Abstract
Patient: Female, 46-year-old
Final Diagnosis: Median arcuate ligament syndrome
Symptoms: Abdominal and/or epigastric pain
Clinical Procedure: —
Specialty: Gastroenterology and Hepatology • Surgery
Objective:
Rare disease
Background:
Median arcuate ligament syndrome (MALS) poses a considerable challenge in terms of diagnosis due to its manifestation of diverse symptoms linked to constriction of the median arcuate ligament surrounding the celiac artery. The present study introduces an earlier diagnostic modality using ultrasound measurements of the flow velocity of the celiac artery during the inspiratory and expiratory phases, with the latter being higher than the former, to avoid prolonged follow-up of postprandial symptomatology.
Case Report:
A 46-year-old female patient presented with acute postprandial abdominal pain, which was alleviated by analgesic medication. The findings from the physical examination and laboratory tests were within normal limits. Further investigations were conducted due to persistent symptoms, revealing an elevation in celiac artery flow velocity during expiration on ultrasound. The diagnosis of median arcuate ligament syndrome (MALS) was confirmed through contrast-enhanced CT and angiography. Subsequently, the patient underwent laparoscopic release of the median arcuate ligament, leading to alleviation of symptoms at the 1-year follow-up assessment.
Conclusions:
Our case report highlights the importance of a dynamic imaging diagnostic strategy for MALS. When encountering challenging postprandial abdominal pain that is hard to diagnose, it could be crucial to utilize abdominal ultrasound to measure the flow velocity of the celiac artery. This approach may serve as a valuable screening method for identifying MALS and, subsequently, prompt the need for further diagnostic tests.
Key words: Abdominal Pain; Median Arcuate Ligament Syndrome; Ultrasonography, Doppler, Color
Introduction
Median arcuate ligament syndrome (MALS), also known as celiac artery compression syndrome, is positioned lower than the variants and is attributed to compression of the celiac artery and overstimulation of the celiac plexus. It is a rare disorder, with an incidence rate of 2 per 100 000 population [1]. There is no sex difference [2,3]. The MAL serves to connect the right and left crura of the diaphragm, where it forms the anterior border of the aortic hiatus [4]. The MAL and ganglionic tissue, which form a broad band with multiple layers overlying the CA [5], and MAL overlap on the celiac trunk has been reported as an anatomical variant [6]. MALS can develop if the MAL is inferior to its insertion at the level of the T12 or L1 vertebrae. The symptoms of MALS include postprandial nausea, abdominal pain, vomiting, and chronic weight loss. Due to this atypical symptomatology, MALS is difficult to diagnose because of the variety of symptoms associated with narrowing of the median arcuate ligament that surrounds the celiac artery. Diagnosis of MALS usually requires exclusion of other conditions to avoid unnecessary tests and procedures, facilitating early treatment. However, measurement of peak flow velocity in the celiac artery during the expiratory phase compared to the inspiratory phase, as shown in this case report, appears to be the definitive diagnostic modality for MALS [7].
Case Report
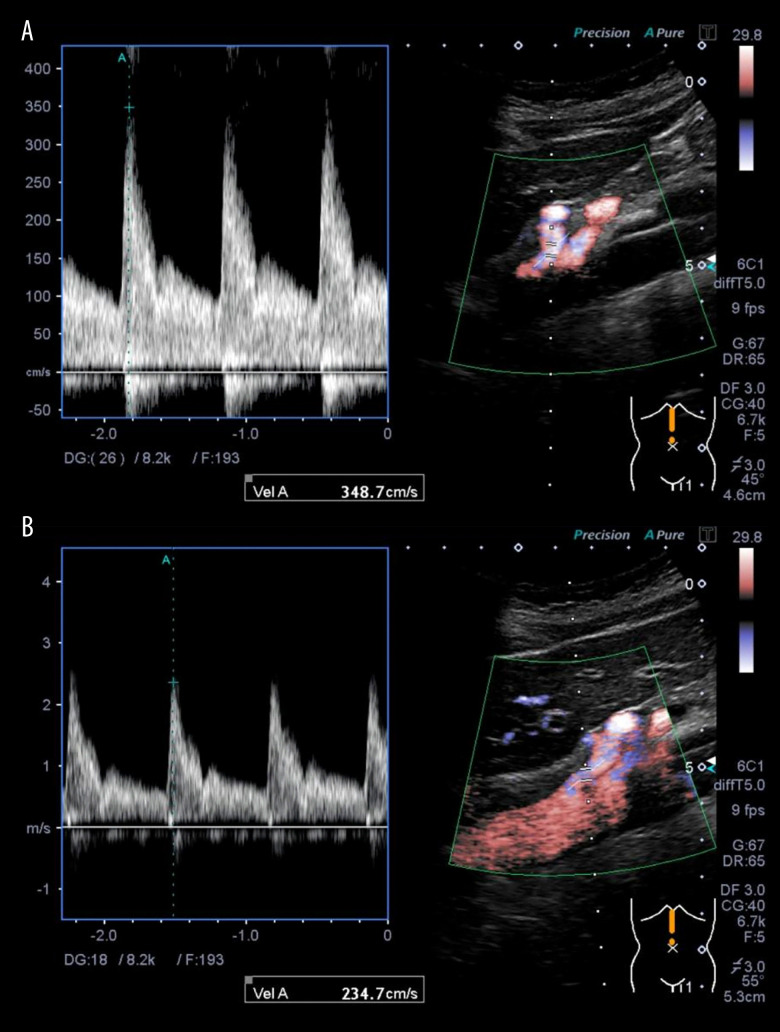
A female patient, aged 46, visited the Emergency Department due to abrupt onset of postprandial abdominal pain, which was relieved by administration of an over-the-counter analgesic. Physical examination was unremarkable, with no tenderness. No abnormalities were detected in laboratory examinations. The ultrasound (US) images showed a significantly increased peak flow velocity of the celiac artery during the expiratory phase of 348 cm/s (Figure 1A) compared to the inspiratory phase of 234 cm/s (Figure 1B). These results could be interpreted that the faster flow velocity of the celiac artery during the inspiratory phase compared to the expiratory phase is due to the compression of the celiac axis only during the expiratory phase. This led to a definitive diagnosis of MALS. To confirm this before surgery, contrast-enhanced computed tomography (CT) showed stenosis of the celiac artery (Figures 2, 3), whereas gastrointestinal endoscopy showed no abnormalities. Furthermore, contrast angiography of the celiac artery fails to visualize the branches that feed the celiac artery because of stenosis, which causes contrast to flow back into the aorta, even with contrast. Therefore, selective superior mesenteric artery contrast has been used to obtain visualization of the right hepatic artery, a branch of the celiac artery (Figure 4). The patient was diagnosed with MALS and underwent laparoscopic release of the median arcuate ligament (intraoperative photo, Figure 5), resulting in relief of symptoms. At 1-year follow-up, her condition remained satisfactory.
Figure 1.
The ultrasound images measured the blood flow velocities in the celiac artery during expiration (A) and inspiration (B). These showed significantly increased peak flow velocity during the expiratory phase.
Figure 2.
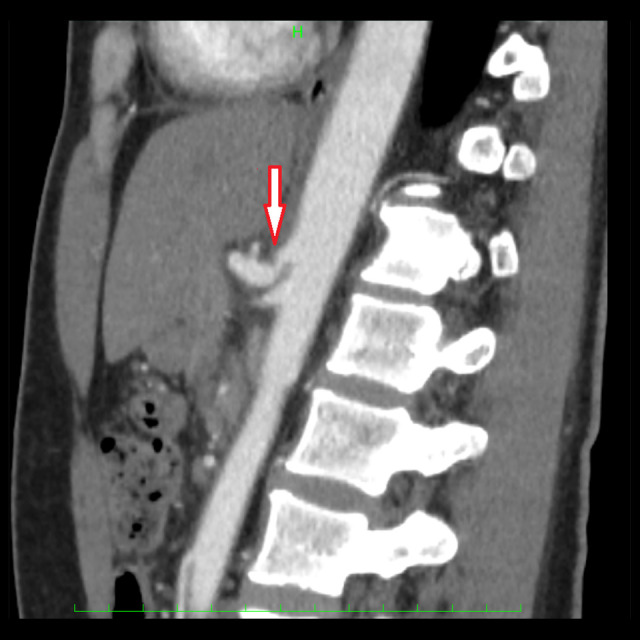
The sagittal view of the contrast-enhanced computed tomography showed the notch of the celiac artery (arrow).
Figure 3.
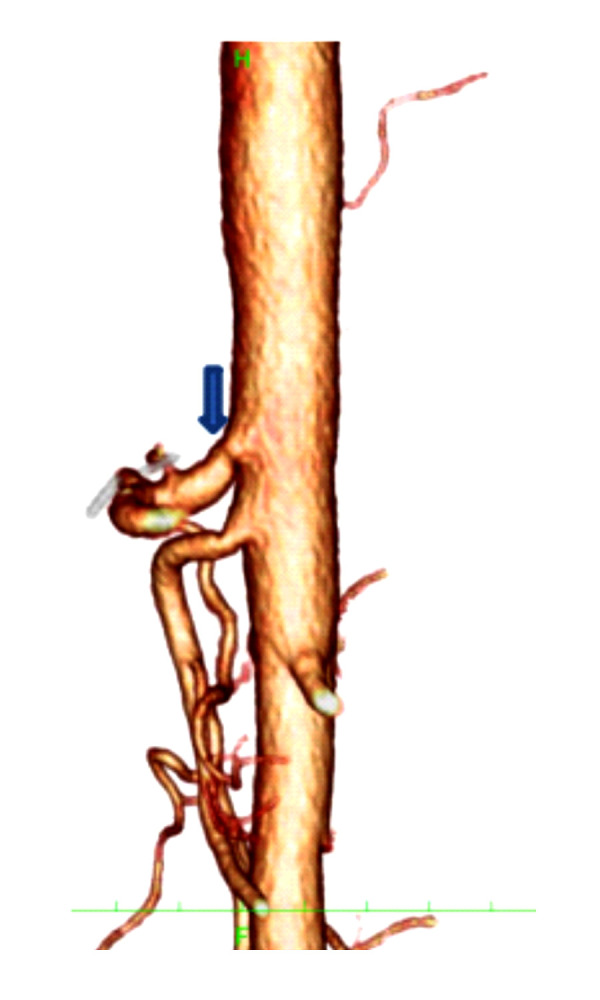
The 3-D computed tomographic scan of Figure 2
Figure 4.
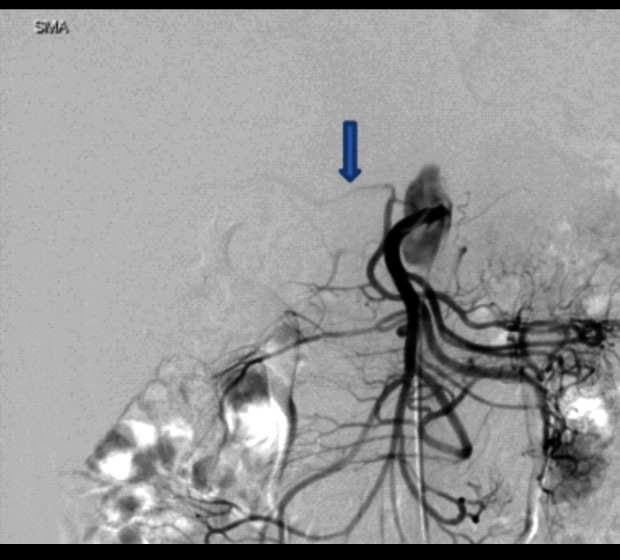
Angiography of the superior mesenteric artery. Arrow: Right hepatic artery.
Figure 5.
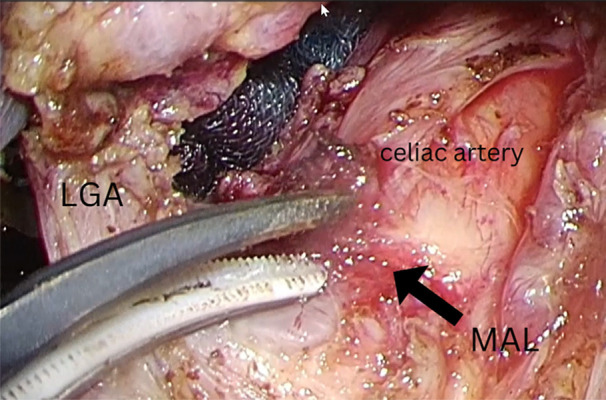
Intraoperative photo showing medial arcuate ligament (arrow), celiac artery(CA), and left gastric artery (LGA).
Discussion
The etiology of MALS is attributed to compression of the celiac plexus at the origin of the celiac artery by the median arcuate ligament. The syndrome is characterized by postprandial intestinal angina. Isolated cases of intra-abdominal hemorrhage due to visceral aneurysm formation and rupture after celiac artery occlusion or stenosis have been reported. To diagnose MALS, US images are required to determine the difference in flow velocity of the celiac artery during inspiration and expiration. The results of US for the diagnosis of MALS have a high specificity when the celiac artery has a flow velocity greater than 226 cm/s during expiration and a reduction greater than 68 cm/s during inspiration [7]. One of the characteristics of MALS is the presence of stenotic post-dilatation of the celiac artery [8]. In addition, other papers have reported the use of US to diagnose MALS, similar to our findings, and observed that arterial velocities were faster during inspiration compared to expiration, from 425 (±130.1) cm/s to 172 (±40.9) cm/s [9]. Angiography of the celiac artery is not considered useful because the catheter tip does not pass through the stenosis, causing contrast to spill into the aorta anterior to the stenosis, resulting in inconclusive contrast enhancement. Intravascular ultrasound (IVUS) can provide evidence of an external occlusion in the celiac artery [10], but its practical use is limited by high costs. Diagnosis requires thorough assessment, including ruling out other potential diagnoses. The criterion standard treatment for MALS is surgery to release the celiac artery [1].
Contrast-enhanced CT findings also easily show stenosis of the celiac artery due to MAL, but there are many cases in which the patient does not present with symptoms, as previously presented. Therefore, the treatment decision should not be based on the imaging findings alone, but on the comparison of the flow velocity of the celiac artery during the inspiratory and expiratory phases, which meets the criteria for MALS as described above, to avoid late treatment with prolonged follow-up, as shown in our case presentation. After the surgical procedure, it is reported that 90% of the individuals experience a reduction in symptoms [11].
The future direction of research based on this case report is to develop a method to confirm the effectiveness of surgery intraoperatively to perform intraoperative confirmation of celiac artery blood flow velocity using intraoperative US under anesthesia ventilation.
Conclusions
Our case report of MALS shows that ultrasound scan confirmed that the velocity of the cerebral artery was significantly faster during inspiration than during expiration. Use of ultrasound to diagnose MALS can help avoid prolonged follow-up of patients with post-prandial symptomatology and chronic weight loss. We emphasize that postprandial abdominal pain can be diagnosed by checking that the blood flow velocity in the celiac artery during the expiratory phase is faster than that during the inspiratory phase, taking MALS into account.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Grotemeyer D, Duran M, Iskandaf F, et al. Vascular surgical therapy and follow-up of 18 patients. Langenbecks Arch Surg. 2009;394:1085–92. doi: 10.1007/s00423-009-0509-5. [DOI] [PubMed] [Google Scholar]
- 2.Kozhimala M, Chan SM, Weininger G, et al. Prevalence and characteristics of patients with median arcuate ligament syndrome in a cohort diagnosed with celiac artery compression. J Am Coll Surg. 2023;236(6):1085–91. doi: 10.1097/XCS.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 3.Essa M, Meyer F, Damm R, Halloul Z. Chronic mesenteric ischemia: Differential vascular-surgical therapy and its outcome in a single-center observational study. Visc Med. 2022;38(4):255–64. doi: 10.1159/000519423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinliewicz M, Ramarkrishnan PK, Henry BM, et al. Laparoscopic decompression as treatment for median arcuate ligament syndrome. Ann R Coll Surg Engl. 2015;97:e96–e99. doi: 10.1308/rcsann.2015.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito K, Takemura N, Oikawa R, et al. Detailed anatomy and procedure of celiac artery decompression in median arcuate ligament syndrome. Langenbecks Arch Surg. 2021;406(5):1717–22. doi: 10.1007/s00423-021-02195-9. [DOI] [PubMed] [Google Scholar]
- 6.Dyches RP, Eaton KJ, Smith HF. The roles of celiac trunk angle and vertebral origin in median arcuate ligament syndrome. Diagnostics (Basel) 2020;10(2):76. doi: 10.3390/diagnostics10020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miura D, Hiwatashi R, Sakita M, Sakata T. A new comprehensive ultrasonic diagnostic method for celiac artery compression syndrome that hybridizes “arterial compression hook sign” and peak systolic velocity. J Ultrasound. 2021;24(3):289–95. doi: 10.1007/s40477-020-00519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JE, Kang M, Jeong OS, Rhee PL. A median arcuate ligament syndrome could be re-termed as a nutcracker celiac ganglion abdominal pain syndrome. J Neurogastroenterol Motility. 2023;29(2):200–7. doi: 10.5056/jnm22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruber H, Loizides A, Peer S, Gruber I. Ultrasound of the median arcuate ligament syndrome: A new approach to diagnosis. Med Ultrason. 2012;14(1):5–9. [PubMed] [Google Scholar]
- 10.Hattori T, Takayama S, Takura K, et al. Diagnosis of median arcuate ligament syndrome with intravascular ultrasound. Jpn J Gastroenterol Surg. 2021;54(21):901–8. [Google Scholar]
- 11.Kazmi SSH, Safi N, Berge ST, et al. A prospective cohort of 52 patients. Vasc Health Risk Manag. 2022;18:139–51. doi: 10.2147/VHRM.S350841. [DOI] [PMC free article] [PubMed] [Google Scholar]