Abstract
Diabetes has arisen as a noteworthy global health issue, marked by escalating incidence and mortality rates. Insulin, crucial for preserving euglycemia, acts as a vital energy provider for various tissues. Iron metabolism notably plays a significant role in the development of insulin resistance, a key factor in the onset of various metabolic disorders. The intricate interaction between iron and insulin signaling encompasses complex regulatory mechanisms at the molecular level, thereby impacting cellular reactions to insulin. The intricate interplay between insulin and glucagon, essential for precise regulation of hepatic glucose production and systemic glucose levels, may be influenced by certain microelements for instance zinc, copper, iron, boron, calcium, cobalt, chromium, iodine, magnesium and selenium. While significant progress has been achieved in elucidating the pathophysiological connections between iron overload and glucose metabolism, our understanding of the involvement of the Fenton reaction and oxidative stress in insulin resistance influencing many chronical conditions remains limited. Furthermore, the exploration of the multifaceted roles of insulin in the human body continues to be a subject of active investigation by numerous scientific researchers. This review comprehensively outlines the potential adverse impact of iron overload on insulin function and glucose metabolism. Additionally, we provide a synthesis of findings derived from various research domains, encompassing population studies, animal models, and clinical investigations, to scrutinize the multifaceted relationship between iron and insulin sensitivity. Moreover, we delineate instances of correlations between serum iron levels and various medical conditions, including the diabetes also gestational diabetes and obesity.
Keywords: Iron, Insulin sensitivity, Obesity, Diabetes
Introduction
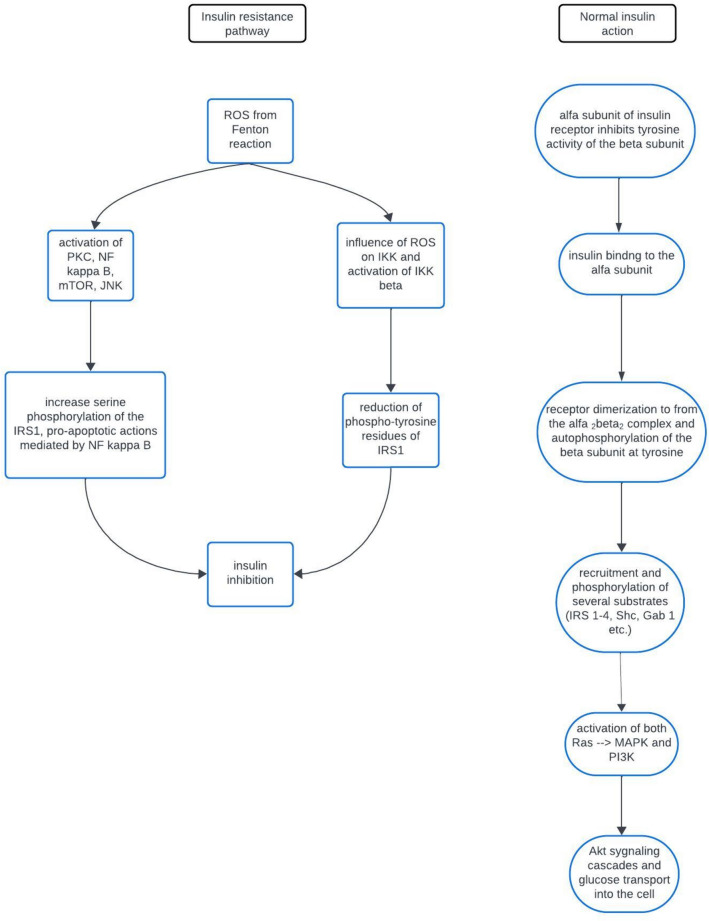
Iron metabolism significantly contributes to the pathogenesis of insulin resistance, a pivotal factor in the development of diverse metabolic disorders. The intricate interplay between iron and insulin signaling involves complex regulatory mechanisms at the molecular level, influencing cellular responses to insulin. Iron, as an essential micronutrient, crucially modulates insulin sensitivity, impacting various processes in glucose homeostasis. Iron's regulatory role in insulin receptor function is a key aspect of this complexity. It modulates insulin receptor phosphorylation, a crucial event initiating downstream signaling cascades. Alterations in iron levels impact the tyrosine phosphorylation of the insulin receptor, thereby influencing kinase activity and subsequent intracellular signaling. This finely tunes insulin sensitivity at the cellular membrane. Furthermore, iron's participation in cellular processes, such as oxidative stress and inflammation, adds complexity to its interplay with insulin signaling. Excess iron catalyzes reactive oxygen species (ROS) production through the Fenton reaction, inducing oxidative stress that interferes with insulin signaling pathways, contributing to insulin resistance (Fig. 1). Beyond the insulin receptor, iron regulation extends to transcription factors in glucose metabolism. Iron-responsive elements (IREs) and iron regulatory proteins (IRPs) collectively control gene expression related to iron homeostasis and cellular energy metabolism, influencing insulin sensitivity. The crosstalk between iron and inflammatory pathways further complicates their interplay. Inflammation, frequently associated with obesity and metabolic syndrome, modulates iron metabolism. Changes in iron levels, in turn, influence immune responses, creating a feedback loop that exacerbates insulin resistance in chronic inflammatory conditions. Elevated iron levels have been implicated in impairing insulin sensitivity, disrupting insulin-mediated glucose uptake. Evidence from population studies, clinical investigations, and animal models supports the association between elevated iron levels and insulin resistance. Iron's influence on adipose tissue macrophages and mitochondrial function introduces additional layers of complexity to comprehending how iron dysregulation contributes to metabolic dysfunction. Unraveling the molecular mechanisms that connect iron metabolism to insulin resistance is crucial for developing targeted therapeutic interventions to address metabolic disorders and enhance overall health outcomes [1–9].
Fig. 1.
Mechanism of occurrence of insulin resistance. ROS- reactive oxygen species. IRS1- insulin receptor substrate 1. PKC- protein kinase C. NF kappa B- nuclear factor kappa-light-chain-enhancer of activated B cells. mTOR- mammalian target of rapamycin. JNK- c-jun-N-terminal kinase. PI3K- phosphatidylinositide 3-kinase. MAPK- mitogen-activated protein kinase. Akt- protein kinase B. IKK/IKK beta- Ikappa B kinase
Crucial role in the storage and regulation of iron in the body is played by ferritin. It acts as an intracellular protein that stores iron in a non-toxic and bioavailable form. The primary function of ferritin is to maintain iron homeostasis by storing excess iron when it is abundant and releasing it when iron levels are low, thereby ensuring a steady supply of iron for cellular processes. Additionally, ferritin helps to protect cells from iron-induced oxidative damage by sequestering excess iron and preventing its participation in harmful reactions that generate ROS. Overall, ferritin plays a critical role in iron metabolism and cellular health by regulating iron storage and preventing iron-mediated oxidative damage. While it's primarily known for its role in iron metabolism, ferritin levels can also be influenced by inflammation. During inflammation, the body releases various cytokines and other inflammatory molecules. These substances can stimulate the production of ferritin in cells, leading to an increase in ferritin levels in the bloodstream. This occurs because ferritin is not only involved in iron storage but also serves as an acute-phase reactant, meaning its production increases in response to inflammation. Several studies have found associations between elevated ferritin levels and insulin resistance. High ferritin levels have been observed in individuals with insulin resistance, obesity, metabolic syndrome, and type 2 diabetes. However, the exact mechanism underlying this association is not fully understood [10–16].
A significant role in iron homeostasis is played by transferrin, a protein that transports iron in the body. Its main role is to bind iron and transport it in the bloodstream to tissues and organs, where it is utilized for various metabolic processes such as hemoglobin production in erythropoiesis or enzyme synthesis. Studies suggest that transferrin, the protein responsible for iron transport in the body, may be associated with insulin resistance. Elevated levels of transferrin may be associated with obesity and inflammatory states, which are often linked to insulin resistance. Obesity and inflammation can affect iron metabolism and transferrin function, which in turn may contribute to insulin sensitivity disturbances [17–21].
Examining the intricate interplay among iron metabolism, insulin resistance and metabolic disorders
Diabetes has emerged as a significant global health concern, underscored by a substantial increase in the number of affected individuals- from 108 million in 1980 to a staggering 422 million in 2014. World Health Organization (WHO) data from 2000 to 2019 indicates a 3% rise in diabetes mortality rates by age [22]. These alarming data are accompanied by many worldwide research suggesting that iron metabolism is a key factor in glucose metabolism changes and developing type 2 diabetes mellitus (T2DM). A population-based study, involving participants except individuals with cardiovascular disease, diabetes, or a body mass index > 35 kg/m2, established a robust correlation between markers of insulin resistance and indicators of iron metabolism in healthy subjects. In multivariable linear regression analyses, β-coefficients (95% confidence intervals) per 1-SD increase in ferritin for glucagon-like peptide 1 (GLP-1), insulin and homeostasis model assessment of insulin resistance (HOMA-IR) were statistically significant, otherwise for fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c). Furthermore, β-coefficients (95% CI) per 1-SD increase in transferrin saturation (TSAT) were for GLP-1, insulin, HOMA-IR, FPG and HbA1c statistically important [23].
In another prospective study, pregnant women who underwent antenatal booking before 20 weeks' gestation and did not exhibit anemia or diabetes mellitus (DM) were recruited at the time of the Oral Glucose Tolerance Test (OGTT) at 28–31 weeks' gestation for the study of serum ferritin, iron and transferrin concentrations. There was no difference in the weight and body mass index (BMI), booking and third trimester haemoglobin in both control group composed of participants chosen as at-risk but nondiabetic cases and non-anaemic women with gestational diabetes mellitus (GDM), while concentrations of serum ferritin, iron, transferrin saturation and the post-natal haemoglobin were significantly higher in the study group. The findings indicate that there is a probable association between increased iron stores and glucose intolerance at the third trimester in non-anaemic women [24].
Cross-sectional data from the 2019 China Health and Nutrition Survey, which analyzed 689 children and adolescents, revealed a significant correlation between serum transferrin and waist circumference (WC) with HOMA-IR. Additionally, BMI, transferrin, and soluble transferrin receptor (sTfR) exhibited significant associations with HbA1c. The highest odds for the risk of insulin resistance (IR) and elevated HbA1c were found in the highest serum transferrin tertile groups in both girls and boys. Meanwhile, the highest odds for elevated HbA1c (HbA1c ≥ 5.7%) were observed in the lowest sTfR tertile group, also in both sexes. The authors noted that serum transferrin and sTfR, but not serum ferritin levels, were significantly correlated with glucose metabolism. These investigations revealed that elevated serum transferrin was associated with IR and higher HbA1c, without any sex disparity, whereas serum sTfR was associated with HbA1c but not IR. All the mentioned results suggest that transferrin and sTfR, rather than ferritin, might have an impact on impaired glucose homeostasis [25].
Conversely, in a Chinese study involving individuals recently diagnosed with T2DM, serum ferritin (SF) levels were significantly higher compared to healthy controls. Notably, serum iron (SI) and transferrin saturation (TSAT) levels were elevated among diabetic patients, and the percentage of transferrin (Trf) levels below normal values was lower in male diabetic patients than in their female counterparts. Subsequent stratification analysis revealed that Trf served as an independent protective factor for beta-cell function in male patients, while SF emerged as an independent risk factor in female patients. Overall, the associations of all four iron biomarkers with beta-cell function or insulin sensitivity were more prominent among women than among men. Importantly, systemic iron status did not independently impact insulin sensitivity [26].
Other researchers uncovered a positive correlation between serum iron levels and elevated fasting glucose and triglycerides. Additionally, the concentration of serum iron increased with the number of metabolic factors, including age, sex, current smoking, current drinking, exercise habits, BMI, and HOMA-IR. The results confirmed a positive correlation between serum iron levels and the risk of developing metabolic syndrome (MetS). However, due to the case–control design of the study, the authors couldn't establish a causal relationship between serum iron concentration and MetS [27]. The results obtained from another study involving participants with overweight or obesity and at least one additional metabolic syndrome factor suggest discernible alterations in iron transport and storage among individuals with overweight/obesity. The findings showed that transferrin and TSAT were negatively correlated. There weren't any significant correlations found between ferritin and TSAT, sTfR, or transferrin and sTfR. TSAT was negatively associated with waist-to-hip ratio (WHR), insulin levels, and HOMA-IR. These results confirmed a link between insulin resistance and iron storage, indicating lower efficiency in iron transport [28].
The animal studies confirmed this. The first group of mice was subjected to a high-fat diet (HFD, 60% fat), while the second group was administered a low-fat diet (LFD, 10% fat) for a duration of 4 months. Subsequently, a subset of the HFD-mice underwent streptozotocin injection to induce a model of T2DM. The subsequent phase of the study involved the administration of quercetin to both the diabetic mice and those on the LFD for an additional 4 months. Upon completion of this experimental timeline, mice with T2DM exhibited an elevated concentration of iron in both serum and pancreatic tissues. Notably, ferritin deposition was observed, particularly in proximity to or directly on islets, as confirmed by immunohistochemical observations, indicating a substantial iron overload within the islets of the pancreatic tissues [29].
The relationship between iron metabolism indices, insulin resistance, and oxidative stress was investigated in patients with GDM in comparison to healthy pregnant women. A statistically significant higher BMI in the GDM group before pregnancy, as compared to the control group, was observed. The results unveiled an elevation in serum iron, serum ferritin, and TSAT in GDM patients, potentially attributed to sustained hyperglycaemia and an increased level of oxidative stress injury [30].
A following study was conducted by Setoodeh et al. to compare the effects of iron overload, insulin resistance, and oxidative stress on metabolic disorders in patients with beta-thalassemia major and healthy individuals. The data revealed a high prevalence of iron overload (indicated by serum ferritin > 1000 ng/ml) in patients with β-thalassemia, underscoring the ineffectiveness of iron-chelating therapy in these individuals. The study also observed a higher mean fasting blood glucose in patients with β-thalassemia, while the index of beta-cell function (HOMA-B %) was comparable in both groups. A notable limitation of the study was the use of fasting blood glucose (FBG) instead of continuous monitoring of serum glucose and the OGTT [31]. Contrastingly, in animal study using mice with global deficiency Irp2 − / − generated by inserting a self-excision cassette containing neomycin (Neor) linked to cre-recombinase (Cre) into exon 3 of the mouse Irp2 − / − gene, was discovered that iron deficiency in β-cells impairs Fe-S cluster biosynthesis, reducing Cdkal1 function and the ms2t6A modification in tRNA Lys UUU. This impairment results in misreading lysine codons in proinsulin, diminishing proinsulin synthesis, and reducing insulin secretion. Additionally, mice lacking iron-regulatory protein 2 (Irp2), a cellular iron regulator, were found to develop T2DM [32].
In addition, outside substances can affect how sensitive your body is to insulin by changing how iron moves around inside cells. In a study with two groups of male C57BL/6 J mice, all fed the same standard diet, one group was given perfluorooctane sulfonate (PFOS) for six weeks at a dose of 2.5 mg/kg body weight. PFOS, a type of organic pollutant, has been shown to slow down one type of enzyme in the cell membrane, called ATP synthase, while speeding up another type called ectopic ATP synthase. This causes some parts of the cell, like the transferrin receptor 2 (TFR2) and the beta-subunit of ATP synthase (ATP5B), to move from the cell membrane to the mitochondria. As a result, both mouse liver mitochondria and normal human liver cells treated with PFOS were found to have too much iron, which made them resistant to insulin in the liver [33].
The link between insulin resistance, diabetes mellitus, diabetic complications, and iron overload extends beyond environmental factors, including genetic predisposition as investigated by Altamura et al. In their study, Lepr db/db mice with T2DM, IR and steatosis were crossbred with a genetic model of hereditary hemochromatosis type 4 (Fpn p.C326S) mice, resulting in the Lepr db/db/Fpn wt/C326S population. The authors demonstrated that increased systemic iron accumulation did not impact obesity, hyperinsulinemia, hyperglycemia, and increased HbA1c normally observed in T2DM Lepr db/db animals. Serum iron levels markedly increased in Lepr db/db/Fpn wt/C326S mice, surpassing iron serum levels in animals with hereditary hemochromatosis type 4. The study highlighted that iron overload in these mice augmented the frequency of retinopathy, suggesting that elevated systemic iron levels can exacerbate microvascular complications observed in diabetic retinopathy [34].
Blood donation serves as a method to reduce systemic iron levels, potentially providing a protective effect by mitigating iron toxicity and preventing lipid peroxidation, particularly in organs abundant in lipids, such as the pancreas. Comparative analyses of iron parameters between 42 consenting regular blood donors, who had donated blood at least twice and not more than thrice in the last 1 year and age-matched non-donors have demonstrated higher serum transferrin receptor and higher serum total iron-binding capacity (TIBC) among blood donors. Concurrently, blood donors exhibit lower mean fasting plasma glucose and mean HOMA-IR compared to controls. This implies that consistent iron depletion contributes to enhanced insulin sensitivity and improved glycemic control [35].
Tackling the intricacies of ferroptosis: from molecular mechanisms to physiological implications in adipose tissue and mitochondrial function
Ferroptosis is a form of regulated cell death that relies on iron and is distinguished by lipid peroxidation, accompanied by an elevated concentration of ferrous ions (Fe2 +). Morphological distinctions between ferroptosis and other modes of cell death, such as apoptosis and necrosis, are observable in mitochondria and the nucleus. These changes encompass reduced mitochondrial volume, elevated mitochondrial membrane density, and increased chromatin concentration with a normal nucleus size. Ferroptosis is intricately associated with the Fenton reaction, serving as a source of ROS. These ROS, in turn, instigate lipid peroxidation, thereby contributing to the distinctive characteristics of ferroptotic cell death [36].
Numerous studies underscore the pivotal role of the relationship between adipose tissue macrophages (ATMs) and iron homeostasis. Proinflammatory M1 macrophages employ iron storage as a strategy to restrict microbial access to this essential element, hindering its utilization for replication. Conversely, anti-inflammatory M2 macrophages serve as local "ferrostats," dynamically regulating iron levels by either supplying or removing excess iron from the cellular environment [37, 38]. Macrophages contribute to leukocyte infiltration, inflammation, and insulin resistance in obese adipose tissue (AT) by producing chemokines and inflammatory cytokines [39]. Examination has revealed that lean adipose tissue hosts a prominent population of high iron-recycling macrophages (MFe hi), crucial for buffering tissue iron concentrations, and a smaller population of low iron-recycling macrophages (MFe lo) [40]. Moreover, literature data suggest that under conditions of elevated iron, a mechanism operates to shield adipocytes from iron overload while concurrently maintaining an anti-inflammatory state. This mechanism involves a shift in macrophage populations, with the relative proportion of high iron-recycling macrophages (MFe hi) increasing primarily through the reconfiguration of low iron-recycling adipose tissue macrophages (MFe lo ATMs) to adopt a high iron-recycling signature. Importantly, this occurs while the overall number of ATMs remains constant [41].
The size of iron stores in adipose cells is contingent upon the type of human adipose tissue and its thermogenic function. Brown adipocytes, characterized by higher metabolic demands, utilize iron for porphyrin and iron-sulfur clusters in mitochondrial enzymes compared to white adipocytes. Yook et al. have elucidated that the differentiation of brown adipocytes, in contrast to white adipocytes, is accompanied by an expansion of the labile iron pool, indicating increased iron uptake but decreased storage. This specific iron metabolism in brown adipocytes is regulated by IRPs, which facilitate iron influx through transferrin receptor 1 (TfR1). The expression of TfR1 was found to be elevated in interscapular brown adipose tissue (IBAT), with IBAT exhibiting a threefold higher iron content than white adipose tissue (WAT). Importantly, mitochondrial biogenesis during brown adipocyte differentiation was identified as a significant driver for increased iron influx, supported by a concurrent significant increase in mitochondrial respiratory chain proteins [42]. IBAT demonstrates the ability to protect against obesity by clearing triglycerides and mitigating insulin resistance, while WAT secretes endocrine factors that contribute to insulin resistance [43]. Furthermore, research by Festa et al. has demonstrated that the differentiation of 3T3-L1 cells into adipocytes results in increased H- and L-ferritin subunit mRNA levels and protein expression. Notably, the accumulation of the H subunit appears to occur preferentially [44]. NADH: ubiquinone oxidoreductase core subunit S8 (NDUFS8) plays a crucial role as an essential core subunit and a component of the iron-sulfur (FeS) fragment within mitochondrial complex I, which is directly involved in the electron transfer process and energy metabolism [45]. In diabetes, the equilibrium between NADH and NAD( +) can be significantly disrupted due to the overproduction of NADH induced by hyperglycemia (activation of the polyol pathway in the Krebs cycle) and the reduction of NAD( +) by various factors. These factors include the overactivation of poly ADP ribose polymerase, utilizing NAD( +) as its substrate, as well as the impairment of NAD( +) regeneration enzymes such as lactate dehydrogenase in erythrocytes and complex I in mitochondria. Consequently, the redox imbalance of NADH/NAD( +) results in oxidative stress [46]. Research involving 12 women and 24 men revealed that adults with T1DM exhibiting better insulin sensitivity (estimated glucose disposal rate [eGDR] above the median) possess a higher serum concentration of NADH dehydrogenase [ubiquinone] iron-sulfur protein 8 (NDUFS8). This association holds true independently of age, duration of diabetes, and smoking habits [47].
The role of hepcidin in obesity, obesity-associated metabolic disorders, insulin resistance and DM2
Hepcidin is a peptide hormone produced in hepatocytes in response to increased body iron stores or inflammation. It binds to the iron export channel ferroportin on the basolateral membrane of enterocytes and macrophages, leading to the inhibition of ferrportin and blocking iron absorption into the bloodstream. Several studies reported that iron stimulates expression of bone morphogenetic protein 6 (BMP6) from liver sinusoidal endothelial cells, which in turn binds to BMP receptors on hepatocytes and induces the SMAD signaling cascade for transcriptional activation of the hepcidin synthesis [48, 49].On the other hand, it has been shown that hepatocellular iron overload suppresses hepcidin by inhibiting the SMAD and STAT3 signaling pathways. Charlebois et al. unveiled that pharmacological iron chelation may impair hepcidin induction by BMP6 and/or IL-6 primarily in human Huh7 hepatoma cells [50]. Another cross-sectional study performed by Rodriguez- Mortera et al. revealed hepcidin, VAI (visceral adiposity index), atherogenic dyslipidemia and IR correlation. The study found that adolescents with obesity, but without overt metabolic syndrome (MetS), had 67% higher levels of hepcidin compared to the control group, while subclinical anemia and iron overload were excluded. Although fasting glycemia was comparable in obese and lean study participants, insulin and HOMA-IR were twofold higher in obese adolescents, emphasizing twofold higher insulin demand to achieve fasting euglycemia in these group of patients with obesity [51]. Furthermore, the research carried out by Wojciechowska et al. exploring the effect of obesity and hypothyroidism on hepcidin concentration in pregnancy revealed pre-pregnancy obesity association with increased maternal hepcidin concentration measured on the day of delivery. Significantly elevated hepcidin level in obese gestating women comparing to both the correct body weight group and the group with a history of hypothyroidism was reported. Conversly, there was no significant differences between maternal serum iron in healthy and obese gravid women [52]. Opposite results were obtained in a study focusing on maternal and fetal iron homeostasis in obese women during pregnancy, which emphasize hepcidin indirect relation with maternal BMI. Hepcidin level, as well as ferritin concentrations were lower in the obese maternal group than in the lean group[53]. Moreover, hepcid has its own negative regulator—matriptase-2 (Tmprss6). Defficiency of matriptase-2 promotes lipolysis and protects against obesity, as described in one animal study using a 3′-Hprt chromosomal engineering targeting vector to generate Tmprss6-/- mutant mice deficient in matriptase-2. Tmprss6 − / − mice show a significant decrease in body fat, improved insulin sensitivity and protection against hepatic steatosis. The fact that hepcidin and matriptase- 2 are involved in iron homeostasis and adipose tissue function is confirmed by rescue experiments, that block hepcidin up-regulation and enable restoration of iron levels in Tmprss6 − / − mice via anti-hemojuvelin (HJV) therapy, finally reverting the obesity-resistant phenotype of Tmprss6 − / − mice. In addition, all experimental models showing high hepcidin levels (Tmprss6 − / − and iron-treated Tmprss6 − / − mice, as well as iron-treated Tmprss6 + / + mice) showed significantly less body weight than wild-type controls after 20 weeks on a high-fat diet [54]. In a subsequent study conducted by Auguet et al. aimed at examining the dependence of hepcidin levels on BMI, it was found that morbidly obese (MO) women had significantly greater circulating hepcidin levels compared to normal-weight control women. Hepcidin levels in both MO women with NAFLD and normal liver histology were significantly higher than in normal-weight subjects, what can be an evidence of lipid liver metabolism and NAFLD risk correlation with the hepatic expression of hepcidin and iron metabolism [55]. Hepcidin as a result of obesity and inflammation has a multilevel relation with IL-6, leading to iron insufficiency and anaemia in some cases of obese patients. Recognizing the above associations might reveal potential links to obesity and iron deficiency, in cases of hepcidin induction, or iron overload, due to hepcidin downregulation when IL-6 activity is less prominent. This understanding could enhance our knowledge of new therapeutic alternatives for obesity and its future complications.
From the other hand, hepcidin secretion is closely linked to IL-6 and body adipose tissue content. IL-6 is produced in chronic inflammation e.g. obesity, activates hepcidin expression and might lead to iron-restricted erythropoiesis and anemia in some cases [56].
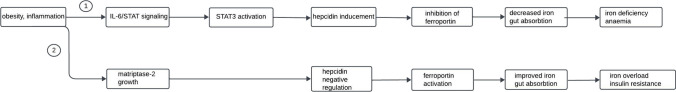
IL6 is a main driving factor for hepcidin secretion in many models of AI (anemia of inflammation) [57]. In agreement with many reserches BMP/SMAD signaling and IL-6/STAT3 signaling are important for transcriptional regulation of hepcidin [58–60]. IL-6 signaling through STAT3 activation and subsequent hepcidin promoter binding induces hepcidin synthesis. Hepcidin can be regulated by inflammation or by alternative pathways leading to STAT3 activation [61]. Elevated hepcidin expression, along with concurrent STAT3 activation, was observed in hepatic adenomas of patients with glycogen storage disease type 1a (GSD1a). Due to the fact that STAT3 is a regulator of glucose-6-phosphatase and other genes involved in gluconeogenesis, it has been hypothesized that adenoma formation and inappropriately high levels of hepcidin mRNA production in GSD1a patients may result from aberrant STAT3 activity. This aberrant activity could be a response to compensatory signals attempting to increase glucose production [62]. Nevertheless, the regulation of hepcidin in patients with DM2 is complex and multifaced. This complexity is evident in the disproportionate association between hepcidin levels and weight, as well as in DM2 patients with subclinical anaemia who often display elevated iron stores [63] (Fig. 2).
Fig. 2.
Two possible partially contrary iron homeostasis and obesity pathways. IL-6- Interleukin- 6. STAT- Signal Transducer and Activator of Transcription Proteins. STAT3- Signal Transducer and Activator of Transcription 3
The impact of iron homeostasis on adipose tissue, adipose differentiation and thermogenic activity
In obesity, the presence of immune cells and adipocytes within WAT leads to chronically elevated levels of IL-6. This cytokine disrupts gastric emptying, inhibits GLP-1 release, and reduces hepatic insulin sensitivity, thereby contributing to disturbances in glucose metabolism. The activation of the classic IL-6 signaling pathway induces anti-inflammatory effects in macrophages, leads to proinflammatory actions in T cells, induces leptin and FFA from adipocytes and further contribute to IR [64]. Exploring the impact of iron on lipid accumulation, lipid release and lipid peroxidation has led to investigations into transferrin gene and increaded protein expression during adipocyte differentiation. Deferoxamine and transferrin knockdown (TF KD) severely inhibited adipocyte differentiation, similarly increasing inflammatory mRNA levels. This effect was reversed in a dose-dependent manner following iron supplementation. However, excess iron exhibited an antiadipogenic effect, although this effect was less pronounced than under iron chelation [65]. Adipocyte hypertrophy and reduced adipogenesis resulting from iron overload contribute to unhealthy expansion of abdominal adipose tissue. This excess visceral adiposity impairs insulin sensitivity, highlighting the importance of early correction of iron excess to potentially prevent further progression of IR [66].
A study performed by Shi et al. investigating adipose browning-whitening transdifferentiation and iron influx pertains focused on the transcription factor forkhead box O1 (FoxO1) and the Tgfβ1 signaling cascade showed that the browning phenotype observed in adO1KO mice was associated with an increased iron content in adipose tissue, accompanied by upregulation of proteins involved in iron uptake and mitochondrial iron import. Iron analysis in the adO1KO mice revealed adipose tissue-liver crosstalk and an increased iron requirement for adipose browning. Additionally, the FoxO1-Tgfβ1 axis was implicated in adipose browning induced by β3-adrenergic receptor agonist CL316243 [67]. A polygenic obese mouse model detected a tendency to deposit iron in the epididymal adipose tissue rather than in the subcutaneous or brown adipose tissue. Furthermore, those male KK/HIJ mice had greater iron deposition in traditional tissues of iron overload such as the liver, heart, and pancreas. The increased iron level was accompanied by elevated oxidative stress and robust tissue remodeling, in at least epididymal adipose tissue, which might be due to accumulated HIF-2α resulting in impaired local insulin signaling [68].
DM2 has a very rare comorbid neurodegenerative disease- Friedreich's ataxia (FRDA), which results from decreased expression of mitochondrial frataxin (FXN), interrupted mitochondrial ultrastructure and oxygen consumption as well as lipid accumulation in BAT. It was reported that the FXN knock-in/knock-out (KIKO) mouse represents hyperlipidemia, reduced energy expenditure and insulin sensitivity. Impaired PKA-mediated lipolysis and expression of genes controlling mitochondrial metabolism, lipid catabolism and adipogenesis were observed in BAT of KIKO mice as well as in FXN-deficient T37i brown and primary adipocytes, whereas adipocyte precursors were the ones more susceptible to ferroptosis [69].
In the following animal study carried out by Deschemin et al.exploring iron homeostasis and thermogenic activity in brown and beige fat in wild-type and iron loaded Hepcidin KO mice, was uncovered that hepcidin-deficient mice exhibited iron overload in both beige inguinal adipose tissue (iWAT) and brown adipose tissue (BAT). WT mice had six times more iron in the BAT than in iWAT. Besides, KO mice demonstrated a threefold increase in total iron levels, in both iWAT and BAT than WT mice, comparing to more than 20-fold increase in iron levels in the liver and pancreatic parenchyma of these mice. The iWAT of Hepcidin KO animals featured with reduced uncoupling protein 1 (UCP 1) levels, decreased mitochondrial respiration and defective thermogenesis [70]. Iron metabolism and iron mobilization from storages to thermogenic fat is an inherent part of adaptive thermogenesis process. ADRB3 stimulation induces two distinct yet coordinated iron-regulatory pathways: one involves creating an intracellular iron gradient to facilitate IRP binding to IRE in adipocytes, while the other triggers acute hypoxia to release stored iron into circulation. ADRB3 stimulation promotes an iron-demanding process leading to activation of the IRP/IRE signaling pathway, which is beige adipogenesis. It has been described that acute hypoxia and stress-induced erythropoiesis facilitate the mobilization of iron, which is essential for the white-to-beige fat conversion, similarly to the effects of hepcidin downregulation. Therefore, iron modulation affecting thermogenesis could potentially be used as a future treatment for obesity [71].
XIST Unveiled: unraveling its dual role in X chromosome inactivation and iron-overload-related T2DM pathogenesis
The long noncoding RNA (lncRNA) X-inactive specific transcript (XIST) plays a crucial role in X chromosome inactivation (XCI) in female placental mammals, ensuring balanced expression dosage of X-linked genes between sexes [72]. Consequently, the expression or modification of XIST can be considered a potential biomarker for the diagnosis and prognosis of various sex-biased diseases, including metabolic disorders like T2DM. An animal study conducted in male Wistar rats divided into four groups: the control group, iron overload group, T2DM group and iron overload-related T2DM group revealed that XIST has the capacity to promote iron overload and contribute to the development of iron-overload-related T2DM by inhibiting the expression of receptor-like kinase 2 (ALK2) through sponging miR-130a-3p. In vitro, increased XIST expression was associated with a reduction in insulin secretion, while XIST knockdown alleviated beta-cell damage induced by iron overload. This suggests a potential role for the XIST/miR-130a-3p/ALK2 axis in influencing the progression of T2DM [73].
Exploring the possible relationship between iron and glucose metabolism in individuals with chronic viral hepatitis C
Chronic viral hepatitis C (CHC) is characterized by excessive iron accumulation in the liver. The novel protein, hepatitis C virus (HCV) core + 1/ARFP, has been identified as capable of decreasing hepcidin expression in hepatocytes. This occurs through the downregulation of transcription mediated by activator protein 1(AP 1), which binds to the site of the human hepcidin promoter [74]. There are several studies highlighting the importance of probable relationship between HCV infection, iron status and insulin sensitivity, suggesting that HCV virus might have developing T2DM potential. In a population study involving HCV-infected patients, iron studies conducted before and after successful treatment with direct-acting antiviral agents (DAAs), and presenting at least one abnormal iron test (serum iron, total iron binding capacity, transferrin-iron saturation, serum ferritin) prior to treatment, revealed that eradication of HCV infection restores normal iron status in most patients with abnormal iron tests, including those with baseline parameters suggestive of hemochromatosis [75]. Additionally, there have been reports indicating that eradication of HCV through interferon (IFN) therapy could ameliorate whole-body insulin resistance and insulin hypersecretion, accompanied by reduced soluble tumor necrosis factor receptor 2 (sTNFR2). In a study involving CHC patients receiving IFN therapy and divided into two groups—sustained responders and non-sustained responders—systemic glucose metabolism indices were assessed before and 6 months after therapy. Among the sustained responders, β-cell function (HOMA-beta) significantly decreased, and the insulin sensitivity index (ISI) composite increased, although there were no significant changes in HOMA-IR and insulinogenic index (II) [76].
In contrast, among a group of patients with CHC divided into diabetic and non-diabetic subgroups, both populations exhibited normal serum insulin levels and insulin resistance. Interestingly, the analysis of the iron profile and glucose homeostasis parameters indicated a significant negative correlation between serum iron and insulin resistance among the diabetic CHC population [77].
The association between CHC and IR has been repeatedly investigated; however, its mechanisms remain unclear, and further research is necessary. In one cross-sectional study, 29 nonobese, normoglycemic males with CHC (genotypes 1 and 3) were compared to 15 obese and age-matched controls. The study employed a 2-step hyperinsulinemic-euglycemic clamp with [6,6-(2)H(2)] glucose to evaluate insulin sensitivity in the liver and peripheral tissues, and magnetic resonance spectroscopy to illustrate liver and intramyocellular lipid content. The authors found that insulin secretion after intravenous glucose application was not impaired in CHC. Peripheral insulin sensitivity was 35% higher in controls compared to CHC during high-dose insulin infusion (264.3 ± 25 [standard error] mU/L). Hepatic glucose production and the absence of suppression of esterified free fatty acids with insulin were similar between CHC and controls, suggesting no adipocyte insulin resistance. This indicates that CHC may represent a unique infective/inflammatory model of muscle insulin resistance, which correlates with subcutaneous, rather than visceral, adiposity [78].
Conversely to previously mentioned studies, serum markers of systemic iron overload were indicated to have low sensitivity in predicting hepatic iron deposits (HIDs) in hepatitis C. The study investigating HIDs in the liver biopsies of a consecutive series of 242 HCV-infected patients with well-compensated liver disease, have unveiled elevated SF and TSAT, however in smaller percentage than the number of stainable HIDs. Raised serum iron indices were more recurring in non-HCV-3 genotypes, while HIDs were more frequent in HCV-3-infected cases than in other genotypes, furthermore in HCV-3 cases there was a close connection between HIDs and severe (grade II–III) steatosis [79].
In the study performed by Wang et al. conducted on HCV-infected insulin-secreting mouse cell line MIN6, they shown that HCV infection induces death of pancreatic β-cells through an endoplasmic reticulum (ER) stress-involved, caspase 3-dependent, special pathway, indicating a connection between HCV infection and developing T2DM. It was revealed that HCV virion itself has a dose- and time-dependent cytopathic effect on the cells, inhibits cell proliferation and induces death of MIN6 cells with apoptotic characteristics, including cell surface exposure of phosphatidylserine, decreased mitochondrial membrane potential, activation of caspase 3 and poly (ADP-ribose) polymerase, and DNA fragmentation in the nucleus. What is significantly interesting, is the fact that HCV-infected cells exhibited a dilated, low-density nucleus with intact plasma and nuclear membrane, denoting a novel apoptosis-like death [80]. Another retrospective study conducted in 300 patients with CHC without hepatoma and antidiabetic treatment, who underwent liver biopsies, OGTT and serum α-fetoprotein (AFP) analysis, unveiled that whole-body insulin resistance is associated with an elevated serum AFP level in patients with CHC [81].
Tangling iron and amyloid beta: exploring the intricate web of neurodegeneration, insulin resistance, and cognitive dysfunction in alzheimer's disease
Iron has the capacity to bind to amyloid beta (Ab), and consequently, iron overload regulates the toxicity of this peptide in the central nervous system (CNS), being associated with the process of neurodegeneration [82]. Brain insulin resistance and insulin-like growth factor 1 (IGF-1) resistance have been reported, along with increased Ab accumulation, tau hyperphosphorylation, and cognitive dysfunction in patients with Alzheimer's disease [83]. A recent study, using multimodal spectroscopic imaging, indicated elevated levels of aggregated protein, iron, and zinc within the Aβ-plaque core [84]. Research has explored the role of apolipoprotein E (ApoE), particularly the ApoE4 isoform, as a significant genetic risk factor for Alzheimer's disease (AD). This isoform, secreted by glia, stimulates neuronal Ab production by enhancing the transcription of amyloid-β precursor protein (APP) [85]. In a case–control study with participation of 23 middle- aged obese subjects without diabetes and 20 healthy nonobese volunteers, in whom iron load was estimated in white and gray matter and the liver by MRI, a connection was established between brain iron overload and impaired cognitive function in obese subjects, highlighting obesity's contribution to increased hepatic iron concentration [86]. An in vivo study revealed that ferrous (Fe2 +) chloride, leading to iron overload, is involved in the disruption of insulin signalling and hyperphosphorylation of tau. Mice with supplemented dietary iron exhibited difficulties in learning and memory, accompanied by decreased tyrosine phosphorylation levels of insulin receptor beta (IR beta), insulin signal substrate 1 (IRS-1), and phosphoinositide 3-kinase p85alfa (PI3K p85alfa) in primary cultured neurons [87]. A connection was reported among a group of 20 obese premenopausal women, linking elevated fatty acid rate of appearance into systemic circulation (FA Ra) and plasma ferritin levels. Plasma hepcidin and FA Ra were higher in the high-normal ferritin tertile than in the low-normal ferritin tertile. Additionally, whole-body insulin sensitivity was lower in women with high-normal ferritin, suggesting that elevated iron stores may lead to insulin resistance by increasing systemic FA availability [88]. Lipocalin 2 (LCN2), also known as neurotrophin gelatinase-associated lipocalin (NGAL), influences iron accumulation in the brain and impacts neuronal cell apoptosis, contributing to the neuropathology of dementia [89]. This aligns with the revealed ability of LCN2 to exacerbate insulin resistance and influence lipid metabolism [90] (Table 1).
Table 1.
The role of iron homeostasis in the pathogenesis of obesity and insulin resistance
| References | Results | Conclusions |
|---|---|---|
| 24. Lao et al | Significantly higher concentration of serum ferritin, iron, TSAT and post-natal haemoglobin in pregnant non-anaemic women with GDM than in at-risk but non-diabetic pregnant women | Probable association between increased irn stores and glucose intolerance at the third trimester in non-anaemic women |
| 25. Wei et al | Significant association between BMI, transferrin, sTfR and HbA1c | Elevated serum transferrin was associated with IR and higher HbA1c, while serum sTfR was associated with HbA1c but not with IR |
| 26. Qin et al | Significantly higher serum ferritin, serum iron and TSAT in patients recently diagnosed with T2DM, lower percentage of transferrin levels below normal values in male diabetic patients than in female patients | Different independent protective factors for beta-call function in male (Trf) and in female (serum ferritin) patients |
| 52. Wojciechowska et al | Significantly elevated hepcidin level in obese gestating women than in correct body weght group | Pre-preganancy obesity is associated with increased maternal hepcidin concentration measured on the ady of delivery |
| 55. Auguet et al | Morbidly obese women had significantly greater hepcidin levels compared to normal-weight control women | Possible lipoid liver metabolism and NAFLD correlation with the hepatic expression of hepcidin |
| 73. Li et al |
XIST has the capacity to promote iron overload and contribute to the development of iron-overloaded-related T2DM by inhibiting the expression of ALK-2 |
Increased XIST expression is connected with a reduction in insulin secretion |
| 75. Hasan et al | HCV-infected patients presenting at least one abnormal iron test before eradication treatment, revealing normal iron status after successful eradication of HCV infection | Correlation between HCV infection and iron status |
| 86. Blasco et al | Increased iron brain load in middle-aged obese subjects without DM than in healthy volunteers | Connection between brain iron overload and impaired cognitive function in obese subjects |
Conclusions
This review provides a nuanced exploration of the intricate interplay between iron and insulin, elucidating their multifaceted roles in anabolic processes, glycemic regulation, and their implications for hepatic glucose production, obesity, and various metabolic disorders. The examination of ferroptosis in adipose tissue and mitochondrial function adds a novel insight to our understanding of regulated cell death in the context of metabolic disorders. The revelation of the long noncoding RNA XIST's dual role in X chromosome inactivation and its potential involvement in iron-overload-related type 2 diabetes mellitus pathogenesis opens intriguing avenues for further research. Additionally, the intricate connections between iron, amyloid beta, and their implications in neurodegeneration, insulin resistance, and cognitive dysfunction in Alzheimer's disease present a comprehensive view of the broader impact of iron metabolism on neurological health. In summary, this scientifically rigorous review not only integrates existing knowledge but also paves the way for future investigations, contributing to the holistic understanding of the complex interplay between iron and insulin in health and disease.
Abbreviations and acronyms
- ROS
Reactive oxygen species
- IREs
Iron-responsive elements
- IRPs
Iron regulatory proteins
- T2DM
Type 2 diabetes mellitus
- GLP-1
Glucagon-like peptide 1
- HOMA-IR
Homeostasis model assessment of insulin resistance
- FPG
Fasting plasma glucose
- TSAT
Transferrin saturation
- HbA1c
Glycated hemoglobin
- DM
Diabetes mellitus
- OGTT
Oral Glucose Tolerance Test
- BMI
Body mass index
- GDM
Gestational diabetes mellitus
- WC
Waist circumference
- sTfR
Soluble transferrin receptor
- IR
Insulin resistance
- SF
Serum ferritin
- SI
Serum iron
- Trf
Transferrin
- MetS
Metabolic syndrome
- WHR
Waist-to-hip ratio
- HOMA-B%
index of beta-cell function
- FBG
Fasting blood glucose
- SPF
Specific pathogen free
- PFOS
Perfluorooctane sulfonate
- BW
Body weight
- TRF2
Transferrin receptor 2
- ATP5B
ATP synthase beta-subunit
- TIBC
Total iron-binding capacity
- ATMs
Adipose tissue macrophages
- AT
Adipose tissue
- TfR1
Transferrin receptor 1
- IBAT
Interscapular brown adipose tissue
- WAT
White adipose tissue
- XIST
X-inactive specific transcript
- XCI
X chrosome inactivation
- CHC
Chronic viral hepatitis C
- HCV
Hepatitis C virus
- DAAs
Direct-acting antiviral agents
- IFN
Interferon
- sTNFR2
Soluble tumour necrosis factor receptor 2
- II
Insulinogenic index
- HIDs
Hepatic iron deposits
- AFP
α-Fetoprotein
- Ab
Amyloid beta
- CNS
Central nervous system
- IGF-1
Insulin-like growth factor 1
- ApoE
Apolipoprotein E
- AD
Alzheimer's disease
- APP
Amyloid-β precursor protein
- IRS-1
Insulin signal substrate 1
- FARa
Fatty acid rate of appearance into systemic circulation
- NGAL
Neurotrophil gelatinase-associated lipocalin
Author contributions
Conceptualization, K.S. and A.PK; Methodology, K.S. and A.PK; Software, A.B. and A. J. K; Validation, A.B. and A.J.K.; Formal Analysis, A.B.; Investigation, K.S., A.B., A.PK.; Resources, A.PK.; Data Curation, K.S. and A.B.; Writing—Original Draft Preparation, K.S. and A.B.; Writing—Review and Editing, A.J.K. and A.PK; Visualization, K.S. and A.B.; Supervision, A.J.K. and A.PK.; Project Administration, A.PK. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Institutional review board
The study was conducted in accordance with the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2012;62:347–60. [DOI] [PubMed] [Google Scholar]
- 2.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2013;99(10):3505–16. [DOI] [PubMed] [Google Scholar]
- 3.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–8. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Real JM, Pickup JC. Innate immunity, insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2008;19(1):10–6. [DOI] [PubMed] [Google Scholar]
- 5.Cooksey RC, Jones D, Gabrielsen S. Dietary iron restriction or iron chelation protects from diabetes and loss of β -cell function in the obese (ob/ob lep-/-) mouse. Am J Physiol Endocrinol Metab. 2004;287(2):E282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Federico A, Diano N, Grange C, Brugnoletti O. The connection between iron metabolism and diabetes: It’s complicated. Nutrients. 2020;12(6):1678.32512782 [Google Scholar]
- 7.Song D, Arikawa E, Galipeau D, Battell M, McNeill JH, Zawalich W. Interaction of ferrous iron and α -cell function: Potential role in diabetes. Diabetes. 2018;67(Supplement 1):246-OR. [Google Scholar]
- 8.Arumugam S, Suyambulingam A. Association between serum ferritin and the duration of Type 2 Diabetes Mellitus in a tertiary care hospital in Chennai. 2024;16(1). 10.7759/cureus.53117. Accessed 28 Jan 2024. [DOI] [PMC free article] [PubMed]
- 9.Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB, Qi L. The role of iron in type 2 diabetes in humans. Biochim Biophys Acta (BBA)- Gen Subjects. 2009;1790(7):671–81. [DOI] [PubMed] [Google Scholar]
- 10.Plays M, Müller S, Rodriguez R. Chemistry and biology of ferritin. Metallomics. 2021;13(5):mfab021. 10.1093/mtomcs/mfab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotla NK, Dutta P, Parimi S, Das NK. The role of ferritin in health and disease: recent advances and understandings. Metabolites. 2022;12(7):609. 10.3390/metabo12070609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautam S, Alam F, Moin S, Noor N, Arif SH. Role of ferritin and oxidative stress index in gestational diabetes mellitus. J Diabetes Metab Disord. 2021;20(2):1615–9. 10.1007/s40200-021-00911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastav SK, Mir IA, Bansal N, Singh PK, Kumari R, Deshmukh A. Serum Ferritin in Metabolic Syndrome-Mechanisms and Clinical Applications. Pathophysiology. 2022;29(2):319–25. 10.3390/pathophysiology29020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran TN, Tran HD, Tran-Huu TT, Tran DM, Tran QN. A cross-sectional study of serum ferritin levels in vietnamese adults with metabolic syndrome. Diabetes Metab Syndr Obes. 2022;12(15):1517–23. 10.2147/DMSO.S360689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han H, Ni P, Zhang S, Ji X, Zhu M, Ma W, Ge H, Chu H. The association of body mass index and weight waist adjustment index with serum ferritin in a national study of US adults. Eur J Med Res. 2023;28(1):374. 10.1186/s40001-023-01343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulhar R, Ashraf MA, Jialal I. Physiology, acute phase reactants. In: Stat Pearls [Internet]. Treasure Island (FL): Stat Pearls Publishing; 2024. [PubMed]
- 17.Klisic A, Kavaric N, Kotur J, Ninic A. Serum soluble transferrin receptor levels are independently associated with homeostasis model assessment of insulin resistance in adolescent girls. Arch Med Sci. 2021;19(4):987–94. 10.5114/aoms/132757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, Cai J, Wang Y, Liu J, Fu S. Non-Enzymatic Glycation of Transferrin and Diabetes Mellitus. Diabetes Metab Syndr Obes. 2021;8(14):2539–48. 10.2147/DMSO.S304796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JD, Lim DM, Park KY, Park SE, Rhee EJ, Park CY, Lee WY, Oh KW. Serum transferrin predicts new-onset type 2 diabetes in koreans: A 4-year retrospective longitudinal study. Endocrinol Metab (Seoul). 2020;35(3):610–7. 10.3803/EnM.2020.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Li Q, Yang Y, Ma L. Iron metabolism and type 2 diabetes mellitus: A meta-analysis and systematic review. J Diabetes Investig. 2020;11(4):946–55. 10.1111/jdi.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu J, Zhang Z, Hu Y, Guo Y, Liu C, Chen Y, Wang D, Su J, Wang S, Ni M, Xu S, Yu J, Hu T, Song G, Ma X, Gu X, Wang J, Xu L. Transferrin receptor levels and its rare variant are associated with human obesity. J Diabetes. 2024;16(1):e13467. 10.1111/1753-0407.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.www.who.int/news-room/fact-sheets/detail/diabetes
- 23.Krisai P, Leib S, Aeschbacher S, Kofler T, Assadian M, Maseli A, Todd J, Estis J, Risch M, Risch L, Conen D. Relationships of iron metabolism with insulin resistance and glucose levels in young and healthy adults. Eur J Intern Med. 2016;32:31–7. 10.1016/j.ejim.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Lao TT, Chan PL, Tam KF. Gestational diabetes mellitus in the last trimester - a feature of maternal iron excess? Diabet Med. 2001;18(3):218–23. 10.1046/j.1464-5491.2001.00453.x. [DOI] [PubMed] [Google Scholar]
- 25.Wei J, Luo X, Zhou S, He X, Zheng J, Sun X, Cui W. Associations between iron status and insulin resistance in Chinese children and adolescents: findings from the China Health and Nutrition Survey. Asia Pac J Clin Nutr. 2019;28(4):819–25. 10.6133/apjcn.201912_28(4).0019. [DOI] [PubMed] [Google Scholar]
- 26.Qin Y, Huang Y, Li Y, Qin L, Wei Q, Chen X, Yang C, Zhang M. Association between systemic iron status and β-cell function and insulin sensitivity in patients with newly diagnosed type 2 diabetes. Front Endocrinol (Lausanne). 2023;3(14):1143919. 10.3389/fendo.2023.1143919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu CW, Lee YC, Kuo CS, Chiang CH, Chang HH, Huang KC. Association of serum levels of zinc, copper, and iron with risk of metabolic syndrome. Nutrients. 2021;13(2):548. 10.3390/nu13020548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaquero MP, Martínez-Maqueda D, Gallego-Narbón A, Zapatera B, Pérez-Jiménez J. Relationship between iron status markers and insulin resistance: an exploratory study in subjects with excess body weight. PeerJ. 2020;31(8):e9528. 10.7717/peerj.9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li D, Jiang C, Mei G, Zhao Y, Chen L, Liu J, Tang Y, Gao C, Yao P. Quercetin alleviates ferroptosis of pancreatic β cells in type 2 diabetes. Nutrients. 2020;12(10):2954. 10.3390/nu12102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Y, Feng Q, Lv Y, Song X, Qu H, Chen Y. The relationship between iron metabolism, stress hormones, and insulin resistance in gestational diabetes mellitus. Nutr Diabetes. 2020;10(1):17. 10.1038/s41387-020-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Setoodeh S, Khorsand M, Takhshid MA. The effects of iron overload, insulin resistance and oxidative stress on metabolic disorders in patients with β- thalassemia major. J Diabetes Metab Disord. 2020;19(2):767–74. 10.1007/s40200-020-00560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos MCFD, Anderson CP, Neschen S, Zumbrennen-Bullough KB, Romney SJ, Kahle-Stephan M, Rathkolb B, Gailus-Durner V, Fuchs H, Wolf E, Rozman J, de Angelis MH, Cai WM, Rajan M, Hu J, Dedon PC, Leibold EA. Irp2 regulates insulin production through iron-mediated Cdkal1-catalyzed tRNA modification. Nat Commun. 2020;11(1):296. 10.1038/s41467-019-14004-5.PMID:31941883;PMCID:PMC6962211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Wang J, Qiu T, Wu J, Sun X, Jiang L, Liu X, Yang G, Cao J, Yao X. Mitochondrial iron overload mediated by cooperative transfer of plasma membrane ATP5B and TFR2 to mitochondria triggers hepatic insulin resistance under PFOS exposure. Ecotoxicol Environ Saf. 2023;253:114662. 10.1016/j.ecoenv.2023.114662. [DOI] [PubMed] [Google Scholar]
- 34.Altamura S, Müdder K, Schlotterer A, Fleming T, Heidenreich E, Qiu R, Hammes HP, Nawroth P, Muckenthaler MU. Iron aggravates hepatic insulin resistance in the absence of inflammation in a novel db/db mouse model with iron overload. Mol Metab. 2021;51:101235. 10.1016/j.molmet.2021.101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oladosu WO, Onwah AL, Oladosu-Olayiwola RO, Ahmed A, Osinubi MO, Gbotosho OA, Okesina AB. Homeostatic model assessment of insulin activity and iron profile among regular blood donors at a tertiary health centre, South-West Nigeria. Int J Appl Basic Med Res. 2020;10(4):252–5. 10.4103/ijabmr.IJABMR_118_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doll S, Conrad M. Iron and ferroptosis: A still ill-defined liaison. IUBMB Life. 2017;69(6):423–34. 10.1002/iub.1616. [DOI] [PubMed] [Google Scholar]
- 37.Cairo G, Recalcati S, Mantovani A, Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol. 2011;32(6):241–7. 10.1016/j.it.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Winn NC, Volk KM, Hasty AH. Regulation of tissue iron homeostasis: the macrophage “ferrostat.” JCI Insight. 2020;5(2):e132964. 10.1172/jci.insight.132964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Orr JS, Kennedy A, Anderson-Baucum EK, Webb CD, Fordahl SC, Erikson KM, Zhang Y, Etzerodt A, Moestrup SK, Hasty AH. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes. 2014;63(2):421–32. 10.2337/db13-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hubler MJ, Erikson KM, Kennedy AJ, Hasty AH. Mfehi adipose tissue macrophages compensate for tissue iron perturbations in mice. Am J Physiol Cell Physiol. 2018;315(3):C319–29. 10.1152/ajpcell.00103.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yook JS, You M, Kim Y, Zhou M, Liu Z, Kim YC, Lee J, Chung S. The thermogenic characteristics of adipocytes are dependent on the regulation of iron homeostasis. J Biol Chem. 2021;296:100452. 10.1016/j.jbc.2021.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szklarz M, Gontarz-Nowak K, Matuszewski W, Bandurska-Stankiewicz E. “Ferrocrinology”-Iron Is an Important Factor Involved in Gluco- and Lipocrinology. Nutrients. 2022;14(21):4693. 10.3390/nu14214693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Festa M, Ricciardelli G, Mele G, Pietropaolo C, Ruffo A, Colonna A. Overexpression of H ferritin and up-regulation of iron regulatory protein genes during differentiation of 3T3-L1 pre-adipocytes. J Biol Chem. 2000;275(47):36708–12. 10.1074/jbc.M004988200. [DOI] [PubMed] [Google Scholar]
- 45.Lin BY, Zheng GT, Teng KW, Chang JY, Lee CC, Liao PC, Kao MC. TAT-Conjugated NDUFS8 can be transduced into mitochondria in a membrane-potential-independent manner and rescue complex i deficiency. Int J Mol Sci. 2021;22(12):6524. 10.3390/ijms22126524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J, Jin Z, Zheng H, Yan LJ. Sources and implications of NADH/NAD(+) redox imbalance in diabetes and its complications. Diabetes Metab Syndr Obes. 2016;10(9):145–53. 10.2147/DMSO.S106087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flotyńska J, Klause D, Kulecki M, Cieluch A, Chomicka-Pawlak R, Zozulińska-Ziółkiewicz D, Uruska A. Higher NADH dehydrogenase [Ubiquinone] iron-sulfur protein 8 (NDUFS8) serum levels correlate with better insulin sensitivity in type 1 diabetes. Curr Issues Mol Biol. 2022;44(9):3872–83. 10.3390/cimb44090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charlebois E, Fillebeen C, Presley J, Cagnone G, Lisi V, Lavallée VP, Joyal JS, Pantopoulos K. Liver sinusoidal endothelial cells induce BMP6 expression in response to non-transferrin-bound iron. Blood. 2023;141(3):271–84. 10.1182/blood.2022016987. [DOI] [PubMed] [Google Scholar]
- 49.Colucci S, Altamura S, Marques O, Müdder K, Agarvas AR, Hentze MW, Muckenthaler MU. Iron-dependent BMP6 regulation in liver sinusoidal endothelial cells is instructed by hepatocyte-derived secretory signals. Hemasphere. 2022;6(10):e773. 10.1097/HS9.0000000000000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Charlebois E, Pantopoulos K. Iron overload inhibits BMP/SMAD and IL-6/STAT3 signaling to hepcidin in cultured hepatocytes. PLoS ONE. 2021;16(6):e0253475. 10.1371/journal.pone.0253475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodríguez-Mortera R, Caccavello R, Hermo R, Garay-Sevilla ME, Gugliucci A. Higher hepcidin levels in adolescents with obesity are associated with metabolic syndrome dyslipidemia and visceral fat. Antioxidants (Basel). 2021;10(5):751. 10.3390/antiox10050751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wojciechowska M, Wisniewski OW, Pruszynska-Oszmalek E, Krauss H, Sassek M, Leciejewska N, Kolodziejski P, Wilczak M. Effect of obesity and hypothyroidism on hepcidin concentration in pregnancy - a pilot study using maternal and umbilical cord blood at delivery day. J Physiol Pharmacol. 2022; 73(5). 10.26402/jpp.2022.5.04 [DOI] [PubMed]
- 53.Korlesky C, Kling PJ, Pham DQD, Ovasapyan AA, Leyns CEG, Weber MB, Coe CL. Cord blood erythropoietin and hepcidin reflect lower newborn iron stores due to maternal obesity during pregnancy. Am J Perinatol. 2019;36(5):511–6. 10.1055/s-0038-1669444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Folgueras AR, Freitas-Rodríguez S, Ramsay AJ, Garabaya C, Rodríguez F, Velasco G, López-Otín C. Matriptase-2 deficiency protects from obesity by modulating iron homeostasis. Nat Commun. 2018;9(1):1350. 10.1038/s41467-018-03853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Auguet T, Aragonès G, Berlanga A, Martínez S, Sabench F, Binetti J, Aguilar C, Porras JA, Molina A, Del Castillo D, Richart C. Hepcidin in morbidly obese women with non-alcoholic fatty liver disease. PLoS ONE. 2017;12(10):e0187065. 10.1371/journal.pone.0187065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silvestri L, Pettinato M, Furiosi V, Bavuso Volpe L, Nai A, Pagani A. Managing the dual nature of iron to preserve health. Int J Mol Sci. 2023;24(4):3995. 10.3390/ijms24043995.PMID:36835406;PMCID:PMC9961779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang CY, Babitt JL. Hepcidin regulation in the anemia of inflammation. Curr Opin Hematol. 2016;23(3):189–97. 10.1097/MOH.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varga E, Pap R, Jánosa G, Sipos K, Pandur E. IL-6 Regulates hepcidin expression via the BMP/SMAD pathway by altering BMP6, TMPRSS6 and TfR2 expressions at normal and inflammatory conditions in BV2 microglia. Neurochem Res. 2021;46(5):1224–38. 10.1007/s11064-021-03322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang N, Yang P, Li Y, Ouyang Q, Hou F, Zhu G, Zhang B, Huang J, Jia J, Xu A. Serum Iron Overload Activates the SMAD Pathway and Hepcidin Expression of Hepatocytes via SMURF1. J Clin Transl Hepatol. 2024;12(3):227–35. 10.14218/JCTH.2023.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu LN, Wang SJ, Chen C, Rausch V, Elshaarawy O, Mueller S. Direct modulation of hepatocyte hepcidin signaling by iron. World J Hepatol. 2021;13(10):1378–93. 10.4254/wjh.v13.i10.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–9. 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100(10):3776–81. 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 63.Ndevahoma F, Mukesi M, Dludla PV, Nkambule BB, Nepolo EP, Nyambuya TM. Body weight and its influence on hepcidin levels in patients with type 2 diabetes: A systematic review and meta-analysis of clinical studies. Heliyon. 2021;7(3):e06429. 10.1016/j.heliyon.2021.e06429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wueest S, Konrad D. The controversial role of IL-6 in adipose tissue on obesity-induced dysregulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2020;319(3):E607–13. 10.1152/ajpendo.00306.2020. [DOI] [PubMed] [Google Scholar]
- 65.Moreno-Navarrete JM, Ortega F, Moreno M, Ricart W, Fernández-Real JM. Fine-tuned iron availability is essential to achieve optimal adipocyte differentiation and mitochondrial biogenesis. Diabetologia. 2014;57(9):1957–67. 10.1007/s00125-014-3298-5. [DOI] [PubMed] [Google Scholar]
- 66.Hinojosa-Moscoso A, Motger-Albertí A, De la Calle-Vargas E, Martí-Navas M, Biarnés C, Arnoriaga-Rodríguez M, Blasco G, Puig J, Luque-Córdoba D, Priego-Capote F, Moreno-Navarrete JM, Fernández-Real JM. The longitudinal changes in subcutaneous abdominal tissue and visceral adipose tissue volumetries are associated with iron status. Int J Mol Sci. 2023;24(5):4750. 10.3390/ijms24054750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi L, Tao Z, Zheng L, Yang J, Hu X, Scott K, de Kloet A, Krause E, Collins JF, Cheng Z. FoxO1 regulates adipose transdifferentiation and iron influx by mediating Tgfβ1 signaling pathway. Redox Biol. 2023;63:102727. 10.1016/j.redox.2023.102727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma X, Pham VT, Mori H, MacDougald OA, Shah YM, Bodary PF. Iron elevation and adipose tissue remodeling in the epididymal depot of a mouse model of polygenic obesity. PLoS ONE. 2017;12(6):e0179889. 10.1371/journal.pone.0179889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turchi R, Tortolici F, Guidobaldi G, Iacovelli F, Falconi M, Rufini S, Faraonio R, Casagrande V, Federici M, De Angelis L, Carotti S, Francesconi M, Zingariello M, Morini S, Bernardini R, Mattei M, La Rosa P, Piemonte F, Lettieri-Barbato D, Aquilano K. Frataxin deficiency induces lipid accumulation and affects thermogenesis in brown adipose tissue. Cell Death Dis. 2020;11(1):51. 10.1038/s41419-020-2253-2. Erratum in: Cell Death Dis. 2020 Mar 3;11(3):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deschemin JC, Ransy C, Bouillaud F, Chung S, Galy B, Peyssonnaux C, Vaulont S. Hepcidin deficiency in mice impairs white adipose tissue browning possibly due to a defect in de novo adipogenesis. Sci Rep. 2023;13(1):12794. 10.1038/s41598-023-39305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yook JS, You M, Kim J, Toney AM, Fan R, Puniya BL, Helikar T, Vaulont S, Deschemin JC, Okla M, Xie L, Ghosh MC, Rouault TA, Lee J, Chung S. Essential role of systemic iron mobilization and redistribution for adaptive thermogenesis through HIF2-α/hepcidin axis. Proc Natl Acad Sci U S A. 2021;118(40):e2109186118. 10.1073/pnas.2109186118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J, Ming Z, Yang L, Wang T, Liu G, Ma Q. Long noncoding RNA XIST: Mechanisms for X chromosome inactivation, roles in sex-biased diseases, and therapeutic opportunities. Genes Dis. 2022;9(6):1478–92. 10.1016/j.gendis.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li W, Feng Q, Wang C, Yin Z, Li X, Li L. LncXIST facilitates iron overload and iron overload-induced islet beta cell injury in type 2 diabetes through miR-130a-3p/ALK2 Axis. Comput Intell Neurosci. 2022;9(2022):6390812. 10.1155/2022/6390812. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Kotta-Loizou I, Vassilaki N, Pissas G, Kakkanas A, Bakiri L, Bartenschlager R, Mavromara P. Hepatitis C virus core+1/ARF protein decreases hepcidin transcription through an AP1 binding site. J Gen Virol. 2013;94(Pt 7):1528–34. 10.1099/vir.0.050328-0. [DOI] [PubMed] [Google Scholar]
- 75.Hasan Y, Brown K. Viral eradication restores normal iron status in chronic hepatitis C patients with abnormal iron studies. Ann Hepatol. 2020;19(4):422–6. 10.1016/j.aohep.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 76.Kawaguchi Y, Mizuta T, Oza N, Takahashi H, Ario K, Yoshimura T, Eguchi Y, Ozaki I, Hisatomi A, Fujimoto K. Eradication of hepatitis C virus by interferon improves whole-body insulin resistance and hyperinsulinaemia in patients with chronic hepatitis C. Liver Int. 2009;29(6):871–7. 10.1111/j.1478-3231.2009.01993.x. [DOI] [PubMed] [Google Scholar]
- 77.Gill M, Ul Qamar M, Ikram F, Naz S, Sadaf H, Hafeez Z. Insulin resistance and chronic hepatitis C: Relationship with serum iron and hepcidin. Cureus. 2020;12(12):e12349. 10.7759/cureus.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milner KL, van der Poorten D, Trenell M, Jenkins AB, Xu A, Smythe G, Dore GJ, Zekry A, Weltman M, Fragomeli V, George J, Chisholm DJ. Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology. 2010;138(3):932-41.e1-3. 10.1053/j.gastro.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 79.Sebastiani G, Vario A, Ferrari A, Pistis R, Noventa F, Alberti A. Hepatic iron, liver steatosis and viral genotypes in patients with chronic hepatitis C. J Viral Hepat. 2006;13(3):199–205. 10.1111/j.1365-2893.2005.00662.x. [DOI] [PubMed] [Google Scholar]
- 80.Wang Q, Chen J, Wang Y, Han X, Chen X. Hepatitis C virus induced a novel apoptosis-like death of pancreatic beta cells through a caspase 3-dependent pathway. PLoS One. 2012;7(6):e38522. 10.1371/journal.pone.0038522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawaguchi Y, Mizuta T, Eguchi Y, Sakurai E, Motomura Y, Isoda H, Kuwashiro T, Oeda S, Iwane S, Takahashi H, Anzai K, Ozaki I. Whole-body insulin resistance is associated with elevated serum α-fetoprotein levels in patients with chronic hepatitis C. Intern Med. 2013;52(21):2393–400. 10.2169/internalmedicine.52.0992. [DOI] [PubMed] [Google Scholar]
- 82.Chung JY, Kim HS, Song J. Iron metabolism in diabetes-induced Alzheimer’s disease: a focus on insulin resistance in the brain. Biometals. 2018;31(5):705–14. 10.1007/s10534-018-0134-2. Erratum in: Biometals. 2018 Sep 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–38. 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Summers KL, Fimognari N, Hollings A, Kiernan M, Lam V, Tidy RJ, Paterson D, Tobin MJ, Takechi R, George GN, Pickering IJ, Mamo JC, Harris HH, Hackett MJ. A Multimodal Spectroscopic Imaging Method To Characterize the Metal and Macromolecular Content of Proteinaceous Aggregates (“Amyloid Plaques”). Biochemistry. 2017;56(32):4107–16. 10.1021/acs.biochem.7b00262. [DOI] [PubMed] [Google Scholar]
- 85.Huang YA, Zhou B, Wernig M, Südhof TC. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Aβ Secretion. Cell. 2017;168(3):427-441.e21. 10.1016/j.cell.2016.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blasco G, Puig J, Daunis-I-Estadella J, Molina X, Xifra G, Fernández-Aranda F, Pedraza S, Ricart W, Portero-Otín M, Fernández-Real JM. Brain iron overload, insulin resistance, and cognitive performance in obese subjects: a preliminary MRI case-control study. Diabetes Care. 2014;37(11):3076–83. 10.2337/dc14-0664. [DOI] [PubMed] [Google Scholar]
- 87.Wan W, Cao L, Kalionis B, Murthi P, Xia S, Guan Y. Iron deposition leads to hyperphosphorylation of tau and disruption of insulin signaling. Front Neurol. 2019;19(10):607. 10.3389/fneur.2019.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ryan BJ, Van Pelt DW, Guth LM, Ludzki AC, Gioscia-Ryan RA, Ahn C, Foug KL, Horowitz JF. Plasma ferritin concentration is positively associated with in vivo fatty acid mobilization and insulin resistance in obese women. Exp Physiol. 2018;103(11):1443–7. 10.1113/EP087283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lim D, Jeong JH, Song J. Lipocalin 2 regulates iron homeostasis, neuroinflammation, and insulin resistance in the brains of patients with dementia: Evidence from the current literature. CNS Neurosci Ther. 2021;27(8):883–94. 10.1111/cns.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Semba T, Nishimura M, Nishimura S, Ohara O, Ishige T, Ohno S, Nonaka K, Sogawa K, Satoh M, Sawai S, Matsushita K, Imazeki F, Yokosuka O, Nomura F. The FLS (fatty liver Shionogi) mouse reveals local expressions of lipocalin-2, CXCL1 and CXCL9 in the liver with non-alcoholic steatohepatitis. BMC Gastroenterol. 2013;23(13):120. 10.1186/1471-230X-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.