Abstract
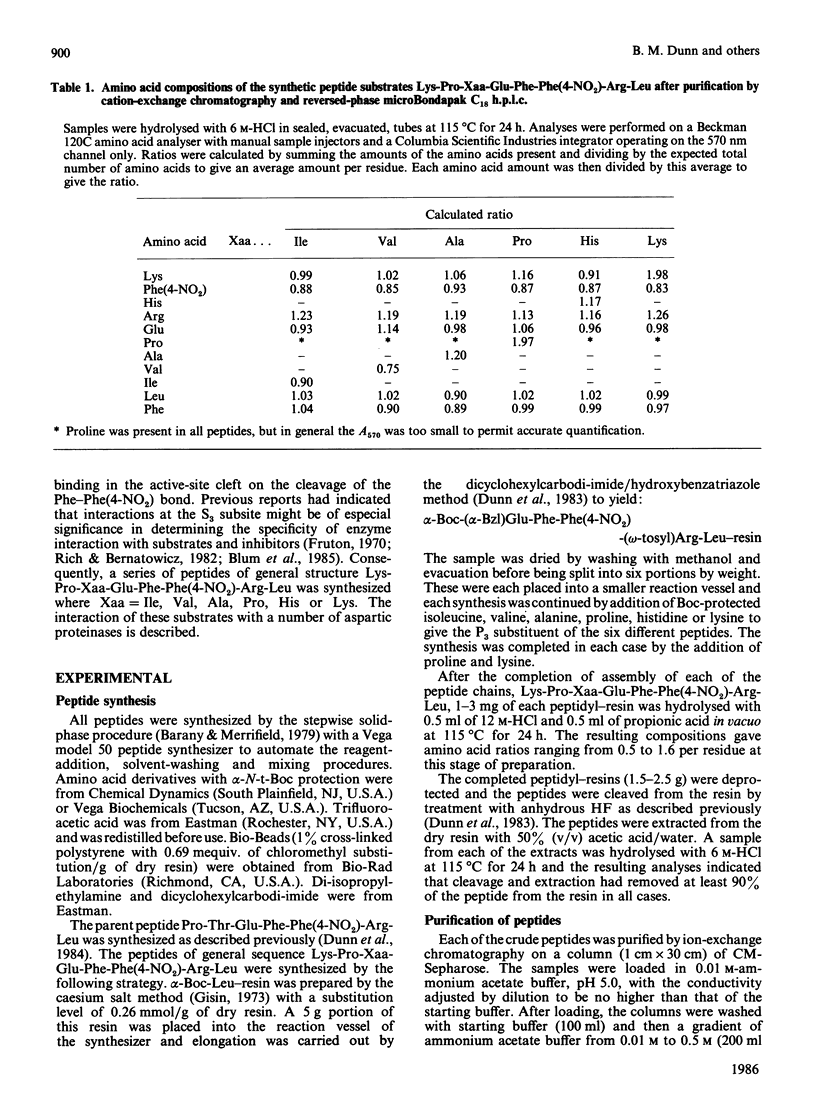
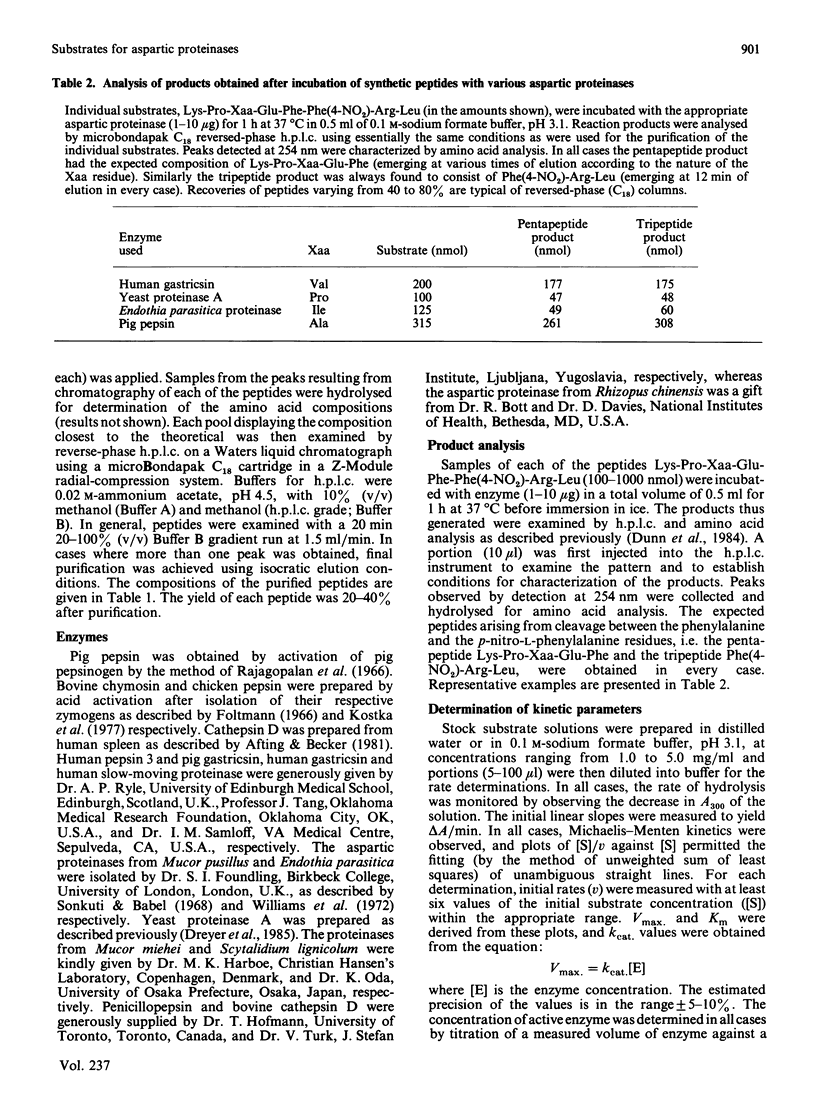
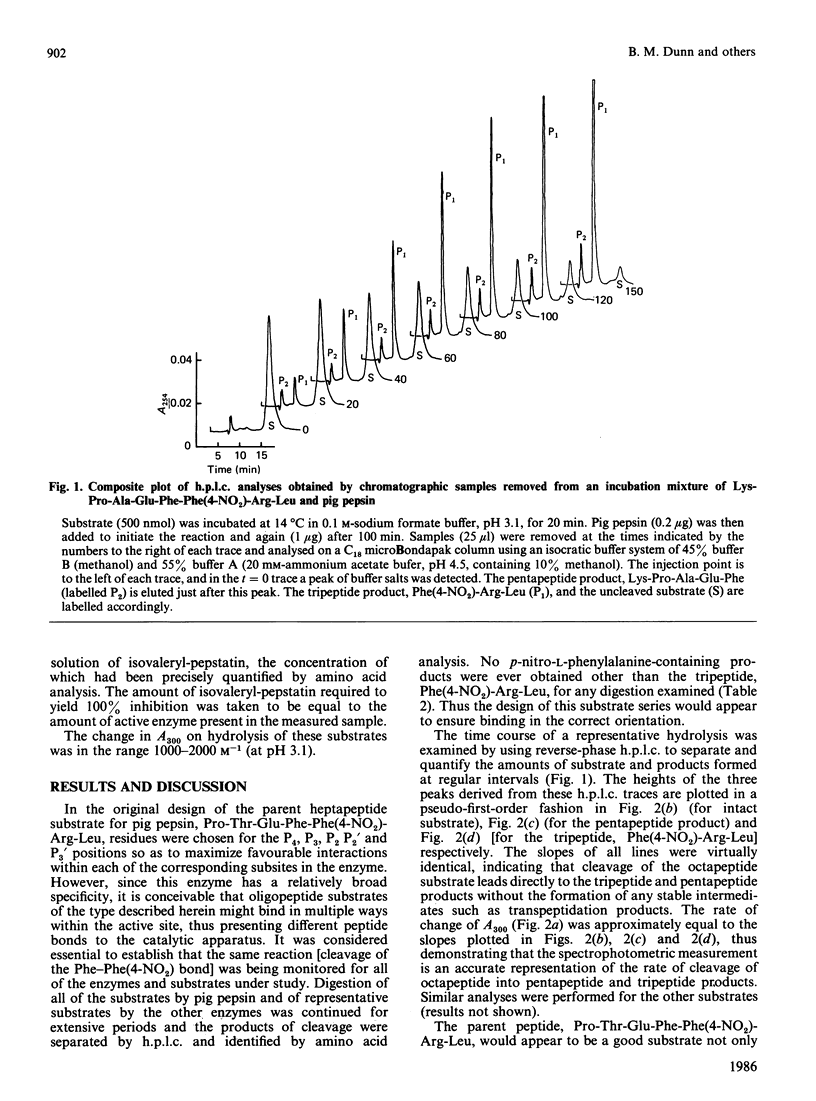
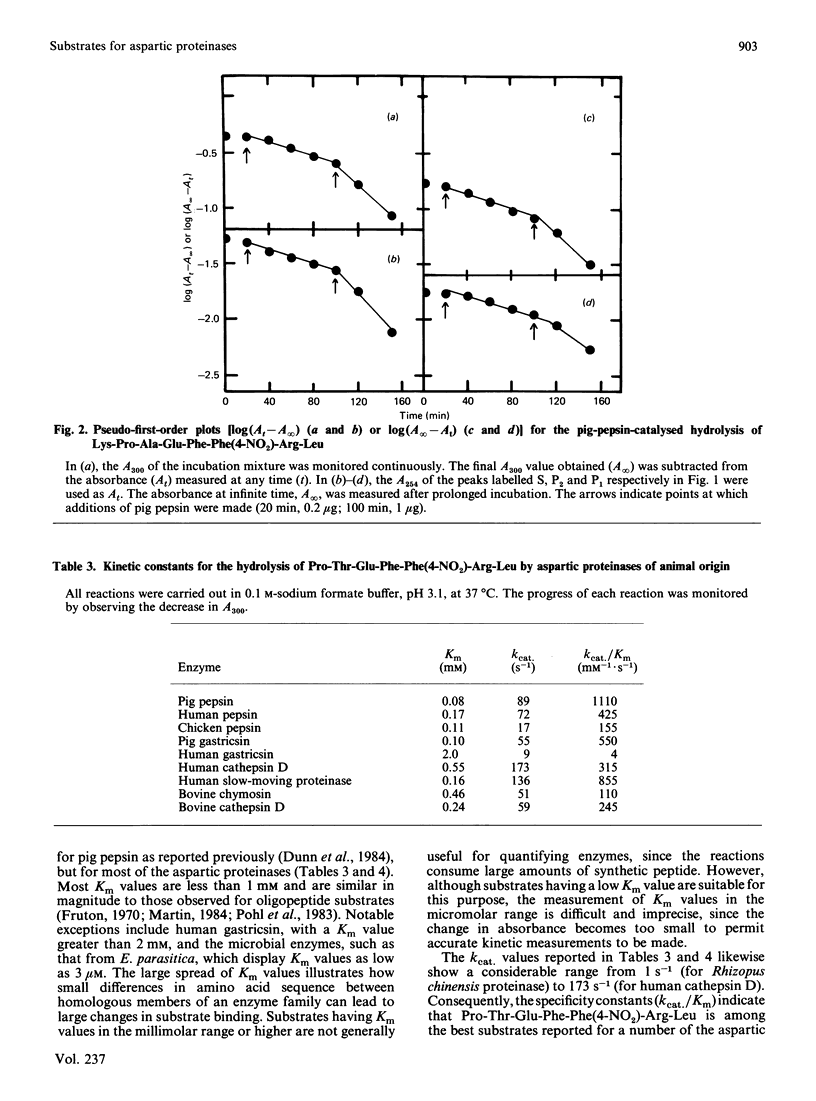
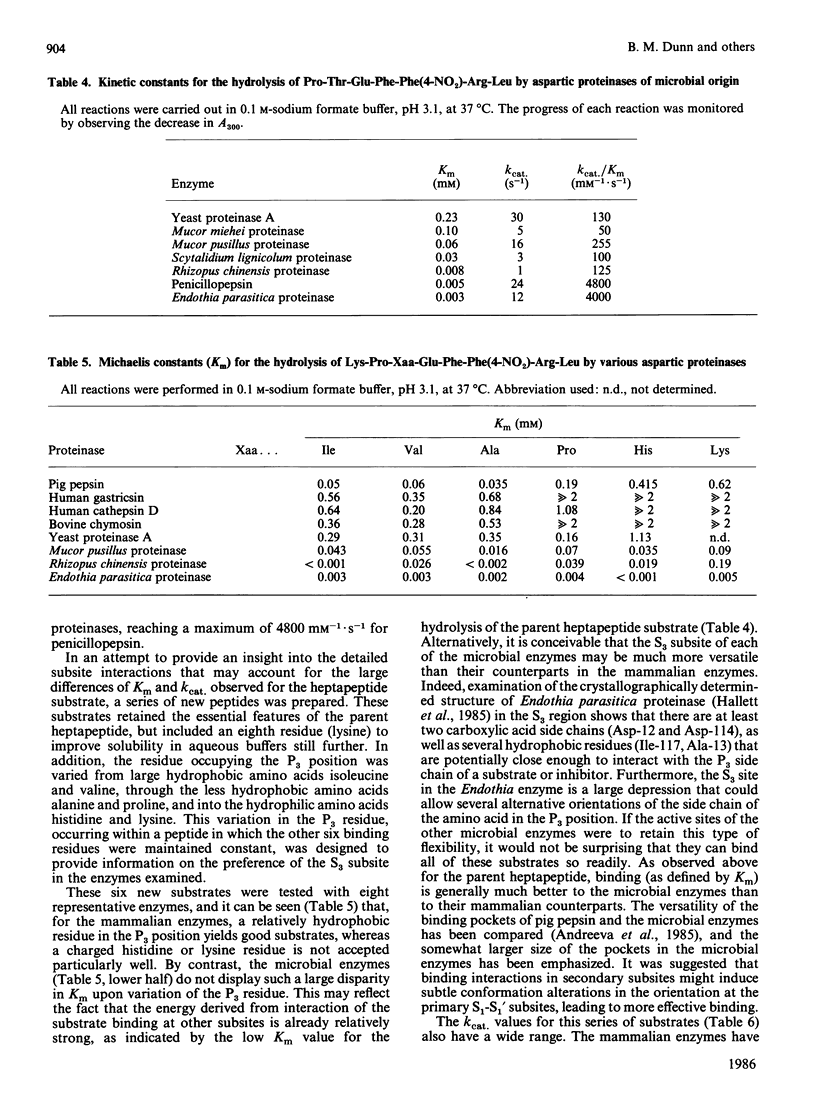
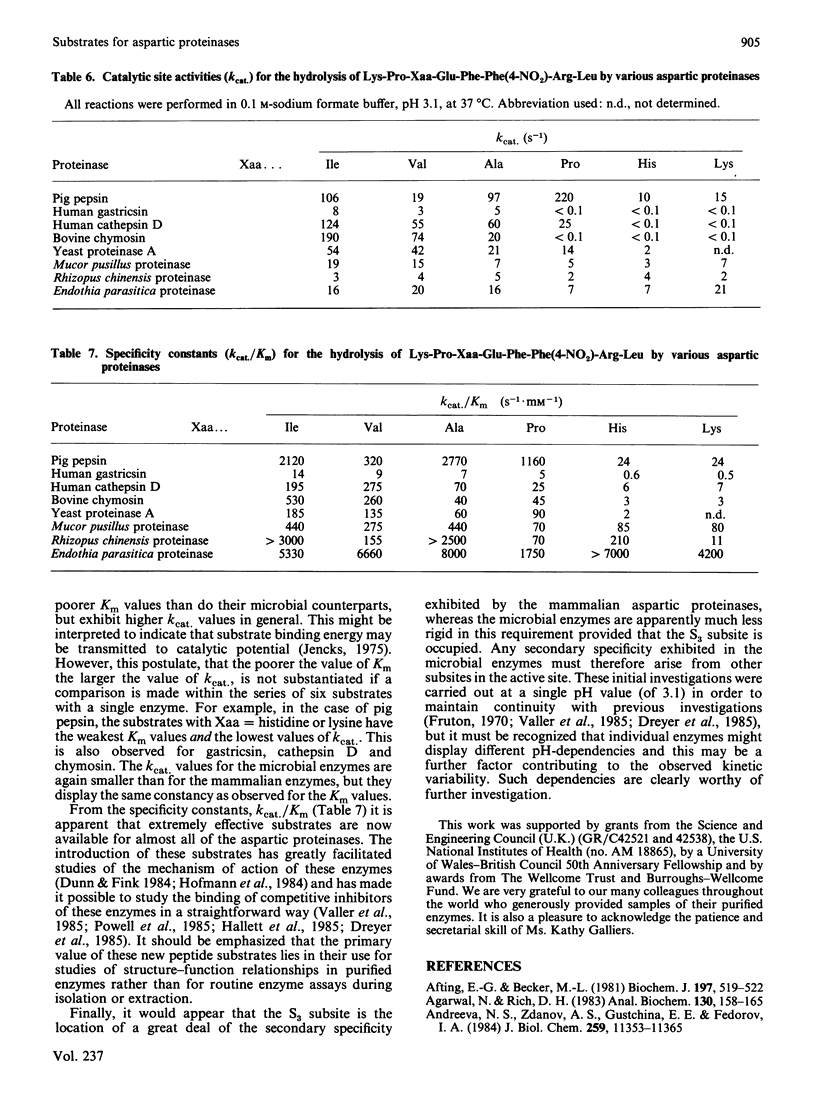
The hydrolysis of the chromogenic peptide Pro-Thr-Glu-Phe-Phe(4-NO2)-Arg-Leu at the Phe-Phe(4-NO2) bond by nine aspartic proteinases of animal origin and seven enzymes from micro-organisms is described [Phe(4-NO2) is p-nitro-L-phenylalanine]. A further series of six peptides was synthesized in which the residue in the P3 position was systematically varied from hydrophobic to hydrophilic. The Phe-Phe(4-NO2) bond was established as the only peptide bond cleaved, and kinetic constants were obtained for the hydrolysis of these peptide substrates by a representative selection of aspartic proteinases of animal and microbial origin. The value of these water-soluble substrates for structure-function investigations is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afting E. G., Recker M. L. Two-step affinity-chromatographic purification of cathepsin D from pig myometrium with high yield. Biochem J. 1981 Aug 1;197(2):519–522. doi: 10.1042/bj1970519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal N., Rich D. H. An improved cathepsin-D substrate and assay procedure. Anal Biochem. 1983 Apr 1;130(1):158–165. doi: 10.1016/0003-2697(83)90663-2. [DOI] [PubMed] [Google Scholar]
- Andreeva N. S., Zdanov A. S., Gustchina A. E., Fedorov A. A. Structure of ethanol-inhibited porcine pepsin at 2-A resolution and binding of the methyl ester of phenylalanyl-diiodotyrosine to the enzyme. J Biol Chem. 1984 Sep 25;259(18):11353–11365. [PubMed] [Google Scholar]
- Blum M., Cunningham A., Bendiner M., Hofmann T. Penicillopepsin, the aspartic proteinase from Penicillium janthinellum: substrate-binding effects and intermediates in transpeptidation reactions. Biochem Soc Trans. 1985 Dec;13(6):1044–1046. doi: 10.1042/bst0131044. [DOI] [PubMed] [Google Scholar]
- Bott R., Subramanian E., Davies D. R. Three-dimensional structure of the complex of the Rhizopus chinensis carboxyl proteinase and pepstatin at 2.5-A resolution. Biochemistry. 1982 Dec 21;21(26):6956–6962. doi: 10.1021/bi00269a052. [DOI] [PubMed] [Google Scholar]
- Dreyer T., Valler M. J., Kay J., Charlton P., Dunn B. M. The selectivity of action of the aspartic-proteinase inhibitor IA3 from yeast (Saccharomyces cerevisiae). Biochem J. 1985 Nov 1;231(3):777–779. doi: 10.1042/bj2310777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B. M., Fink A. L. Cryoenzymology of porcine pepsin. Biochemistry. 1984 Oct 23;23(22):5241–5247. doi: 10.1021/bi00317a023. [DOI] [PubMed] [Google Scholar]
- Dunn B. M., Kammermann B., McCurry K. R. The synthesis, purification, and evaluation of a chromophoric substrate for pepsin and other aspartyl proteases: design of a substrate based on subsite preferences. Anal Biochem. 1984 Apr;138(1):68–73. doi: 10.1016/0003-2697(84)90770-x. [DOI] [PubMed] [Google Scholar]
- Dunn B. M., Lewitt M., Pham C. Inhibition of pepsin by analogues of pepsinogen-(1-12)-peptide with substitutions in the 4-7 sequence region. Biochem J. 1983 Feb 1;209(2):355–362. doi: 10.1042/bj2090355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltmann B. A review on prorennin and rennin. C R Trav Lab Carlsberg. 1966;35(8):143–231. [PubMed] [Google Scholar]
- Fruton J. S. The specificity and mechanism of pepsin action. Adv Enzymol Relat Areas Mol Biol. 1970;33:401–443. doi: 10.1002/9780470122785.ch9. [DOI] [PubMed] [Google Scholar]
- Hofmann T., Fink A. L., Dunn B. M. Cryoenzymology of penicillopepsin; with an appendix: mechanism of action of aspartyl proteinases. Biochemistry. 1984 Oct 23;23(22):5247–5256. doi: 10.1021/bi00317a024. [DOI] [PubMed] [Google Scholar]
- Hofmann T., Hodges R. S. A new chromophoric substrate for penicillopepsin and other fungal aspartic proteinases. Biochem J. 1982 Jun 1;203(3):603–610. doi: 10.1042/bj2030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M. N., Sielecki A. R. Structure and refinement of penicillopepsin at 1.8 A resolution. J Mol Biol. 1983 Jan 15;163(2):299–361. doi: 10.1016/0022-2836(83)90008-6. [DOI] [PubMed] [Google Scholar]
- Jencks W. P. Binding energy, specificity, and enzymic catalysis: the circe effect. Adv Enzymol Relat Areas Mol Biol. 1975;43:219–410. doi: 10.1002/9780470122884.ch4. [DOI] [PubMed] [Google Scholar]
- Martin P. Hydrolysis of the synthetic chromophoric hexapeptide Leu-Ser-Phe(NO2)-Nle-Ala-Leu-OMe catalyzed by bovine pepsin A. Dependence on pH and effect of enzyme phosphorylation level. Biochim Biophys Acta. 1984 Nov 23;791(1):28–36. doi: 10.1016/0167-4838(84)90277-2. [DOI] [PubMed] [Google Scholar]
- Martin P., Trieu-Cuot P., Collin J. C., Ribadeau Dumas B. Purification and characterization of bovine gastricsin. Eur J Biochem. 1982 Feb;122(1):31–39. doi: 10.1111/j.1432-1033.1982.tb05844.x. [DOI] [PubMed] [Google Scholar]
- Pearl L., Blundell T. The active site of aspartic proteinases. FEBS Lett. 1984 Aug 20;174(1):96–101. doi: 10.1016/0014-5793(84)81085-6. [DOI] [PubMed] [Google Scholar]
- Pohl J., Baudys M., Kostka V. Chromophoric peptide substrates for activity determination of animal aspartic proteinases in the presence of their zymogens: a novel assay. Anal Biochem. 1983 Aug;133(1):104–109. doi: 10.1016/0003-2697(83)90228-2. [DOI] [PubMed] [Google Scholar]
- Rajagopalan T. G., Stein W. H., Moore S. The inactivation of pepsin by diazoacetylnorleucine methyl ester. J Biol Chem. 1966 Sep 25;241(18):4295–4297. [PubMed] [Google Scholar]
- Rich D. H., Bernatowicz M. S. Synthesis of analogues of the carboxyl protease inhibitor pepstatin. Effect of structure in subsite P3 on inhibition of pepsin. J Med Chem. 1982 Jul;25(7):791–795. doi: 10.1021/jm00349a005. [DOI] [PubMed] [Google Scholar]
- Rich D. H., Sun E. T. Mechanism of inhibition of pepsin by pepstatin. Effect of inhibitor structure on dissociation constant and time-dependent inhibition. Biochem Pharmacol. 1980 Aug 15;29(16):2205–2212. doi: 10.1016/0006-2952(80)90199-9. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Somkuti G. A., Babel F. J. Purification and properties of Mucor pusillus acid protease. J Bacteriol. 1968 Apr;95(4):1407–1414. doi: 10.1128/jb.95.4.1407-1414.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., James M. N., Hsu I. N., Jenkins J. A., Blundell T. L. Structural evidence for gene duplication in the evolution of the acid proteases. Nature. 1978 Feb 16;271(5646):618–621. doi: 10.1038/271618a0. [DOI] [PubMed] [Google Scholar]
- Valler M. J., Kay J., Aoyagi T., Dunn B. M. The interaction of aspartic proteinases with naturally-occurring inhibitors from actinomycetes and Ascaris lumbricoides. J Enzyme Inhib. 1985;1(1):77–82. doi: 10.3109/14756368509031284. [DOI] [PubMed] [Google Scholar]
- Williams D. C., Witaker J. R., Caldwell P. V. Hydrolysis of peptide bonds of the oxidized B-chain of insulin by Endothia parasitica protease. Arch Biochem Biophys. 1972 Mar;149(1):52–61. doi: 10.1016/0003-9861(72)90298-6. [DOI] [PubMed] [Google Scholar]


