Abstract
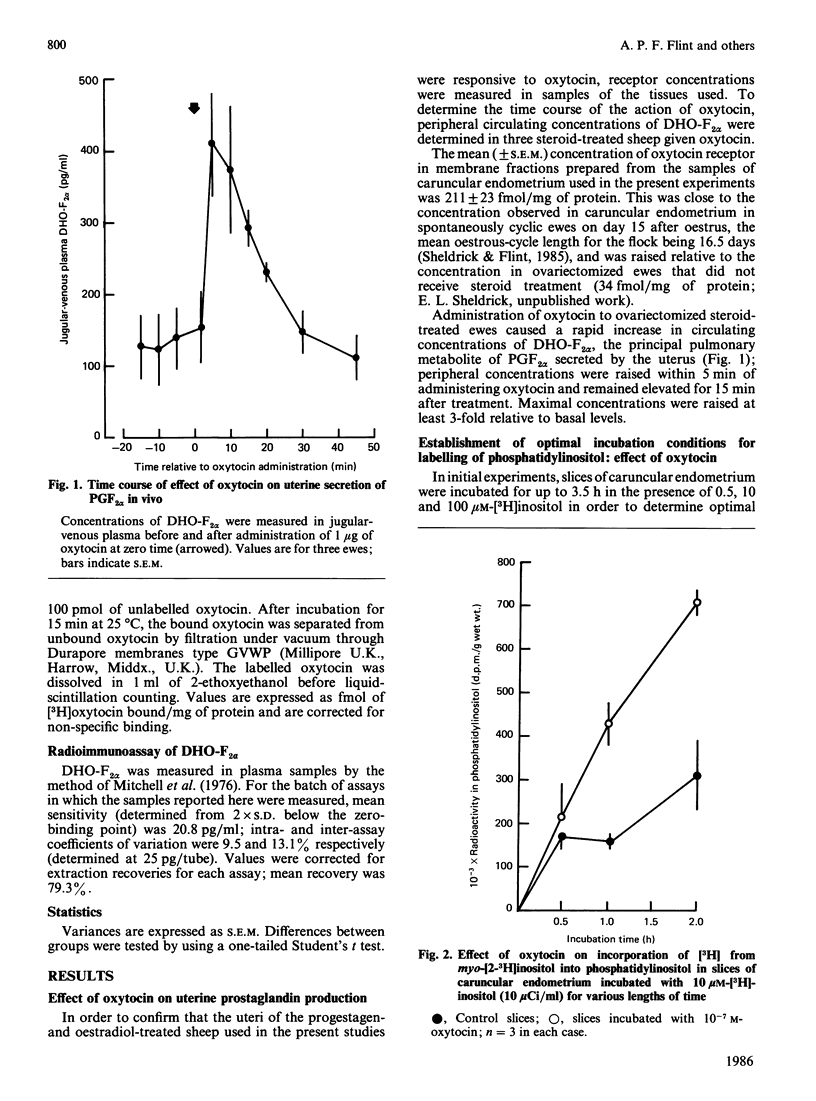
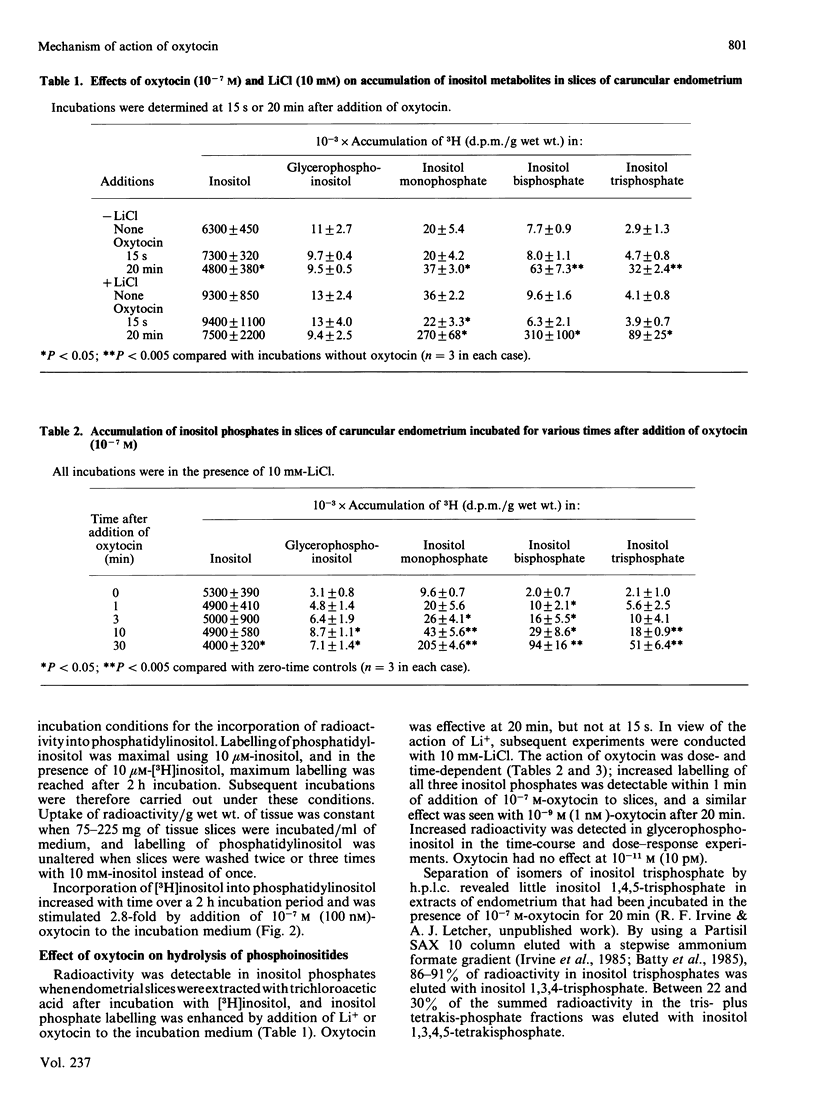
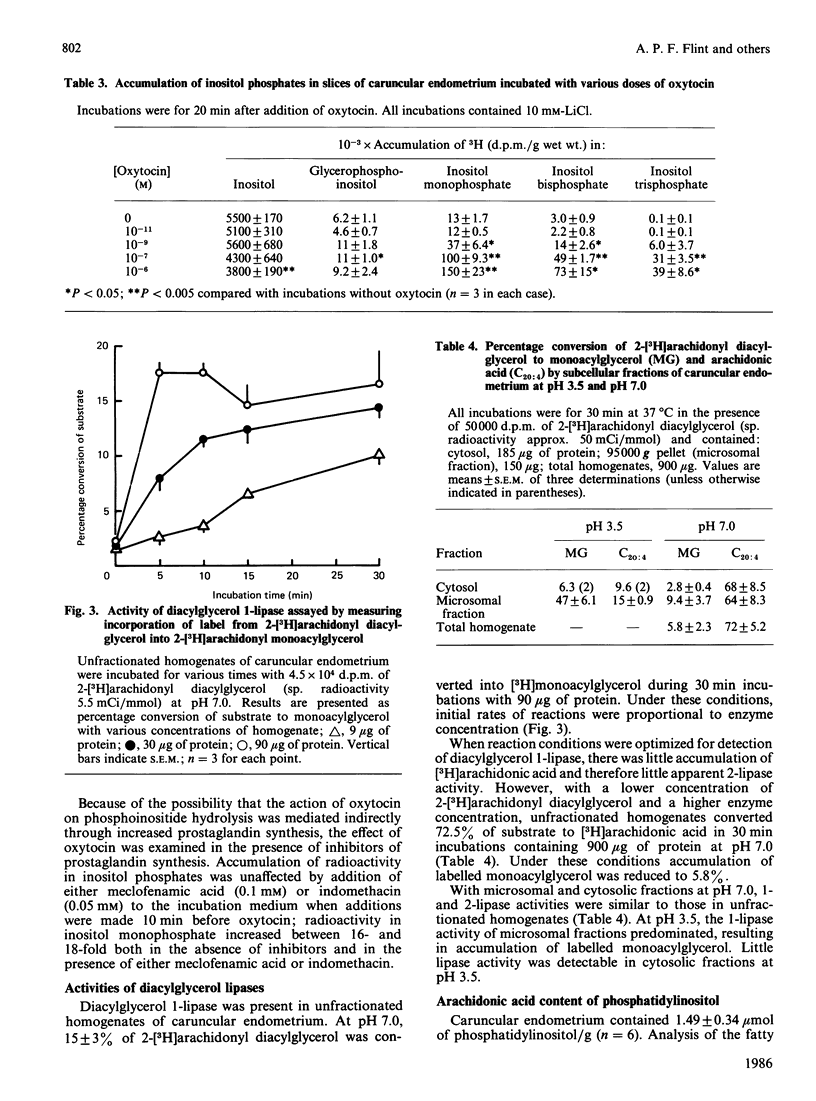
Slices of caruncular endometrium from steroid-treated ovariectomized sheep were incubated with myo-[2-3H]inositol to label tissue phosphatidylinositol. Effects of oxytocin were determined on the rate of incorporation of radioactivity into phosphatidylinositol and on the hydrolysis of phosphoinositides to inositol phosphates and diacylglycerol. Incorporation of radioactivity into phosphatidylinositol was linear during 2 h incubations; 10(-7) M (100 nM)-oxytocin caused a 2.8-fold increase in the rate of incorporation. In the presence of Li+, addition of 10(-7) M-oxytocin to slices in which phosphatidylinositol was pre-labelled caused mean increase of 40-fold in the incorporation of radioactivity into inositol mono-, bis- and tris-phosphates. Inositol 1,3,4-trisphosphate was quantitatively the major trisphosphate formed. The action of oxytocin on phosphoinositide hydrolysis was dose- and time-dependent, occurring at concentrations within the range observed in plasma during episodes of secretion in vivo, and with a time course comparable with that of the action of oxytocin on uterine prostaglandin production. The effect of oxytocin on incorporation of radioactivity into inositol phosphates was not affected by inhibitors of prostaglandin synthesis. Diacylglycerol 1- and 2-lipases in caruncular endometrium converted up to 72% of added 2-[3H]arachidonyldiacylglycerol into [3H]arachidonic acid during 30 min incubations at pH 7.0. Caruncular endometrium contained 1.49 mumol of phosphatidylinositol/g, representing approx. 0.2 mumol/g of phosphatidylinositol arachidonic acid. It is proposed that the stimulation of endometrial prostaglandin synthesis by oxytocin is accounted for by increased hydrolysis of phosphoinositides to diacylglycerol and inositol phosphates with subsequent release of arachidonic acid from diacylglycerol.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Balla T., Enyedi P., Spät A., Antoni F. A. Pressor-type vasopressin receptors in the adrenal cortex: properties of binding, effects on phosphoinositide metabolism and aldosterone secretion. Endocrinology. 1985 Jul;117(1):421–423. doi: 10.1210/endo-117-1-421. [DOI] [PubMed] [Google Scholar]
- Bauduin H., Galand N., Boeynaems J. M. In vitro stimulation of prostaglandin synthesis in the rat pancreas by carbamylcholine, caerulein and secretin. Prostaglandins. 1981 Jul;22(1):35–51. doi: 10.1016/0090-6980(81)90052-6. [DOI] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney R. C. Measurement of phospholipase A2 activity in human endometrium during the menstrual cycle. J Endocrinol. 1985 Nov;107(2):183–189. doi: 10.1677/joe.0.1070183. [DOI] [PubMed] [Google Scholar]
- Brinsfield T. H., Hawk H. W. Control by progesterone of the concentration of lipid droplets in epithelial cells of the sheep endometrium. J Anim Sci. 1973 May;36(5):919–922. doi: 10.2527/jas1973.365919x. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabot M. C., Gatt S. Hydrolysis of neutral glycerides by lipases of rat brain microsomes. Biochim Biophys Acta. 1976 Apr 22;431(1):105–115. doi: 10.1016/0005-2760(76)90264-2. [DOI] [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau L. Y., Tai H. H. Release of arachidonate from diglyceride in human platelets requires the sequential action of a diglyceride lipase and a monoglyceride lipase. Biochem Biophys Res Commun. 1981 Jun;100(4):1688–1695. doi: 10.1016/0006-291x(81)90713-0. [DOI] [PubMed] [Google Scholar]
- Cooke R. G., Homeida A. M. Suppression of prostaglandin F-2 alpha release and delay of luteolysis after active immunization against oxytocin in the goat. J Reprod Fertil. 1985 Sep;75(1):63–68. doi: 10.1530/jrf.0.0750063. [DOI] [PubMed] [Google Scholar]
- Cooke R. G., Knifton A. Oxytocin-induced oestrus in the goat. Theriogenology. 1981 Jul;16(1):95–97. doi: 10.1016/0093-691x(81)90117-5. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J. Concentrations of glycerides and phospholipids in rat heart and gastrocnemius muscles. Effects of alloxan-diabetes and perfusion. Biochem J. 1967 Aug;104(2):416–422. doi: 10.1042/bj1040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing I., Poyser N. L. Estimation of phospholipase A2 activity in guinea-pig endometrium on days 7 and 16 of the estrous cycle. Prostaglandins Leukot Med. 1983 Sep;12(1):107–117. doi: 10.1016/0262-1746(83)90073-2. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Berridge M. J. Relationship between hormonal activation of phosphatidylinositol hydrolysis, fluid secretion and calcium flux in the blowfly salivary gland. Biochem J. 1979 Jan 15;178(1):45–58. doi: 10.1042/bj1780045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough R. J., Moore L. G., McGowan L. T., Peterson A. J., Smith J. F., Tervit H. R., Watkins W. B. Temporal relationship between plasma concentrations of 13,14-dihydro-15-keto-prostaglandin F and neurophysin I/II around luteolysis in sheep. Prostaglandins. 1980 Aug;20(2):199–208. doi: 10.1016/s0090-6980(80)80039-6. [DOI] [PubMed] [Google Scholar]
- Findlay J. K., Ackland N., Burton R. D., Davis A. J., Walker F. M., Walters D. E., Heap R. B. Protein, prostaglandin and steroid synthesis in caruncular and intercaruncular endometrium of sheep before implantation. J Reprod Fertil. 1981 Jul;62(2):361–377. doi: 10.1530/jrf.0.0620361. [DOI] [PubMed] [Google Scholar]
- Flint A. P., Sheldrick E. L. Evidence for a systemic role for ovarian oxytocin in luteal regression in sheep. J Reprod Fertil. 1983 Jan;67(1):215–225. doi: 10.1530/jrf.0.0670215. [DOI] [PubMed] [Google Scholar]
- Gröbner P., Loidl P. ADP-ribosyltransferase in isolated nuclei during the cell cycle of Physarum polycephalum. Biochem J. 1985 Nov 15;232(1):21–24. doi: 10.1042/bj2320021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Higgins J. A., Dawson R. M. Asymmetry of the phospholipid bilayer of rat liver endoplasmic reticulum. Biochim Biophys Acta. 1977 Nov 1;470(3):342–356. doi: 10.1016/0005-2736(77)90126-2. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Holub B. J., Kuksis A., Thompson W. Molecular species of mono-, di-, and triphosphoinositides of bovine brain. J Lipid Res. 1970 Nov;11(6):558–564. [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Dawson R. M. Transfer of arachidonic acid between phospholipids in rat liver microsomes. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1399–1405. doi: 10.1016/0006-291x(79)91222-1. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk C. J., Creba J. A., Downes C. P., Michell R. H. Hormone-stimulated metabolism of inositol lipids and its relationship to hepatic receptor function. Biochem Soc Trans. 1981 Oct;9(5):377–379. doi: 10.1042/bst0090377. [DOI] [PubMed] [Google Scholar]
- Mauco G., Chap H., Simon M. F., Douste-Blazy L. Phosphatidic and lysophosphatidic acid production in phospholipase C-and thrombin-treated platelets. Possible involvement of a platelet lipase. Biochimie. 1978 Sep 29;60(6-7):653–661. doi: 10.1016/s0300-9084(78)80784-6. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Jafferji S. S., Jones L. M. The possible involvement of phosphatidylinositol breakdown in the mechanism of stimulus-response coupling at receptors which control cell-surface calcium gates. Adv Exp Med Biol. 1977;83:447–464. doi: 10.1007/978-1-4684-3276-3_41. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Billah M. M. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979 Oct;7(5):861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Stimulated inositol lipid metabolism: an introduction. Cell Calcium. 1982 Oct;3(4-5):285–294. doi: 10.1016/0143-4160(82)90017-3. [DOI] [PubMed] [Google Scholar]
- Mitchell M. D., Flint A. P., Turnbull A. C. Plasma concentrations of 13,14-dihydro-15-keto-prostaglandin F during pregnancy in sheep. Prostaglandins. 1976 Feb;11(2):319–329. doi: 10.1016/0090-6980(76)90154-4. [DOI] [PubMed] [Google Scholar]
- Mitchell M. D., Flint A. P., Turnbull A. C. Stimulation by oxytocin of prostaglandin f levels in uterine venous effluent in pregnant and puerperal sheep. Prostaglandins. 1975 Jan;9(1):47–56. doi: 10.1016/s0090-6980(75)80115-8. [DOI] [PubMed] [Google Scholar]
- Rholam M., Nicolas P., Cohen P. Salt-dependent structural changes of neurohormones: lithium ions induce conformational rearrangements of ocytocin to a vasopressin-like structure. Biochemistry. 1985 Jun 18;24(13):3345–3349. doi: 10.1021/bi00334a040. [DOI] [PubMed] [Google Scholar]
- Richards D. E., Irvine R. F., Dawson R. M. Hydrolysis of membrane phospholipids by phospholipases of rat liver lysosomes. Biochem J. 1979 Aug 15;182(2):599–606. doi: 10.1042/bj1820599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. S., McCracken J. A., Gavagan J. E., Soloff M. S. Oxytocin-stimulated release of prostaglandin F2alpha from ovine endometrium in vitro: correlation with estrous cycle and oxytocin-receptor binding. Endocrinology. 1976 Oct;99(4):1107–1114. doi: 10.1210/endo-99-4-1107. [DOI] [PubMed] [Google Scholar]
- SELDINGER S. I. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953 May;39(5):368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- Schams D., Prokopp S., Barth D. The effect of active and passive immunization against oxytocin on ovarian cyclicity in ewes. Acta Endocrinol (Copenh) 1983 Jul;103(3):337–344. doi: 10.1530/acta.0.1030337. [DOI] [PubMed] [Google Scholar]
- Sharma S. C., Fitzpatrick R. J. Effect of oestradiol-17beta and oxytocin treatment on prostaglandin F alpha release in the anoestrous ewe. Prostaglandins. 1974 Apr 25;6(2):97–105. doi: 10.1016/0090-6980(74)90021-5. [DOI] [PubMed] [Google Scholar]
- Sheldrick E. L., Flint A. P. Endocrine control of uterine oxytocin receptors in the ewe. J Endocrinol. 1985 Aug;106(2):249–258. doi: 10.1677/joe.0.1060249. [DOI] [PubMed] [Google Scholar]
- Sheldrick E. L., Mitchell M. D., Flint A. P. Delayed luteal regression in ewes immunized against oxytocin. J Reprod Fertil. 1980 May;59(1):37–42. doi: 10.1530/jrf.0.0590037. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Thorburn G. D., Cox R. I., Currie W. B., Restall B. J., Schneider W. Prostaglandin F and progesterone concentrations in the utero-ovarian venous plasma of the ewe during the oestrous cycle and early pregnancy. J Reprod Fertil Suppl. 1973 Jul;18:151–158. [PubMed] [Google Scholar]


