Abstract
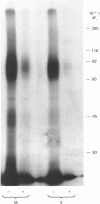
Studies with a panel of monoclonal antibodies (MAbs) reactive towards the presumptive rabbit liver growth-hormone (GH) receptor show that the rabbit serum GH-binding proteins share seven antigenic determinants (three at the hormone-binding site and four located elsewhere) with the liver cytosolic GH-binding proteins and the putative GH 'receptors' associated with the hepatocyte membrane. The rabbit serum binding proteins have an affinity for GH similar to the membrane GH receptors [for human GH, Ka = 2.45 (+/- 0.15) X 10(9) M-1 (mean +/- S.E.M., n = 8)] and high capacity relative to membrane 'GH receptors'. Analogues of the postulated membrane 'receptor' subtypes 1 and 2 exist in the serum, but not subtype 3, which is also absent from liver cytosol. The serum and cytosolic binding proteins have identical cation-dependence properties; hGH binding is Ca2+-dependent, whereas oGH binding is Ca2+-independent. Affinity labelling of hGH-affinity-purified serum binding proteins with 125I-hGH demonstrated a major GH-binding subunit, of Mr 55,000, identical with the major component purified from membranes. In view of their high affinity and capacity, the serum binding proteins could control availability of GH to membrane receptors. It is suggested that the cytosolic binding proteins may be newly synthesized serum binding proteins. The existence of a close relationship between subsets of membrane-associated GH-binding sites, the serum GH-binding proteins and cytosolic GH-binding proteins dictates a reappraisal of earlier ligand-binding studies, which did not distinguish between binding-site subsets in the liver.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard R., Bundesen P. G., Rylatt D. B., Waters M. J. Evidence from the use of monoclonal antibody probes for structural heterogeneity of the growth hormone receptor. Biochem J. 1985 Oct 15;231(2):459–468. doi: 10.1042/bj2310459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard R., Bundesen P. G., Rylatt D. B., Waters M. J. Monoclonal antibodies to the rabbit liver growth hormone receptor: production and characterization. Endocrinology. 1984 Nov;115(5):1805–1813. doi: 10.1210/endo-115-5-1805. [DOI] [PubMed] [Google Scholar]
- Baumann G., Stolar M. W., Buchanan T. A. Slow metabolic clearance rate of the 20,000-dalton variant of human growth hormone: implications for biological activity. Endocrinology. 1985 Oct;117(4):1309–1313. doi: 10.1210/endo-117-4-1309. [DOI] [PubMed] [Google Scholar]
- Baxter R. C., Zaltsman Z. Induction of hepatic receptors for growth hormone (GH) and prolactin by GH infusion is sex independent. Endocrinology. 1984 Nov;115(5):2009–2014. doi: 10.1210/endo-115-5-2009. [DOI] [PubMed] [Google Scholar]
- Ciccia-Torres G. N., Dellacha J. M. Influence of divalent cations on the detection of somatogenic and lactogenic binding sites in mouse liver cells. Biochem J. 1985 Jun 15;228(3):761–764. doi: 10.1042/bj2280761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A. D., Tanenbaum R., Rabinowitz D. Existence of two forms of immunoreactive growth hormone in human plasma. J Clin Endocrinol Metab. 1972 Dec;35(6):868–878. doi: 10.1210/jcem-35-6-868. [DOI] [PubMed] [Google Scholar]
- Gorden P., Hendricks C. M., Roth J. Evidence for "big" and "little" components of human plasma and pituitary growth hormone. J Clin Endocrinol Metab. 1973 Jan;36(1):178–184. doi: 10.1210/jcem-36-1-178. [DOI] [PubMed] [Google Scholar]
- Grichting G., Levy L. K., Goodman H. M. Relationship between binding and biological effects of human growth hormone in rat adipocytes. Endocrinology. 1983 Sep;113(3):1111–1120. doi: 10.1210/endo-113-3-1111. [DOI] [PubMed] [Google Scholar]
- Hughes J. P., Friesen H. G. The nature and regulation of the receptors for pituitary growth hormone. Annu Rev Physiol. 1985;47:469–482. doi: 10.1146/annurev.ph.47.030185.002345. [DOI] [PubMed] [Google Scholar]
- Hughes J. P. Identification and characterization of high and low affinity binding sites for growth hormone in rabbit liver. Endocrinology. 1979 Aug;105(2):414–420. doi: 10.1210/endo-105-2-414. [DOI] [PubMed] [Google Scholar]
- Hughes J. P., Tokuhiro E., Simpson J. S., Friesen H. G. 20K is bound with high affinity by one rat and one of two rabbit growth hormone receptors. Endocrinology. 1983 Nov;113(5):1904–1906. doi: 10.1210/endo-113-5-1904. [DOI] [PubMed] [Google Scholar]
- Kleinberg D. L., Todd J. Evidence that human growth hormone is a potent lactogen in primates. J Clin Endocrinol Metab. 1980 Nov;51(5):1009–1013. doi: 10.1210/jcem-51-5-1009. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Shiu R. P. Prolactin receptors in human breast cancer cells in long-term tissue culture. Cancer Res. 1979 Nov;39(11):4381–4386. [PubMed] [Google Scholar]
- Sigel M. B., Thorpe N. A., Kobrin M. S., Lewis U. J., Vanderlaan W. P. Binding characteristics of a biologically active variant of human growth hormone (20K) to growth hormone and lactogen receptors. Endocrinology. 1981 Apr;108(4):1600–1603. doi: 10.1210/endo-108-4-1600. [DOI] [PubMed] [Google Scholar]
- Spencer E. M., Lewis L. J., Lewis U. J. Somatomedin generating activity of the 20,000-dalton variant of human growth hormone. Endocrinology. 1981 Oct;109(4):1301–1302. doi: 10.1210/endo-109-4-1301. [DOI] [PubMed] [Google Scholar]
- Stolar M. W., Amburn K., Baumann G. Plasma "big" and "big-big" growth hormone (GH) in man: an oligomeric series composed of structurally diverse GH monomers. J Clin Endocrinol Metab. 1984 Aug;59(2):212–218. doi: 10.1210/jcem-59-2-212. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Tsushima T., Sasaki N., Imai Y., Matsuzaki F., Friesen H. G. Characteristics of solubilized human-somatotropin-binding protein from the liver of pregnant rabbits. Biochem J. 1980 May 1;187(2):479–492. doi: 10.1042/bj1870479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. J., Friesen H. G. Purification and partial characterization of a nonprimate growth hormone receptor. J Biol Chem. 1979 Jul 25;254(14):6815–6825. [PubMed] [Google Scholar]
- Yalow R. S. Heterogeneity of peptide hormones. Recent Prog Horm Res. 1974;30(0):597–633. doi: 10.1016/b978-0-12-571130-2.50020-1. [DOI] [PubMed] [Google Scholar]
- Ymer S. I., Herington A. C. Evidence for the specific binding of growth hormone to a receptor-like protein in rabbit serum. Mol Cell Endocrinol. 1985 Jul;41(2-3):153–161. doi: 10.1016/0303-7207(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Ymer S. I., Stevenson J. L., Herington A. C. Identification of a rabbit liver cytosolic binding protein for human growth hormone. Biochem J. 1984 Aug 1;221(3):617–622. doi: 10.1042/bj2210617. [DOI] [PMC free article] [PubMed] [Google Scholar]