox state and stimulation of gluconeogenesis by glucagon and norepinephrine in the perfused rat liver. J Biochem. 1980 Jan;87(1):153–166. doi: 10.1093/oxfordjournals.jbchem.a132721. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]


