Abstract
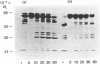
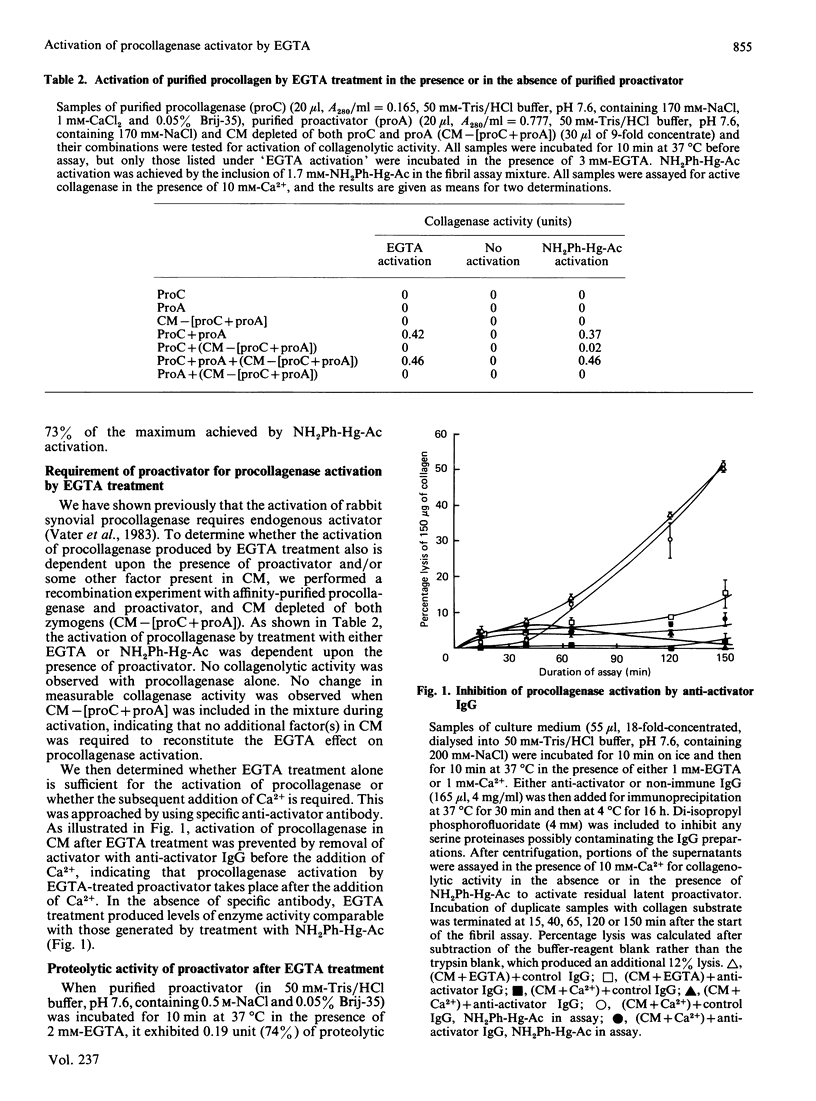
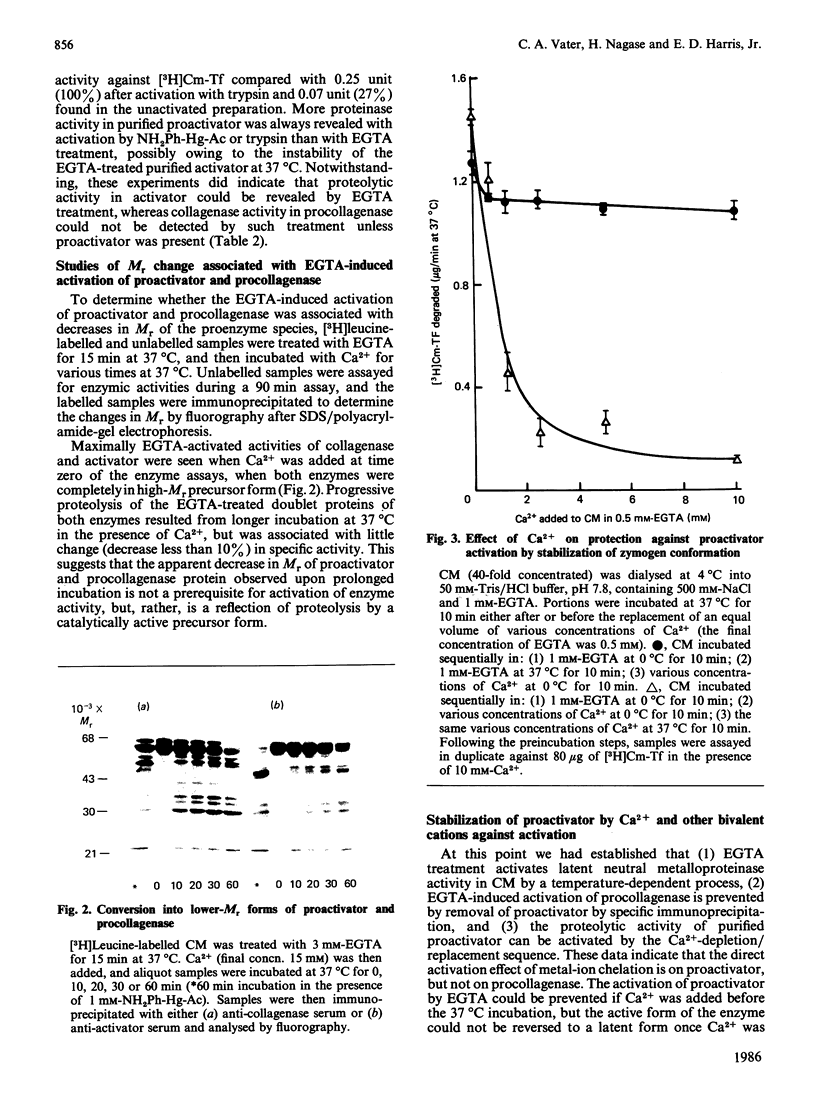
A new mechanism for activation of the proactivator of procollagenase [Vater, Nagase & Harris (1983) J. Biol. Chem. 258, 9374-9382] has been found. Collagenolytic and other proteolytic enzyme activities in the medium of cultured rabbit synovial fibroblasts were found to be activated by a new mechanism: short-term incubation at 37 degrees C performed in the presence of EGTA followed by replacement of Ca2+ during enzyme assay. The crucial event in procollagenase activation is the production of a functional activator enzyme. Activation of procollagenase in the culture medium did not occur when proactivator was removed by immunoprecipitation. Proteolytic activity of proactivator was fully activated, whereas procollagenase alone could not be activated by the same sequence. EGTA treatment of the culture medium at 0 degrees C did not result in enzyme activation if Ca2+ was replaced before incubation at 37 degrees C. Certain other bivalent metal ions (e.g. Sn2+, Cd2+, Zn2+ and Mn2+) could substitute for Ca2+ to stabilize the proactivator as a zymogen and therefore prevent the appearance of proteolytic activity in culture medium. Isolation of proactivator and procollagenase from EGTA-treated radiolabelled culture medium by immunoprecipitation and subsequent analyses by fluorography revealed that a time-dependent proteolysis of both molecules occurred after replacement of Ca2+ and incubation at 37 degrees C. However, comparison of enzyme activity with fluorographic analyses showed that the maximal activation of both enzymes was achieved before any detectable decrease in Mr. The results suggest that the activation of proactivator and the subsequent activation of procollagenase may be initiated by conformational changes in structure of the proactivator molecule produced by removal of stabilizing bivalent metal ions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer E. A., Stricklin G. P., Jeffrey J. J., Eisen A. Z. Collagenase production by human skin fibroblasts. Biochem Biophys Res Commun. 1975 May 5;64(1):232–240. doi: 10.1016/0006-291x(75)90243-0. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Cobb C. M., Taylor R. E., Fullmer H. M. Synthesis and release of procollagenase by cultured fibroblasts. J Biol Chem. 1976 May 25;251(10):3162–3168. [PubMed] [Google Scholar]
- Birkedal-Hansen H., Taylor R. E. Detergent-activation of latent collagenase and resolution of its component molecules. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1173–1178. doi: 10.1016/s0006-291x(82)80120-4. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., McMillan R. M., Fahey J. V., Harris E. D., Jr Collagenase production by synovial fibroblasts treated with phorbol myristate acetate. Arthritis Rheum. 1979 Oct;22(10):1109–1116. doi: 10.1002/art.1780221010. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Mercer E., Tyler J. A. The activation of latent pig synovial collagenase. Biochim Biophys Acta. 1981 Jan 15;657(1):73–83. doi: 10.1016/0005-2744(81)90131-5. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Tyler J. A. Purification of pig synovial collagenase to high specific activity. Biochem J. 1979 Dec 1;183(3):647–656. doi: 10.1042/bj1830647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang T. F., Sargeant R. B., Hougie C. The effect of calcium ions on the properties of factor IX and its activated form. Br J Haematol. 1974 Jun;27(2):281–287. doi: 10.1111/j.1365-2141.1974.tb06794.x. [DOI] [PubMed] [Google Scholar]
- Drabikowski W., Kuznicki J., Grabarek Z. Similarity in Ca2+-induced changes between troponic-C and protein activator of 3':5'-cyclic nucleotide phosphodiesterase and their tryptic fragments. Biochim Biophys Acta. 1977 Nov 23;485(1):124–133. doi: 10.1016/0005-2744(77)90199-1. [DOI] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLIMCHER M. J., FRANCOIS C. J., RICHARDS L., KRANE S. M. THE PRESENCE OF ORGANIC PHOSPHORUS IN COLLAGENS AND GELATINS. Biochim Biophys Acta. 1964 Dec 9;93:585–602. doi: 10.1016/0304-4165(64)90342-3. [DOI] [PubMed] [Google Scholar]
- Gisslow M. T., McBride B. C. A rapid sensitive collagenase assay. Anal Biochem. 1975 Sep;68(1):70–78. doi: 10.1016/0003-2697(75)90680-6. [DOI] [PubMed] [Google Scholar]
- Grabarek Z., Drabikowski W., Vinokurov L., Lu R. C. Digestion of troponin C with trypsin in the presence and absence of Ca2+. Identification of cleavage points. Biochim Biophys Acta. 1981 Dec 29;671(2):227–233. doi: 10.1016/0005-2795(81)90138-0. [DOI] [PubMed] [Google Scholar]
- Halme J., Tyree B., Jeffrey J. J. Collagenase production by primary cultures of rat uterine cells. Partial purification and characterization of the enzyme. Arch Biochem Biophys. 1980 Jan;199(1):51–60. doi: 10.1016/0003-9861(80)90255-6. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, DiBona D. R., Krane S. M. Collagenases in human synovial fluid. J Clin Invest. 1969 Nov;48(11):2104–2113. doi: 10.1172/JCI106177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. An endopeptidase from rheumatoid synovial tissue culture. Biochim Biophys Acta. 1972 Feb 28;258(2):566–576. doi: 10.1016/0005-2744(72)90249-5. [DOI] [PubMed] [Google Scholar]
- Kárpáti J., Váradi K., Elödi S. Effect of granulocyte proteases on human coagulation factors IX and X. The protective effect of calcium. Hoppe Seylers Z Physiol Chem. 1982 May;363(5):521–525. [PubMed] [Google Scholar]
- McMillan R. M., Vater C. A., Hasselbacher P., Hahn J., Harris E. D., Jr Induction of collagenase and prostaglandin synthesis in synovial fibroblasts treated with monosodium urate crystals. J Pharm Pharmacol. 1981 Jun;33(6):382–383. doi: 10.1111/j.2042-7158.1981.tb13809.x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cawston T. E., Galloway W. A., Barnes M. J., Bunning R. A., Mercer E., Reynolds J. J., Burgeson R. E. Metalloproteinases from rabbit bone culture medium degrade types IV and V collagens, laminin and fibronectin. Biochem J. 1981 Dec 1;199(3):807–811. doi: 10.1042/bj1990807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Brinckerhoff C. E., Vater C. A., Harris E. D., Jr Biosynthesis and secretion of procollagenase by rabbit synovial fibroblasts. Inhibition of procollagenase secretion by monensin and evidence for glycosylation of procollagenase. Biochem J. 1983 Aug 15;214(2):281–288. doi: 10.1042/bj2140281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Cawston T. E., De Silva M., Barrett A. J. Identification of plasma kallikrein as an activator of latent collagenase in rheumatoid synovial fluid. Biochim Biophys Acta. 1982 Mar 18;702(1):133–142. doi: 10.1016/0167-4838(82)90036-x. [DOI] [PubMed] [Google Scholar]
- Nagase H., Jackson R. C., Brinckerhoff C. E., Vater C. A., Harris E. D., Jr A precursor form of latent collagenase produced in a cell-free system with mRNA from rabbit synovial cells. J Biol Chem. 1981 Dec 10;256(23):11951–11954. [PubMed] [Google Scholar]
- Ohyama H., Hashimoto K. Collagenase of human skin basal cell epithelioma. J Biochem. 1977 Jul;82(1):175–183. doi: 10.1093/oxfordjournals.jbchem.a131667. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Salamino F., Sparatore B., Michetti M., Horecker B. L. Cytosolic Ca2+-dependent neutral proteinases from rabbit liver: activation of the proenzymes by Ca2+ and substrate. Proc Natl Acad Sci U S A. 1984 Jan;81(1):53–56. doi: 10.1073/pnas.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S., Sakamoto M., Matsumoto A., Goldhaber P., Glimcher M. J. Latent collagenase from the culture medium of embryonic chick bones. FEBS Lett. 1978 Apr 1;88(1):53–58. doi: 10.1016/0014-5793(78)80605-x. [DOI] [PubMed] [Google Scholar]
- Sellers A., Reynolds J. J. Identification and partial characterization of an inhibitor of collagenase from rabbit bone. Biochem J. 1977 Nov 1;167(2):353–360. doi: 10.1042/bj1670353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer J. L., Adams S. A., Grant G. A., Eisen A. Z. Purification and properties of a gelatin-specific neutral protease from human skin. J Biol Chem. 1981 May 10;256(9):4662–4668. [PubMed] [Google Scholar]
- Seltzer J. L., Welgus H. G., Jeffrey J. J., Eisen A. Z. The function of Ca+ in the action of mammalian collagenases. Arch Biochem Biophys. 1976 Mar;173(1):355–361. doi: 10.1016/0003-9861(76)90270-8. [DOI] [PubMed] [Google Scholar]
- Shinkai H., Nagai Y. A latent collagenase from embryonic human skin explants. J Biochem. 1977 May;81(5):1261–1268. [PubMed] [Google Scholar]
- Vaes G. The release of collagenase as an inactive proenzyme by bone explants in culture. Biochem J. 1972 Jan;126(2):275–289. doi: 10.1042/bj1260275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater C. A., Hahn J. L., Harris E. D., Jr Preparation of a monospecific antibody to purified rabbit synovial fibroblast collagenase. Coll Relat Res. 1981 Nov;1(6):527–542. doi: 10.1016/s0174-173x(81)80034-9. [DOI] [PubMed] [Google Scholar]
- Vater C. A., Mainardi C. L., Harris E. D., Jr Activation in vitro of rheumatoid synovial collagenase from cell cultures. J Clin Invest. 1978 Nov;62(5):987–992. doi: 10.1172/JCI109228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater C. A., Nagase H., Harris E. D., Jr Purification of an endogenous activator of procollagenase from rabbit synovial fibroblast culture medium. J Biol Chem. 1983 Aug 10;258(15):9374–9382. [PubMed] [Google Scholar]
- Walsh M., Stevens F. C. Characterization of tryptic fragments obtained from bovine brain protein modulator of cyclic nucleotide phosphodiesterase. J Biol Chem. 1977 Nov 10;252(21):7440–7443. [PubMed] [Google Scholar]
- Werb Z., Burleigh M. C. A specific collagenase from rabbit fibroblasts in monolayer culture. Biochem J. 1974 Feb;137(2):373–385. doi: 10.1042/bj1370373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr A latent form of collagenase in the involuting rat uterus and its activation by a serine proteinase. Biochem J. 1977 Mar 1;161(3):535–542. doi: 10.1042/bj1610535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Separation of collagenase and a metal-dependent endopeptidase of rat uterus that hydrolyzes a heptapeptide related to collagen. Biochim Biophys Acta. 1979 Dec 7;571(2):313–320. doi: 10.1016/0005-2744(79)90101-3. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Glanville R. W., Crossley M. J., Evanson J. M. Purification of rheumatoid synovial collagenase and its action on soluble and insoluble collagen. Eur J Biochem. 1975 Jun;54(2):611–622. doi: 10.1111/j.1432-1033.1975.tb04173.x. [DOI] [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]