Abstract
Background
Among heart failure patients with obesity, the prognosis is better than those with normal weight, a phenomenon known as the obesity paradox. However, it is unclear whether lipoprotein levels play a mediating role in the machine of the obesity paradox.
Methods
The study included 1663 heart failure patients hospitalized from January, 2019 through August, 2022. Kaplan-Meier survival analysis and Log-rank tests were performed for three endpoints in order to determine cumulative event-free survival. We investigated the correlation between Body Max Index (BMI) and outcomes by multifactorial Cox models. Mediation analysis was applied to study the presence and magnitude of mediation effects of triglyceride, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, apolipoprotein A1 and apolipoprotein B, with the association between BMI and endpoints.
Results
In MACCEs, the median follow-up period was 679 days. In Cox model, compared with the underweight group, a high BMI level was significantly associated with lower all-cause mortality (HR=0.47, 95%CI 0.31~0.69, p<0.001, obese vs underweight), cardiovascular mortality (HR=0.46, 95%CI 0.30~0.73, p<0.001, obese vs underweight) and the incidence of MACCEs (HR=0.68, 95%CI 0.53~0.88, p=0.003, obese vs underweight). Mediation analysis revealed that TG was the strongest mediator between BMI and endpoints, with proportions of mediated effects of 6.6% (95%CI 2.2%~18.0%, p=0.0258, in all-cause death),7.0% (95%CI 2.3%~18.9%, p=0.0301, in cardiovascular death) and 10.2% (95%CI 3.3%~27.4%, p=0.0185, in MACCEs).
Conclusions
There is an "obesity paradox" in patients with heart failure, and lipoprotein levels especially triglyceride mediate the association between BMI and cardiovascular outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04155-9.
Keywords: Heart failure, Obesity, Lipoprotein, Mediating effect
Introduction
Heart failure (HF) is a prevalent and intricate clinical syndrome that impacts a global population exceeding 40 million individuals, thereby leading to substantial rates of morbidity, mortality, and financial burdens [1].The incidence of heart failure in the United States is projected to escalate by 46% between 2012 and 2030, leading to the number of people suffering from heart failure will exceed 8 million by 2030 [2]. Obesity has emerged as a burgeoning concern within the realm of public health. It has been widely acknowledged as an independent risk factor for cardiovascular ailments, exhibiting a conspicuous correlation with conditions such as coronary heart disease(CHD),heart failure, and atrial fibrillation(AF) [3]. Metabolic abnormalities, notably obesity, hyperglycemia, and hyperlipidemia, are causally linked with the progression of type 2 diabetes, atherosclerosis, and cardiovascular disease culminating in HF [4]. Numerous studies have consistently demonstrated a counterintuitive association between obesity and HF, which overweight and obese individuals exhibit improved prognoses and reduced risk of complications compared to those with normal or low weight, a phenomenon referred as the "obesity paradox." [5, 6] This paradoxical relationship has also been observed within the Chinese population of HF patients [7, 8]. Lipoproteins are essential for the transportation of dietary fats, such as triglycerides, cholesterol, and fatty acids within the bloodstream [9]. In the context of chronic heart failure, the presence of bacterial endotoxins can aggravate the condition by stimulating immune activation. Interestingly, obese individuals with HF exhibit elevated levels of lipoproteins, which can potentially bind to inflammatory endotoxins and function as a neutralizing agent. This interaction may serve to regulate immune function and safeguard the body [10]. Consequently, this mechanism could potentially explain the phenomenon known as the obesity paradox. However, the confirmation of the mediating role of lipoprotein levels in the association between obese patients with HF and improved clinical outcomes remains unsubstantiated in real-world studies. Hence, a retrospective cohort study was undertaken to examine the mediating influence of lipoprotein levels on the relationship between BMI and cardiovascular outcomes in patients with heart failure.
Materials and methods
Study population
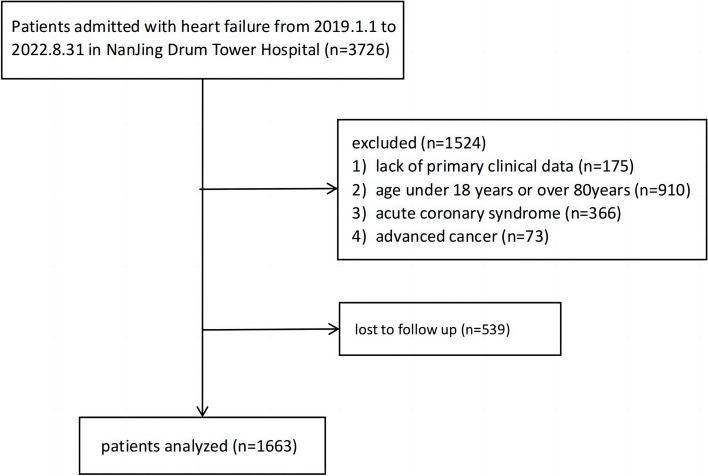
There were 1663 patients with HF who were hospitalized in the Department of Cardiovascular Medicine of Nanjing Drum Tower Hospital from January, 2019 to August, 2022 in this retrospective cohort study (Fig. 1).They were categorized according to the quartiles of BMI into the underweight group(BMI< 22.39kg/m2), normal group(22.39kg/m2≤BMI<24.70kg/m2), overweight group(24.70kg/m2≤BMI< 27.58kg/m2), and obese group(BMI≥27.58kg/m2). According to the 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure, the diagnostic criteria for HF were based on the definitions established in the guidelines [11]. The inclusion criteria for this study were (1) meeting the clinical diagnostic criteria for chronic heart failure (2) between 18-80 years (3) complete clinical data is available. Exclusion criteria were (1) history of combined malignant tumors (2) congenital heart disease (3) after heart and other major organ transplantation (4) acute coronary syndrome (5) patients with missing data and lost to follow up.
Fig.1.
Flow diagram of patient selection
Data collection
The electronic hospitalization system was used by trained physicians to collect basic patient information, personal past history, utilization of drugs, laboratory test results and cardiac ultrasound data. Basic patient information included age, gender, BMI, NYHA, HR, SBP and DBP. Past history included history of smoking, alcohol consumption, hypertension, DM, CHD, AF and hyperlipidemia. Utilization of drugs included diuretics, digoxin, ACEI/ARB/ARIN, SGLT2i, beta-blockers, antiplatelet agents, statins, calcium channel blockers and aldosterone antagonists. Laboratory test results included AST, ALT, LD,TB,TP,ALB/GLB,Glu,Cr,UA,CRP,eGFR,TG,TC,HDL-C,LDL-C,Apo-A1,Apo-B,Ca,P,K,Na,Cl,WBC,GRA,RBC,HGB,PLT,BNP,and HbA1c.Echocardiographic data included LVEF, IVSTd, LVPWTd, LVDd and LAD.
Clinical follow-up and setting of primary endpoint events
All patients in the study were followed up by a specialized clinician by telephone or in an outpatient clinic. The primary endpoint events for follow-up included (1) all-cause death: defined as cardiovascular and noncardiovascular deaths (2) cardiovascular death: stroke and myocardial infarction considered fatal, congestive heart failure, malignant arrhythmias, and other structural and functional heart conditions (3) major adverse cardiac and cerebral events(MACCEs): nonfatal myocardial infarction, stroke, heart failure exacerbation, and cardiac transplant.
Statistical analysis
SPSS 25.0, R 4.2.2 and SAS 9.4 software were used for data analysis. Categorical data were expressed as n (%), and comparisons between groups were made using the chi-square test. Normal-distributed quantitative data were expressed as mean±standard deviation, and comparisons between groups were made using the t-test or the ANOVA analysis; skewed-distribution quantitative data were expressed as M (P25, P75), and comparisons between groups were made using the rank sum test. Kaplan-Meier survival curves and log-rank tests were used to analyze cumulative event-free survival for the three endpoints. Multifactorial Cox models were constructed by adjusting for age, sex, LVEF, eGFR, CPR, hypertension, and DM to analyze the correlation between BMI and the event rates of the three endpoints and hazard ratios (HR)and 95% confidence intervals(95%CI) were calculated separately.
An analysis of the dose-response relationship between all-cause death, cardiovascular death and MACCEs was performed using restricted cubic spline method when BMI was used as a continuous variable and the optimal cutoff value of BMI was taken according to the Akaike Information criterion. Mediation effect analysis [12] was used to construct mediation models by adjusting for age, sex, LVEF, eGFR, CPR, hypertension and DM with the continuous variable BMI as the independent variable, TG, TC, HDL-C, LDL-C, Apo-A1, and Apo-B as the mediator variables, and all-cause death, cardiovascular death and MACCEs as the dependent variables, respectively. All statistical analyses considered a two-tailed P<0.05 to determine statistically significant differences.
Our findings were additionally subjected to several sensitivity analyses to assess their robustness. First, to rule out possible effects of high BNP levels and high glucose levels, we performed subgroup analyses based on median BNP and glucose. Second, lipid markers such as triglycerides may affect liver function, so we performed additional sensitivity analyses based on median ALT and AST. Third, we divided patients into two separate categories according to whether they had comorbid hyperlipidemia and whether they were on lipid-lowering therapy (with or without statins) to perform sensitivity analyses. Finally, risk factors with p < 0.05 and considered clinically significant in the univariate analysis between the group of MACCEs and UN-MACCEs included HR, DBP, NYHA Class, AF, LAD, LDH, TP, ALB/GLB, UA, Cr, Ca, Cl, GRA, RBC, HGB, PLT, HbA1c,and the use of ACEI/ARB/ARIN, SGLT2i, Beta -blocker, MRA, diuretic and digoxin were included in the sensitivity analyses by Cox regression models in order to avoid overstratification in the main analysis.
Results
General clinical characteristics of the patient and follow-up outcomes
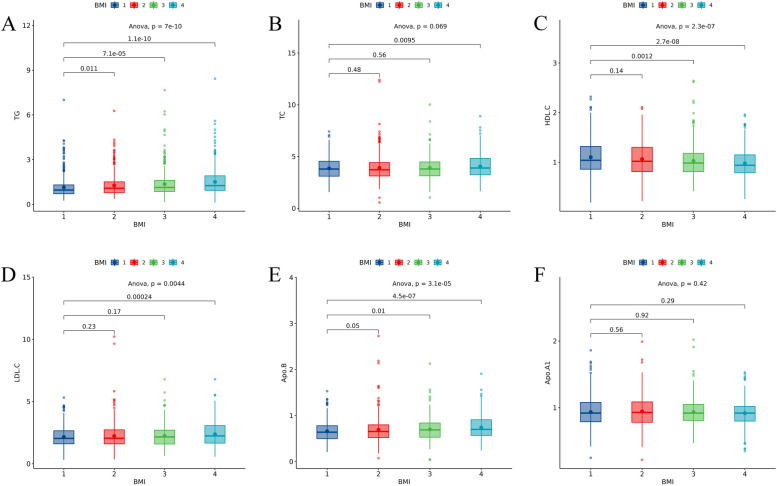
There were 1663 patients enrolled in this study, 538 of whom had MACCEs events and 1125 did not. Patients were on average 63 years old, of which 550 were female, accounting for 33.1%. Table 1 illustrates the baseline clinical characteristics of the two groups. The median follow-up time was 679 days in the MACCEs. The analysis of the differences in TG, TC, HDL-C, LDL-C, Apo-A1, and Apo-B among different BMI groups is shown in Fig. 2. All-cause death occurred in 231(13.9%) patients, which included 183(11.0%) patients with cardiovascular death, and MACCEs cumulatively occurred in 538(32.4%) patients. For different BMI levels, Additional file 1Figure S1 shows the variability of all-cause death, cardiovascular death and MACCEs, all of which were statistically significant (p<0.001).
Table 1.
Basic clinical characteristics of patients with different MACCEs outcomes
| Characteristic |
Total (n = 1663) |
UN-MACCEs (n = 1125) |
MACCEs (n = 538) |
P Value |
|---|---|---|---|---|
| Age (years) | 66 (56,72) | 64 (54,71) | 69 (61,74) | <0.001** |
| Female, n (%) | 550 (33.07) | 360 (32.00) | 190 (35.32) | 0.179 |
| BMI (kg/m2) | 24.70 (22.40,27.57) | 24.91 (22.66,27.89) | 24.22 (21.65,26.87) | <0.001** |
| BMI (kg/m2) | <0.001** | |||
| <22.39 | 415 (24.95) | 244 (21.69) | 171 (31.78) | |
| 22.39~24.69 | 414 (24.89) | 290 (25.78) | 124 (23.05) | |
| 24.70~27.57 | 418 (25.14) | 282 (25.07) | 136 (25.28) | |
| ≥27.58 | 416 (25.02) | 309 (27.47) | 107 (19.89) | |
| Heart Rate (bmp) | 77 (68,89) | 78 (68,90) | 77 (68,88) | 0.365 |
| SBP (mmHg) | 129 (114,146) | 130 (116,146) | 128 (112,145) | 0.075 |
| DBP (mmHg) | 77 (68,89) | 78 (69,90) | 75 (66,86) | <0.001** |
| NYHA Class, n (%) | <0.001** | |||
| I | 123 (7.4) | 99 (8.80) | 24 (4.46) | |
| II | 669 (40.23) | 506 (44.98) | 163 (30.30) | |
| III | 710 (42.69) | 442 (39.29) | 268 (49.81) | |
| IV | 161 (9.68) | 78 (6.93) | 83 (15.43) | |
| Medical history, n (%) | ||||
| Hypertension | 972 (58.45) | 655 (58.22) | 317 (58.92) | 0.786 |
| CHD | 613 (36.86) | 399 (35.47) | 214 (39.78) | 0.088 |
| DM | 525 (31.57) | 316 (28.09) | 209 (38.85) | <0.001** |
| Hyperlipidemia | 244 (14.67) | 185 (16.44) | 59 (10.97) | 0.003* |
| AF | 612 (36.8) | 391 (34.76) | 221 (41.08) | 0.012* |
| Smoke, n (%) | 491 (29.52) | 345 (30.67) | 146 (27.14) | 0.140 |
| Alcohol, n (%) | 283 (17.02) | 196 (17.42) | 87 (16.17) | 0.525 |
| IVSTd (cm) | 0.90 (0.80,1.02) | 0.90 (0.80,1.00) | 0.90 (0.80,1.04) | 0.731 |
| LVPWTd (cm) | 0.90 (0.80,1.00) | 0.90 (0.80,1.00) | 0.90 (0.80,1.00) | 0.667 |
| LVDd(cm) | 5.89 (5.35,6.53) | 5.89 (5.35,6.50) | 5.89 (5.37,6.65) | 0.236 |
| LAD(cm) | 4.66 (4.30,5.10) | 4.66 (4.25,5.00) | 4.72 (4.40,5.33) | <0.001** |
| LVEF (%) | 41 (32,51) | 41 (33,52) | 41 (31,50) | 0.007* |
| BNP (pg/mg) | 347 (151,734) | 300 (127,659) | 475 (213,949) | <0.001** |
| ALT (U/L) | 20.1 (13.8,31.3) | 20.7 (14.5,31.6) | 18.5 (12.8,29.4) | 0.002* |
| AST (U/L) | 21.3 (17.0,28.4) | 21.3 (17.1,28.0) | 21.2 (16.5,29.2) | 0.869 |
| LDH (U/L) | 215 (184,258) | 213 (181,254) | 219 (187,271) | <0.001** |
| TBIL (μmol/L) | 13.6 (9.4,19.3) | 13.5 (9.5,18.9) | 14.1 (9.2,19.6) | 0.834 |
| Total Protein (g/L) | 64.4 (60.6,68.8) | 64.7 (61.1,69.1) | 63.4 (59.6,67.8) | <0.001** |
| ALB/GLB | 1.56 (1.37 ,1.74) | 1.57 (1.40,1.75) | 1.50 (1.29,1.68) | <0.001** |
| Glucose (mmol/L) | 4.95 (4.48,5.86) | 4.92 (4.49,5.72) | 5.05 (4.45,6.13) | 0.084 |
| Cr (μmol/L) | 76 (63,95) | 73 (62,89) | 83 (67,113) | <0.001** |
| UA (μmol/L) | 422 (335,523) | 407 (328,498) | 446 (350,566) | <0.001** |
| TG (mg/dL) | 1.10 (0.81,1.57) | 1.15 (0.85,1.66) | 1.02 (0.74,1.38) | <0.001** |
| TC (mg/dL) | 3.79 (3.18,4.54) | 3.90 (3.29,4.68) | 3.54 (2.95,4.29) | <0.001** |
| HDL-C (mg/dL) | 1.00 (0.81,1.23) | 1.00 (0.83,1.24) | 0.98 (0.78,1.23) | 0.102 |
| LDL-C (mg/dL) | 2.12 (1.61,2.76) | 2.21 (1.69,2.85) | 1.93 (1.47,2.59) | <0.001** |
| Apo-A1 (g/L) | 0.92 (0.80,1.07) | 0.93 (0.82,1.08) | 0.87 (0.75,1.02) | <0.001** |
| Apo-B (g/L) | 0.67 (0.53,0.83) | 0.69 (0.55,0.86) | 0.62 (0.49,0.78) | <0.001** |
| Ca (mmol/L) | 2.31 (2.23,2.41) | 2.32 (2.24,2.42) | 2.29 (2.19,2.39) | <0.001** |
| P (mmol/L) | 1.10 (0.99,1.22) | 1.10 (0.99,1.22) | 1.10 (0.98,1.22) | 0.981 |
| K (mmol/L) | 3.93 (3.70,4.20) | 3.93 (3.70,4.17) | 3.96 (3.69,4.25) | 0.129 |
| Na (mmol/L) | 141.3 (139.7,142.8) | 141.3 (139.8,142.8) | 141.2 (139.5,142.8) | 0.241 |
| Cl (mmol/L) | 105.0 (102.9,106.9) | 105.1 (103.2,106.9) | 104.7 (102.0,107.1) | 0.037* |
| CRP (mg/L) | 3.90 (2.70,6.65) | 3.90 (2.60,6.50) | 4.10 (2.82,7.77) | 0.009* |
| eGFR (ml/min/1.73m2) | 88.7 (69.1,108.0) | 92.8 (75.8,110.4) | 78.8 (53.9,98.5) | <0.001** |
| WBC (10^9/L) | 6.1 (5.0,7.3) | 6.1 (5.1,7.3) | 6.0 (4.8,7.4) | 0.083 |
| GRA (%) | 63.2 (57.5,69.4) | 62.4 (56.5,68.0) | 65.4 (58.9,72.1) | <0.001** |
| RBC (10^12/L) | 4.40 (3.95,4.81) | 4.45 (4.07,4.89) | 4.21 (3.73,4.65) | <0.001** |
| HGB (g/L) | 134 (120.,147) | 136 (123,149) | 129 (112,142) | <0.001** |
| PLT (10^9/L) | 177 (141,216) | 179 (145,219) | 169 (130,210) | <0.001** |
| HbA1c(%) | 6.0 (5.6,6.7) | 6.0 (5.6,6.6) | 6.1 (5.7,7.0) | <0.001** |
| Drug therapy, n(%) | ||||
| ACEI/ARB/ARNI | 1169 (70.29) | 834 (74.13) | 335 (62.27) | <0.001** |
| Beta-blocker | 1301 (78.23) | 888 (78.93) | 413 (76.77) | 0.316 |
| MRA | 1071 (64.4) | 726 (64.53) | 345 (64.13) | 0.871 |
| SGLT2i | 328 (19.72) | 248 (22.04) | 80 (14.87) | <0.001** |
| Diuretic | 1071 (64.4) | 669 (59.47) | 402 (74.72) | <0.001** |
| Digoxin | 197 (11.85) | 112 (9.96) | 85 (15.80) | <0.001** |
| Antiplatelet agent | 674 (40.53) | 457 (40.62) | 217 (40.33) | 0.911 |
| Statins | 1005 (60.43) | 676 (60.09) | 329 (61.15) | 0.678 |
| CCB | 256 (15.39) | 167 (14.84) | 89 (16.54) | 0.369 |
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, NYHA new york heart association, CHD coronary heart disease, DM diabetes mellitus, AF atrial fibrillation, ACEI angiotensin converting enzyme inhibitor, ARB angiotensin receptor inhibitor, ARNI angiotensin receptor neprilysin inhibitor, MRA mineralocorticoidreceptor, SGLT2i Sodium-glucose cotransporter 2 inhibitor, CCB calcium channel blocker. BNP B-type natriuretic peptide, ALT aspartate transaminase, AST alanine aminotransferase, LDH lactate dehydrogenase, TBIL total bilirubin, ALB albumin, GLB globulin, Cr creatinine, UA uric acid, TG triglyceride, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, CRP C-creative protein, eGFR estimated glomerular filtration rate, IVSTD interventricular septal thickness at diastole, LVPWTd left ventricular posterior wall end-diastolic thickness, LVDd left ventricular end-diastolic diameter, LAD left atrial diameter, LVEF left ventricular ejection fraction, WBC white blood cell, GRA granulocyte, RBC red blood cell, HGB hemoglobin, PLT platelet count, HbA1c hemoglobin A1c
*P<0.05 **P<0.001
Fig. 2.
Differences of each lipid indicators in different BMI groups. BMI1: underweight group, BMI2: normal group, BMI3: overweight group, BMI4: obese group
Predictive power of BMI for primary endpoint events
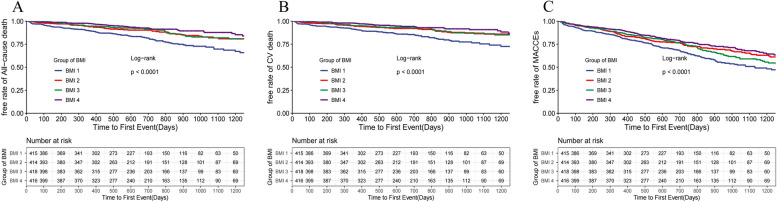
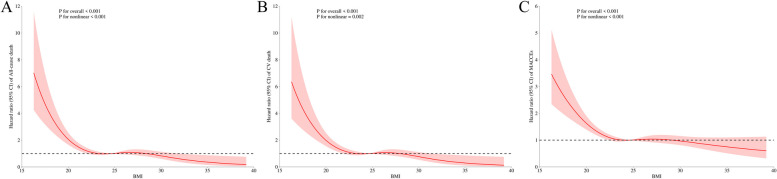
According to Kaplan-Meier survival curves, it demonstrated that the four groups of HF patients with different BMI levels had significantly difference in the three primary endpoints (p<0.001, log-rank test) and survival increased clearly with increasing BMI levels (Fig. 3). After adjusting for confounders in model 3, it was found that high BMI levels were associated with lower all-cause mortality (HR=0.47, 95%CI 0.31~0.69, p<0.001, obese vs underweight), cardiovascular mortality (HR=0.46, 95%CI 0.30~0.73, p<0.001, obese vs underweight) and the incidence of MACCEs (HR=0.68, 95%CI 0.53~0.88, p=0.003, obese vs underweight) (Table 2). In addition, the MACCEs was used as the endpoint event in a Cox model constructed in four groups with different BMI levels as a rank variable. The results showed that the risk of MACCEs in patients gradually decreased with increasing BMI levels (HR=0.892,95%CI 0.822~0.968, p=0.006) (Fig. 4). Based on restricted cubic spline analysis, we tested whether BMI had a linear relationship with endpoint events, and the results showed that their relationship was nonlinear(p<0.001). The RCS curves (Fig. 5) showed that the risk of all-cause death(A), cardiovascular death(B)and MACCEs(C)in patients was gradually decreased with the increase of BMI.
Fig. 3.
Kaplan-Meier survival curves for all-cause death (A), cardiovascular death (B), and MACCEs (C) in four groups of patients with BMI levels. BMI1: underweight group, BMI2: normal group, BMI3: overweight group, BMI4: obese group
Table2.
The results of cox models in three endpoints under different BMI level
| Model 1a | Model 2b | Model 3c | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Group | Events(n,%) | HR(95%CI) | p Value | HR(95%CI) | p Value | HR(95%CI) | p Value |
| All-cause death | Underweight | 91(21.9) | Reference | Reference | Reference | |||
| Normal | 53(12.8) |
0.56 (0.40, 0.78) |
<0.001 |
0.58 (0.41,0.81) |
0.001 |
0.55 (0.39,0.77) |
<0.001 | |
| Overweight | 49(11.7) |
0.50 (0.36,0.71) |
<0.001 |
0.53 (0.38,0.76) |
<0.001 |
0.51 (0.35,0.72) |
<0.001 | |
| Obese | 38(9.1) |
0.38 (0.26,0.55) |
<0.001 |
0.48 (0.33,0.71) |
<0.001 |
0.47 (0.31,0.69) |
<0.001 | |
| CV death | Underweight | 73(17.6) | Reference | Reference | Reference | |||
| Normal | 41(9.9) |
0.54 (0.37,0.79) |
0.002 |
0.55 (0.38,0.81) |
0.002 |
0.53 (0.36,0.78) |
0.001 | |
| Overweight | 39(9.3) |
0.50 (0.34,0.74) |
<0.001 |
0.53 (0.36,0.78) |
0.001 |
0.51 (0.34,0.76) |
<0.001 | |
| Obese | 30(7.2) |
0.37 (0.24,0.57) |
<0.001 |
0.46 (0.30,0.71) |
<0.001 |
0.46 (0.30,0.73) |
<0.001 | |
| MACCEs | Underweight | 171(41.2) | Reference | Reference | Reference | |||
| Normal | 124(30.0) |
0.70 (0.55,0.88) |
0.002 |
0.72 (0.57,0.90) |
0.005 |
0.69 (0.55,0.87) |
0.002 | |
| Overweight | 136(32.5) |
0.75 (0.60,0.94) |
0.012 |
0.79 (0.63,0.99) |
0.039 |
0.75 (0.60,0.95) |
0.015 | |
| Obese | 107(25.7) |
0.57 (0.45,0.73) |
<0.001 |
0.71 (0.56,0.91) |
0.007 |
0.68 (0.53,0.88) |
0.003 | |
aModel 1 unadjusted
bModel 2 adjusted for age, sex
cModel 3 adjusted for age, sex, LVEF, hypertension, DM, eGFR, CRP
Fig. 4.
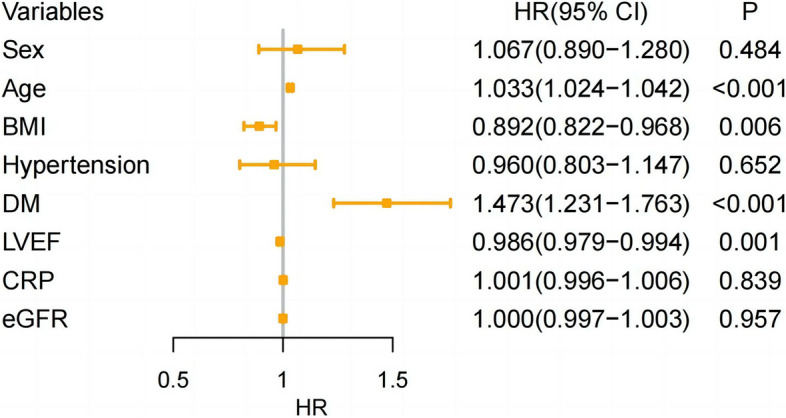
Multifactorial Cox regression modeling with MACCEs outcome as endpoint event
Fig. 5.
Restricted cubic spline showing the relationship between BMI as a continuous variable and the risk ratios for all-cause mortality (A), cardiovascular death (B), and MACCEs (C)
Sensitivity analysis
First, when grouped according to median BNP and median glucose for analysis (Additional file 1 Table S2), their results were similar to the results of the multifactorial adjusted Cox model. Second, the results grouped by median ALT and AST (Additional file 1 Table S3) showed that in the range of ALT >20.1 U/L, the difference in all-cause deaths and MACCEs outcomes was not significant in the obesity group compared with underweight group, and the other results were similar to those reported. Third, the results of the analyses of whether patients had comorbid hyperlipidemia and whether they were on lipid-lowering therapy (with or without statins) were consistent with those reported (Additional file 1 Table S4), except that among patients with comorbid hyperlipidemia, the difference between the overweight and obese groups compared with the low-body group was not significant. Finally, the results in the stratified analyses of covariates using Cox regression were consistent with reported (Additional file 1 Table S5).
Analysis of mediating effects of lipoprotein levels
The results of the mediation analyses after adjusting for confounding factors showed that TG was the mediator with the strongest association between BMI and cardiovascular outcomes, with the proportion of mediated effects of TG in model 3 after adjustment for confounders being 6.6% (95%CI 2.2%~18.0%, p=0.0258, in all-cause deaths),7.0% (95%CI 2.3%~18.9%, p=0.0301, in cardiovascular deaths) and 10.2% (95%CI 2.3%~27.4%, p=0.0185, in MACCEs), respectively. In model 1, TC and Apo-B also showed strong mediating effects, with TC mediating 3.4% (95%CI 1.1%~21.1%, p=0.0398) and 6.3% (95%CI 2.4%~15.4%, p=0115) in all-cause death and MACCEs, and apo-B only showed a strong mediating effect in MACCEs (mediation ratio 10.6%, 95%CI 4.7%~22.0%, p=0.0018). Notably, after adjusting for confounders in Models 2 and 3, Apo-B still showed a strong mediating effect in MACCEs (Model 2: 5.7%, 95%CI 1.6%~18.6%, p=0.0447; Model 3:6.1%, 95%CI 1.7%~19.6%, p=0.0470) (Table 3).
Table 3.
Results of mediation effects analysis in the total population
| variables | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| Percentage of mediation effect | P value | Percentage of mediation effect | P value | Percentage of mediation effect | P value | |
| All-cause death | ||||||
| TG |
9.1 (3.6,21.1) |
0.0076* |
6.2 (1.9,18.4) |
0.0375* |
6.6 (2.2,18.0) |
0.0258* |
| TC |
3.4 (1.1,10.3) |
0.0398* |
1.8 (0.3,10.1) |
0.1443 | - | - |
| HDL-C | - | - | - | - | - | - |
| LDL-C |
2.8 (0.6,12.9) |
0.1030 |
1.1 (0.1,17.4) |
0.2486 | - | - |
| Apo-AI | - | - |
2.7 (0.2,28.7) |
0.2282 |
2.3 (0.2,18.9) |
0.1881 |
| Apo-B |
1.5 (0.0,45.1) |
0.3077 | - | - |
1.1 (0.0,31.8) |
0.2979 |
| CV death | ||||||
| TG |
9.1 (3.4,22.1) |
0.0123* |
6.7 (2.1,19.6) |
0.0364* |
7.0 (2.3,19.6) |
0.0301* |
| TC |
2.9 (0.7,10.9) |
0.0737 |
1.6 (0.2,10.7) |
0.1650 | - | - |
| HDL-C | - | - | - | - | - | - |
| LDL-C |
1.6 (0.1,22.3) |
0.2396 | - | - | - | - |
| Apo-AI | - | - |
3.8 (0.5,24.2) |
0.1693 |
3.5 (0.6,19.0) |
0.1395 |
| Apo-B | - | - | - | - | - | - |
| MACCEs | ||||||
| TG |
14 (6.1,29.0) |
0.0026* |
10.2 (3.0,29.5) |
0.0288* |
10.2 (3.3,27.4) |
0.0185* |
| TC |
6.3 (2.4,15.4) |
0.0115* |
2.4 (0.2,25.5) |
0.2245 |
1.9 (0.1,33.8) |
0.2724 |
| HDL-C | - | - | - | - | - | - |
| LDL-C |
8.4 (3.7,18.2) |
0.0031* |
4.4 (1.0,17.3) |
0.0810 |
3.8 (0.7,18.8) |
0.1223 |
| Apo-AI | - | - | - | - | - | - |
| Apo-B |
10.6 (4.7,22.0) |
0.0018* |
5.7 (1.6,18.6) |
0.0447* |
6.1 (1.7,19.6) |
0.0470* |
*p<0.05
aModel 1 unadjusted
bModel 2 adjusted for age, sex
cModel 3 adjusted for age, sex, LVEF, hypertension, DM , eGFR, CRP
In addition, according to the Akaike information criterion, we took BMI=24kg/m2 as the cutoff value and divided it into two groups(Additional file 1 Table S1): BMI≤24kg/m2 and BMI>24kg/m2, to explore the mediating effect of TG and other indicators in the range of BMI ≤ 24kg/m2 (n=692,41.6%). Other factors were not found to be significantly different from the results, except for TC, which showed a strong mediating effect in MACCEs in model 1 (mediation ratio of 4.8%, 95%CI 1.4%~15.1%, p=0.0407). However, it has to be mentioned that the mediating proportions of TG in model 3 were 3.6% (95%CI 1.0%~12.2%, p=0.0599, in all-cause mortality), 3.8% (95%CI 0.9%~15.5%, p=0.0922, in cardiovascular death) and 4.4% (95%CI 1.2%~14.5%, p=0.0535, in MACCEs),and although the differences were not significant(p>0.05), the range of p-value below 0.1, which still demonstrated strong mediation, and this is consistent with the results for the total population. In addition, LDL-C and Apo-B also showed strong mediating effects in MACCEs(p<0.1) (Table 4).
Table 4.
Results of mediation effects analysis in the range of BMI≤24kg/m2
| variables | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| Percentage of mediation effect | P value | Percentage of mediation effect | P value | Percentage of mediation effect | P value | |
| All-cause death | ||||||
| TG |
3.9 (0.7,0.84) |
0.1201 |
2.9 (0.3,23.5) |
0.1889 |
3.6 (1.0,12.2) |
0.0599* |
| TC |
3.4 (0.7,14.5) |
0.0922* |
2.9 (0.5,15.4) |
0.1250 |
2.3 (0.3,14.2) |
0.1473 |
| HDL-C | - | - | - | - | - | - |
| LDL-C |
1.1 (0.0,39.0) |
0.3120 | - | - |
1.1 (0.0,21.4) |
0.2672 |
| Apo-AI |
1.4 (0.0,81.2) |
0.3647 |
3.1 (0.2,34.4) |
0.2367 |
1.1 (0.0,68.7) |
0.3521 |
| Apo-B | - | - | - | - | - | - |
| CV death | ||||||
| TG |
3.5 (0.4,25.0) |
0.1794 |
3.3 (0.3,27.1) |
0.1995 |
3.8 (0.9,15.5) |
0.0922* |
| TC |
2.9 (0.4,17.1) |
0.1471 |
2.9 (0.4,18.3) |
0.1559 |
2.6 (0.3,18.1) |
0.1645 |
| HDL-C | - | - | - | - | - | - |
| LDL-C | - | - | - | - |
1.1 (0.0,35.4) |
0.3061 |
| Apo-AI |
1.4 (0.0,95.0) |
0.3929 |
3.9 (0.3,36.1) |
0.2230 |
3.1 (0.2,29.5) |
0.2142 |
| Apo-B | - | - | - | - | - | - |
| MACCEs | ||||||
| TG |
5.0 (1.1,20.2) |
0.0867* |
3.7 (0.5,23.2) |
0.1556 |
4.4 (1.2,14.5) |
0.0535* |
| TC |
4.8 (1.4,15.1) |
0.0407* |
4.4 (1.2,15.1) |
0.0527* |
4.4 (1.1,16.3) |
0.0650* |
| HDL-C | - | - | - | - | - | - |
| LDL-C |
4.0 (1.0,14.6) |
0.0598* |
3.3 (0.7,14.7) |
0.0931* |
4.4 (1.1,16.1) |
0.0621* |
| Apo-AI | - | - |
1.9 (0.1,22.9) |
0.2311 | - | - |
| Apo-B |
4.3 (1.0,17.2) |
0.0804* |
3.1 (0.4,19.0) |
0.1480 |
4.8 (1.2,16.9) |
0.0609* |
*p<0.1
aModel 1 unadjusted
bModel 2 adjusted for age, sex
cModel 3 adjusted for age, sex, LVEF hypertension, DM eGFR, CRP
Discussion
We are the first retrospective cohort study to use mediation effect analysis to explore possible mechanisms of the obesity paradox in HF. First, we found that high BMI level was associated with lower rate of endpoint events and this result validates the obesity paradox. Second, there are a mediating role for lipid levels especially triglycerides in all-cause death, cardiovascular death and MACCEs, which mediate the causal relationship between BMI and outcomes.
Heart failure, as a multifaceted clinical syndrome, is caused by structural or functional abnormalities in the heart that result in diminished cardiac output and/or heightened intracardiac pressure [11, 13]. Despite notable progress in medical therapy and revascularization approaches, HF remains an escalating worldwide epidemic. The global prevalence of HF has surged from 33.5 million in 1990 to 64.3 million in 2017 [14]. The annual incidence of HF in Europe and North America is estimated to range from 2 to 3 per 1000 individuals, with a notable increase in occurrence among older age groups [15]. In China, presently, it is estimated that approximately 330 million individuals are affected by cardiovascular diseases, including 8.9 million individuals with HF [16]. Obesity is characterized by BMI exceeding 30kg/m2 and the excessive accumulation of adipose tissue(AT)in various regions of the body [17]. Data from the U.S. Health and Nutrition Examination Survey reveals that the prevalence of obesity among American adults has already reached a rate of 42.4 percent [18]. A majority of adults is afflicted with overweight or obesity in China [19]. Additionally, obesity is recognized as a significant risk factor for various ailments including cardiovascular disease, diabetes, hypertension and fatty liver, which it has emerged as a pressing health concern [20–22]. Obesity is a contributing factor to the onset of HF due to its detrimental impact on the functioning of the left ventricle [23]. The presence of obesity can induce modifications in the structure and function of the cardiac muscle, resulting in compromised myocardial performance, cardiac hypertrophy, and ultimately HF [24]. Furthermore, obesity holds significant relevance as a risk factor for HF, irrespective of whether it presents as heart failure with preserved ejection fraction (HEpEF) or heart failure with reduced ejection fraction (HErEF) [25].
However, within individuals with HF, patients with a higher BMI do not exhibit a worse prognosis, and even their prognosis may be more favorable compared to those with a normal or low BMI. This paradox is known as the obesity paradox. The initial discovery of this phenomenon was made by Horwich et al [26] in a study. Their findings indicated that HF patients with higher BMI levels exhibited higher survival rates. Numerous studies conducted in recent years [27–30] have consistently demonstrated that obesity confers a favorable outcome in HF patients, which it may potentially offer a certain degree of protection. Moreover, obesity exhibited a reduction in mortality rates among patients regardless of both HFrEF and HFpEF [31]. In this study, we included LVEF as confounders in models, including Cox regression models and mediation models, and obtained the same results. Furthermore, multivariate Cox models were constructed in HFrEF+HFmrEF and HFpEF groups to explore the association between BMI level and outcome events and the obesity paradox was found in both groups (Additional file 1 Table S7). The phenomenon of the obesity paradox has been observed in Chinese populations with HF [8]. For instance, a study conducted by Hao Sufang et al [7] revealed that BMI can independently predict mortality in HF patients, with low BMI being associated with higher mortality rates. We similarly obtained comparable results in Chinese HF patients, meaning that the findings are consistent with those in Europe and the United States. Several studies have employed alternative approaches to evaluate obesity among individuals with HF, such as fat content and body surface area (BSA). These studies have examined obesity using other metrics outside BMI, and they have also produced outcomes that the obesity paradox.
There must be some mechanism underlying this strange phenomenon. Lipoproteins, which consist of lipids and proteins, play a crucial role in the transportation of triglycerides and cholesterol within the bloodstream [32], as well as it is frequently utilized to evaluate the risk of patients [33]. For example, since HDL particles carry molecules other than Apo1 (superoxide dismutase, sphingosine-1-phosphate), this makes HDL more potent than HDL-C in terms of antioxidant, anti-inflammatory, and anti-apoptotic functions. Hence, HDL may provide better prognostic information [34, 35]. The endotoxin-lipoprotein hypothesis, proposed in a study conducted by Niebauer et al offers a potential explanation for the obesity paradox [10]. Immune activation in inflammatory diseases is facilitated by bacterial lipopolysaccharide, which can cause these diseases to progress in a harmful direction [36]. Bacterial endotoxin is a potent trigger for the discharge of inflammatory cytokines by circulating immune cells, which the source of immune activation of chronic heart failure may be attributed to bacterial endotoxin, thereby exacerbating the condition of chronic heart failure [37]. However, elevated levels of cholesterol may confer benefits in the context of chronic heart failure. This is due to the capacity of circulating lipoproteins and triglyceride lipoproteins to bind and detoxify bacterial endotoxins, thereby those play a role in modulating immune function and safeguarding the body against bacterial endotoxins. In addition, aerobic exercise, smoking cessation, balanced diet and other healthy activities can increase the level of HDL-C, and these healthy activities may also bring certain benefits to patients [38]. Our study found that both TC and apo-B showed strong mediating effects before adjusting for confounders. In contrast, this mediating effect of TC became insignificant in Models 2 and 3 and possibly related to the smaller sample size. There appears to be an obesity paradox regardless of whether patients are taking statins or not (Additional file 1 Table S4). This poses a challenge that the interpretation of lipid levels may be influenced by the sample size and by other possible mechanisms, which need to be further studied in the future. In obese individuals, the presence of circulating lipoproteins with higher cholesterol levels may effectively counteract inflammatory endotoxins and impede the inflammatory response, ultimately mitigating cardiac inflammation and offering cardiovascular protection [5, 10, 39]. Similarly, in the mediation model we constructed, it can be found that TG is an influential factor that exerts the strongest mediating effect. It was also found that the mediating effect of TG was more pronounced in patients with HFrEF+HFmrEF and without MI (Additional file 1 Table S6), which provided more detailed patient grouping information (such as different types of HF and comorbidities) for further understanding the mechanism of the obesity paradox. In conclusion, the protective mechanism of high lipoprotein levels in obese patients may be an explanation for the obesity paradox.
As such, it is imperative that we concentrate on the obesity paradox in HF and the potential mechanisms behind it. This study provides a foundation for further research aimed at deciphering the mechanism underlying the obesity paradox. In the meantime, our research will contribute to a better understanding of obese patients with HF and offer a solid scientific basis for improving their long-term prognosis.
Limitations
The limitations of this study are as follows. First, the retrospective study itself suffered from recall bias of patients, which may have led to inappropriate grouping. Second, only the BMI at admission was calculated without focusing on the changes during follow-up. Third, we only used the BMI to assess the obesity status and did not measure their metabolic status, waist-to-hip ratio. Fourth, single-center findings do not apply well to all HF patients. Therefore, it is necessary to conduct further large prospective cohort studies to confirm our findings.
Conclusion
In conclusion, this research provides evidence supporting the obesity paradox, highlighting the protective nature of obesity for HF. Additionally, the mediating role of lipoprotein levels, especially TG, elucidates a potential mechanism for the protective effect of obesity on HF.
Supplementary Information
Acknowledgements
The authors would like to thank all patients who participated in this study.
Abbreviations
- HF
Heart failure
- CHF
Chronic heart failure
- BMI
Body mass index
- MACCEs
Major adverse cardiac and cerebral events
- TG
Triglyceride
- TC
Total cholesterol
- HDL-C
High density lipoprotein cholesterol
- LDL-C
Low density lipoprotein cholesterol
- Apo-A1
Apolipoprotein A1
- Apo-B
Apolipoprotein B
- CHD
Coronary heart disease
- AF
Atrial fibrillation
- NYHA
New york heart association
- HR
Heart rate
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- DM
Diabetes mellitus
- ACEI
Angiotensin-converting enzyme inhibitor
- ARB
Angiotensin receptor inhibitor
- ARIN
Angiotensin receptor neprilysin inhibitor
- SGLT2i
sodIum-glucose cotransporter 2 inhibitor
- ALB
Albumin
- GLB
Globulin
- UA
Uric acid
- Cr
Creatinine
- CRP
C-creative protein
- eGFR
Estimated glomerular filtration rate
- WBC
White blood cell
- GRA
Granulocyte
- RBC
Red blood cell
- HGB
Hemoglobin
- PLT
Platelet count
- BNP
B-type natriuretic peptide
- HbA1c
Hemoglobin A1c
- LVEF
Left ventricular ejection fraction
- IVSTd
Interventricular septal thickness at diastole
- LVPWTd
Left ventricular posterior wall end-diastolic thickness
- LVDd
Left ventricular end-diastolic diameter
- LAD
Left atrial diameter
- LDH
Lactate dehydrogenase
- TBIL
Total bilirubin
- MRA
Mineralocorticoidreceptor
- CCB
Calcium channel blocker
- AT
Adipose tissue
- HEpEF
Heart failure with preserved ejection fraction
- HErEF
Heart failure with reduced ejection fraction
- BSA
Body surface area
- LPS
Lipopolysaccharide
Author contributions
YW: Writing-original draft, Methodology. XLL and BCW: Data curation. XT, LC, HYC and ZYZ: Investigation. XB, BX and RG: Writing-review & editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University(2023-LCYJ-PY-34); the National Natural Science Foundation of China (82100478); the Jiangsu Planned Projects for Postdoctoral Research Funds (2021K287B); and the Nanjing Medical Science and Technology Development Project (YKK21070).
Availability of data and materials
The information and data of the study population were extracted from the hospital information system. The datasets are not publicly available because the privacy of the participants should be protected. The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The need for informed consent was waived by Nanjing Drum Tower Hospital ethical committee because of the retrospective nature of the study.This was a retrospective study in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Nanjing Drum Tower Hospital(2023-428-01).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi Wang, Xiaoli Liu and Baochuan Wu contributed equally to this work.
Contributor Information
Xue Bao, Email: baoxue@njglyy.com.
Biao Xu, Email: xubiao62@nju.edu.cn.
Rong Gu, Email: gurong.nju@163.com.
Reference
- 1.Roalfe AK, Taylor CJ, Hobbs FDR. Long term changes in health-related quality of life for people with heart failure: the ECHOES study. ESC Heart Fail. 2023;10(1):211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145(8):e153–639. [DOI] [PubMed] [Google Scholar]
- 3.Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2021;143(21):e984–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niderla-Bielińska J, Ścieżyńska A, Moskalik A, Jankowska-Steifer E, Bartkowiak K, Bartkowiak M, Kiernozek E, Podgórska A, Ciszek B, Majchrzak B et al: A Comprehensive miRNome Analysis of Macrophages Isolated from db/db Mice and Selected miRNAs Involved in Metabolic Syndrome-Associated Cardiac Remodeling. Int J Mol Sci 2021, 22(4). [DOI] [PMC free article] [PubMed]
- 5.Horwich TB, Fonarow GC, Clark AL. Obesity and the Obesity Paradox in Heart Failure. Prog Cardiovasc Dis. 2018;61(2):151–6. [DOI] [PubMed] [Google Scholar]
- 6.Shalaby G, Samarin K, Alabbasi R, Fallatah AA, Roblah T, Abdulwahab RA, Althomali RN, Babateen EM, Alhodian FY, Khaled S. Obesity Influences on Patients With Non-valvular Cardiomyopathy in Relation to Early In-Hospital Outcomes and Health System Burden. Cureus. 2022;14(5): e24859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.郝素芳, 侯翠红, 裴娟慧, 冉玉琴, 张澍, 浦介麟: 体重指数对慢性心力衰竭患者全因死亡风险的预测作用. 中国循环杂志. 2013;28(01):51–4.
- 8.吴穷, 李慧, 任妍, 张凤如: 体质量指数对慢性心力衰竭患者预后的影响. 内科理论与实践. 2016;11(03):176–9.
- 9.Abrahams C, Woudberg NJ, Lecour S. Anthracycline-induced cardiotoxicity: targeting high-density lipoproteins to limit the damage? Lipids Health Dis. 2022;21(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Coats AJ, Anker SD. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353(9167):1838–42. [DOI] [PubMed] [Google Scholar]
- 11.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032. [DOI] [PubMed] [Google Scholar]
- 12.VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health. 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 13.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. [DOI] [PubMed] [Google Scholar]
- 14.Bragazzi NL, Zhong W, Shu J, Abu Much A, Lotan D, Grupper A, Younis A, Dai H. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. 2021;28(15):1682–90. [DOI] [PubMed] [Google Scholar]
- 15.Roger VL. Epidemiology of Heart Failure: A Contemporary Perspective. Circ Res. 2021;128(10):1421–34. [DOI] [PubMed] [Google Scholar]
- 16.中国心血管健康与疾病报告2020概要. 中国循环杂志. 2021;36(06):521–45.
- 17.Piché ME, Tchernof A, Després JP. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ Res. 2020;126(11):1477–500. [DOI] [PubMed] [Google Scholar]
- 18.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- 19.中国居民营养与慢性病状况报告 (2020年) . 营养学报. 2020;42(06):521.
- 20.The Lancet Diabetes E: Should we officially recognise obesity as a disease? Lancet Diabetes Endocrinol 2017, 5(7):483. [DOI] [PubMed]
- 21.Elbaz-Greener G, Rozen G, Carasso S, Yarkoni M, Wijeysundera HC, Alcalai R, Gotsman I, Rahamim E, Planer D, Amir O. The Relationship Between Body Mass Index and In-hospital Survival in Patients Admitted With Acute Heart Failure. Front Cardiovasc Med. 2022;9: 855525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ. Obesity paradox in cardiovascular disease: where do we stand? Vasc Health Risk Manag. 2019;15:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Bandt JP, Monin C: Obesity, Nutrients and the Immune System in the Era of COVID-19. Nutrients 2021, 13(2). [DOI] [PMC free article] [PubMed]
- 24.Palomer X, Román-Azcona MS, Pizarro-Delgado J, Planavila A, Villarroya F, Valenzuela-Alcaraz B, Crispi F, Sepúlveda-Martínez Á, Miguel-Escalada I, Ferrer J, et al. SIRT3-mediated inhibition of FOS through histone H3 deacetylation prevents cardiac fibrosis and inflammation. Signal Transduct Target Ther. 2020;5(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385(16):1451–61. [DOI] [PubMed] [Google Scholar]
- 26.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38(3):789–95. [DOI] [PubMed] [Google Scholar]
- 27.Carbone S, Lavie CJ, Arena R. Obesity and Heart Failure: Focus on the Obesity Paradox. Mayo Clin Proc. 2017;92(2):266–79. [DOI] [PubMed] [Google Scholar]
- 28.Marcks N, Aimo A, Januzzi JL Jr, Vergaro G, Clerico A, Latini R, Meessen J, Anand IS, Cohn JN, Gravning J, et al. Re-appraisal of the obesity paradox in heart failure: a meta-analysis of individual data. Clin Res Cardiol. 2021;110(8):1280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gajulapalli RD, Kadri A, Gad M, Chahine J, Nusairat L, Rader F. Impact of Obesity in Hospitalized Patients with Heart Failure: A Nationwide Cohort Study. South Med J. 2020;113(11):568–77. [DOI] [PubMed] [Google Scholar]
- 30.Pandey A, Berry JD, Drazner MH, Fang JC, Tang WHW, Grodin JL. Body Mass Index, Natriuretic Peptides, and Risk of Adverse Outcomes in Patients With Heart Failure and Preserved Ejection Fraction: Analysis From the TOPCAT Trial. J Am Heart Assoc. 2018;7(21): e009664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tadic M, Cuspidi C. Obesity and heart failure with preserved ejection fraction: a paradox or something else? Heart Fail Rev. 2019;24(3):379–85. [DOI] [PubMed] [Google Scholar]
- 32.Giordani S, Marassi V, Placci A, Zattoni A, Roda B, Reschiglian P: Field-Flow Fractionation in Molecular Biology and Biotechnology. Molecules 2023, 28(17). [DOI] [PMC free article] [PubMed]
- 33.Burguete-García AI, Ramírez Valverde AG, Espinoza-León M, Vázquez IS, Estrada Ramírez EY, Maldonado-López I, Martínez AL, Diaz Benítez CE, Araujo RK, Fernández-Madinaveitia D, et al. Severe Quantitative Scale of Acanthosis Nigricans in Neck is Associated with Abdominal Obesity, HOMA-IR, and Hyperlipidemia in Obese Children from Mexico City: A Cross-Sectional Study. Dermatol Res Pract. 2022;2022:2906189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badimón JJ, Santos-Gallego CG, Badimón L. Importance of HDL cholesterol in atherothrombosis: how did we get here? Where are we going? Rev Esp Cardiol. 2010;63(Suppl 2):20–35. [DOI] [PubMed] [Google Scholar]
- 35.Santos-Gallego CG, Rosenson RS. Role of HDL in those with diabetes. Curr Cardiol Rep. 2014;16(9):512. [PubMed] [Google Scholar]
- 36.Espinosa-Riquer ZP, Segura-Villalobos D, Ramírez-Moreno IG, Pérez Rodríguez MJ, Lamas M, Gonzalez-Espinosa C: Signal Transduction Pathways Activated by Innate Immunity in Mast Cells: Translating Sensing of Changes into Specific Responses. Cells 2020, 9(11). [DOI] [PMC free article] [PubMed]
- 37.Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;356(9233):930–3. [DOI] [PubMed] [Google Scholar]
- 38.Santos-Gallego CG, Ibanez B, Badimon JJ. HDL-cholesterol: is it really good? Differences between apoA-I and HDL. Biochem Pharmacol. 2008;76(4):443–52. [DOI] [PubMed] [Google Scholar]
- 39.Jackson AO, Meng J, Tang H, Yin K. High-density lipoprotein-mediated cardioprotection in heart failure. Heart Fail Rev. 2021;26(4):767–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The information and data of the study population were extracted from the hospital information system. The datasets are not publicly available because the privacy of the participants should be protected. The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.