Abstract
Objective
This study leveraged data from 11 independent international diabetes models to evaluate the impact of unrelated future medical costs on the outcomes of health economic evaluations in diabetes mellitus.
Methods
Eleven models simulated the progression of diabetes and occurrence of its complications in hypothetical cohorts of individuals with type 1 (T1D) or type 2 (T2D) diabetes over the remaining lifetime of the patients to evaluate the cost effectiveness of three hypothetical glucose improvement interventions versus a hypothetical control intervention. All models used the same set of costs associated with diabetes complications and interventions, using a United Kingdom healthcare system perspective. Standard utility/disutility values associated with diabetes-related complications were used. Unrelated future medical costs were assumed equal for all interventions and control arms. The statistical significance of changes on the total lifetime costs, incremental costs and incremental cost-effectiveness ratios (ICERs) before and after adding the unrelated future medical costs were analysed using t-test and summarized in incremental cost-effectiveness diagrams by type of diabetes.
Results
The inclusion of unrelated costs increased mean total lifetime costs substantially. However, there were no significant differences between the mean incremental costs and ICERs before and after adding unrelated future medical costs. Unrelated future medical cost inclusion did not alter the original conclusions of the diabetes modelling evaluations.
Conclusions
For diabetes, with many costly noncommunicable diseases already explicitly modelled as complications, and with many interventions having predominantly an effect on the improvement of quality of life, unrelated future medical costs have a small impact on the outcomes of health economic evaluations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40258-024-00914-z.
Key Points for Decision Makers
| Incorporating unrelated future medical costs may have little impact on the findings from the cost-effectiveness analyses of many diabetes interventions, at least those whose benefits include quality-adjusted life-year gains and cost offsets, but do not include significant increases in life expectancy. | |
| Some health technology assessment guidelines require the inclusion of unrelated future medical costs in cost-effectiveness analysis. This study demonstrates that, at least in the interventions and disease area studied, the inclusion of unrelated future medical costs is unlikely to impact decision making. |
Introduction
Unrelated future medical costs are healthcare expenses that may incur in the future for conditions that are not directly related to the intervention under evaluation. These costs have long been excluded from most economic analysis. When an intervention extends life, however, these costs will continue to accrue and thus affect estimates of cost effectiveness. For instance, successful smoking cessation initiatives may reduce rates of lung cancer, increase average life expectancy, and reduce lung cancer treatment costs, but the additional longevity may increase rates of other age-related health conditions not commonly considered in economic analysis of lung cancer.
While rare in economic analysis, it is essential to include unrelated future medical costs in economic evaluations (e.g., cost-effectiveness/cost-utility/cost-minimization analyses) in order to ensure both internal and external consistency [1, 2], especially when adopting a broad societal perspective [3]. By considering these costs, we acknowledge that additional medical care is required during the incremental life-years gained through an intervention, maintaining the integrity of the analysis. The consideration of future unrelated medical costs will vary by intervention, given different incremental longevity and different timing of mortality reductions (e.g., early on and not heavily discounted or later and deeply discounted). Incorporating these costs additionally improves the accuracy of estimates of the true financial impact, including opportunity costs produced by interventions over future consumption.
Empirical research has shown that the impact of omitting unrelated future costs can be large [4]. Van Baal et al. [5], for example, found that including unrelated future medical costs reduced the cost effectiveness of smoking cessation interventions across the board, but the impact declined with smoker age. de Vries et al. [6] found that inclusion led to vaccine interventions no longer being cost effective, especially for those at higher risk of infection. In the diabetes mellitus space—where typically patients are middle-aged to older, treatment is multi-modal (e.g., often including anti-hypertensive and dyslipidaemia therapies) and treat-to-goal algorithms are standard—estimated gains in life expectancy tend to be small, which may limit the impact of including unrelated future medical costs. Roberts et al. [7] corroborated this in a sensitivity analysis, finding virtually no effect on cost-effectiveness estimates. Meltzer et al. [8], however, found that inclusion of these costs along with consumption and productivity costs lowered ICER estimates by 57%.
While many practice guidelines emphasize inclusion of unrelated future medical costs [9–11], there have been instances where the inclusion of unrelated future medical costs is either not explicitly recommended [12, 13], or is disallowed [14]. Research is recommended to establish a formal process to evaluate the economic impact of including future unrelated medical costs in cost-effectiveness analysis [2].
Diabetes has been recognized as a serious public health concern with a considerable impact on human life and health expenditures. Several economic evaluation models of diabetes have been developed to evaluate the long-term disease outcomes and cost effectiveness of diabetes interventions, with many of them not including unrelated future medical costs [15–17]. As part of the Mount Hood 2022 Diabetes Challenge, 11 diabetes models simulated a set of standardized scenarios designed to evaluate how cost and cost-effectiveness outcomes vary in response to adding unrelated future medical costs. The detailed challenge instruction is included as Supplement 1 (see electronic supplementary material [ESM]). Our study aimed to leverage these cross-model simulation results to further analyse the impact of unrelated future medical costs in health economic evaluations of diabetes interventions.
Methods
2022 Mount Hood Diabetes Challenge
Eleven diabetes modelling groups participated in the challenges. Four models participated in the Type 1 Diabetes (T1D) challenge: IQVIA (formerly known as the CORE Diabetes Model) [18], COSMO-T1D [19], PRIME [20] and ECHO-T1D. Seven models participated in the Type 2 Diabetes (T2D) challenge: IQVIA [18], CARDIFF [21], CHIME [22], PRIME [23], ECHO-T2D [24], MICADO [25] and UKPDS [26]. The models are summarized individually in Supplement 2 (see ESM). All modelling groups contributed towards the preparation of this article and approved the use of their results/outputs.
Challenge 2 consisted of simulating three hypothetical blood glucose interventions—a low cost (£12 annually) option with an HbA1c reduction of 0.5% and no body mass index (BMI) reduction, a middle option (£320 annually) with an HbA1c reduction of 0.9% and a 1-kg/m2 increase in BMI and a more effective option (£3810 annually) with a 1.5% HbA1c reduction and 1-kg/m2 decrease in BMI—and a control arm with no treatment effects or costs. All changes were considered permanent for simplicity, treatment adherence was assumed to be 100%, and the time horizon was lifetime. Each modelling team compiled and submitted a complete set of health, cost and cost-effectiveness results to the conference organizers in advance, importantly including estimates with and without unrelated future medical costs. Results provided by the modelling groups were pooled, analysed and presented at the meeting.
Standardized Input Data
To ensure valid comparisons across the models, the modelling groups were requested to use the standardized profiles described in the challenge instructions, including baseline characteristics, utility value set and complication costs, to the extent possible. If a model required additional input parameters or assumptions that differed from the instructions, the modelling groups were requested to document these additional inputs, data sources and/or assumptions applied and submit them with their results.
Baseline Characteristics
Baseline patient characteristics are presented in Table S2 in Supplement 1 (see ESM) and were chosen to reflect typical patients with T2D enrolled in a randomized controlled trial for diabetes, specifically being sourced from the Action in Diabetes and Vascular Disease–PreterAx and DiamicroN Controlled Evaluation (ADVANCE) trial for T2D [27] and from the Swedish National Diabetes Register for T1D [19]. All risk factors (except age) were held constant over the simulation period, after applying treatment effects for HbA1c and in some cases BMI.
Health State Utility Values
The challenge instructions provided a set of utility/disutility values for a wide range of likely health states including diabetes-related complications (Table S1 in Supplement 1, see ESM) for T1D and T2D. The health state utility value set was from a published systematic review and literature [28, 29]. The modelling groups were requested to follow the additive quality-of-life model when combining the health utility values in the modelling simulation.
Costs
The perspective of the cost analysis was the health care system in the United Kingdom (UK). All costs were presented in UK currency (£GBP 2017–2018). The challenge instructions specified the costs of diabetes-related complications in the UK (Table S3 in Supplement 1, see ESM). This standardized unit cost vector was applied for both T1D and T2D, as well as both male and female patients. The challenge instructions also specified age- and sex-specific annual unrelated future medical costs, based on estimates by Briggs et al. [30]. For models where age- and sex-specific values could not be accommodated, the modelling groups were directed to use mean values if possible and to document methods used when submitting results. Instructions also requested that these costs be unaffected by the occurrence of diabetes-related complications. Unrelated future medical costs are presented in Table 1. A discount rate of 3.5% was applied to both costs and quality-adjusted life-years (QALYs).
Table 1.
Total annual expenditure on unrelated future medical cost by age group and sex (£GBP 2017–2018 price) [28]
| Age group | Men | Women | Differences between men and women |
|---|---|---|---|
| 65–69 years | 1737 | 1659 | 78 |
| 70–74 years | 2085 | 1989 | 96 |
| 75–79 years | 2742 | 2565 | 177 |
| 80–84 years | 3189 | 2962 | 227 |
| 85+ years | 3694 | 3339 | 355 |
Statistical Analyses and Outcomes
The differences on the mean lifetime costs, incremental costs and ICERs of intervention and control groups before and after considering the unrelated future medical costs were calculated for each model and were summarized by diabetes type. Statistical significance of the differences was tested using t-test. Whether adding unrelated medical costs changed the model conclusions at a threshold of £20,000 per QALY gained was summarized using an incremental cost-effectiveness diagram.
The shifting on ICERs before and after adding the unrelated future medical costs was also analysed using linear regression, controlling for the amount of unrelated future medical costs added to the evaluation using the regression model:
For each model and intervention, the amount of incremental unrelated future medical costs was calculated as the difference between total lifetime costs with and without modelling unrelated future medical costs.
Results
Incremental Life Expectancy and QALYs Gained
Table 2 shows the incremental life expectancy and QALYs gained of each intervention compared with control from 11 diabetes models. All three interventions had only a minor effect on life expectancy and QALYs, with a maximum incremental life expectancy of 0.52 years and a QALY gain of 1.25.
Table 2.
Incremental life expectancy and QALYs gained for each intervention compared with control
| Model name | Incremental life expectancy (years) | Incremental QALYs | ||||
|---|---|---|---|---|---|---|
| Intervention 1 | Intervention 2 | Intervention 3 | Intervention 1 | Intervention 2 | Intervention 3 | |
| Type 1 diabetes | ||||||
| COSMO-T1D | 0.09 | 0.12 | 0.26 | 0.12 | 0.17 | 0.39 |
| ECHO-T1D | 0.13 | 0.08 | 0.39 | 0.26 | 0.23 | 0.79 |
| IQVIA | 0.21 | 0.33 | 0.52 | 0.48 | 0.70 | 1.25 |
| PRIME | 0.15 | 0.23 | 0.51 | 0.35 | 0.44 | 1.17 |
| Type 2 diabetes | ||||||
| CARDIFF | 0.05 | 0.07 | 0.14 | 0.04 | −0.01 | 0.21 |
| CHIME | 0.11 | 0.14 | 0.10 | 0.14 | 0.30 | 0.32 |
| ECHO-T2D | 0.06 | 0.09 | 0.21 | 0.08 | 0.05 | 0.31 |
| IQVIA | 0.09 | 0.11 | 0.20 | 0.11 | 0.09 | 0.35 |
| MICADO | 0.01 | −0.03 | 0.14 | 0.07 | 0.09 | 0.27 |
| PRIME | 0.09 | 0.13 | 0.26 | 0.10 | 0.08 | 0.37 |
| UKPDS | 0.05 | 0.08 | 0.16 | 0.05 | 0.07 | 0.15 |
QALYs quality-adjusted life-years
Total Lifetime Costs
Total lifetime costs increased substantially for all interventions and control arms (Supplement 3, see ESM). The t-test showed there was a statistically significant increase in the mean total lifetime costs after adding the unrelated future medical costs for T2D but no significant changes for T1D (Table 3).
Table 3.
T-test analysis on the total lifetime costs before and after adding the unrelated future medical costs (£GBP 2017–2018)
| Diabetes type | Variable | Observation | Mean | SE | SD | 95% CI | P value (difference <0) | P value (difference != 0) | P value (difference >0) | |
|---|---|---|---|---|---|---|---|---|---|---|
| T1D | Before | 16 | 54,156 | 8560 | 34,241 | 35,911 | 72,402 | |||
| After | 16 | 68,436 | 8408 | 33,633 | 50,514 | 86,358 | ||||
| Difference | − 14,280 | 11,999 | − 38,785 | 10,226 | 0.12 | 0.24 | 0.88 | |||
| T2D | Before | 28 | 71,511 | 13,277 | 70,255 | 44,269 | 98,753 | |||
| After | 28 | 105,422 | 13,971 | 73,930 | 76,755 | 134,089 | ||||
| Difference | − 33,911 | 19,274 | − 72,553 | 4730 | 0.04 | 0.08 | 0.96 | |||
Difference = Before–After
Bolded p-value indicates statistical significance at 0.05 threshold
95% CI 95% confidence interval, SD standard deviation, SE standard error, T1D type 1 diabetes, T2D type 2 diabetes
Incremental Costs
The incremental costs of each intervention compared with control changed slightly with and without including the unrelated future medical costs (Supplement 4, see ESM). However, the t-test showed that the changes were not statistically significant for either T1D or T2D (Table 4).
Table 4.
T-test analysis on the incremental costs before and after adding the unrelated future medical costs (£GBP 2017–2018)
| Diabetes type | Variable | Observation | Mean | SE | SD | 95% CI | p-Value (difference <0) | p-Value (difference != 0) | p-Value (difference >0) | |
|---|---|---|---|---|---|---|---|---|---|---|
| T1D | Before | 12 | 23,550 | 9988 | 34,599 | 1567 | 45,534 | |||
| After | 12 | 23,944 | 10,043 | 34,791 | 1839 | 46,049 | ||||
| Difference | − 394 | 14,164 | − 29,769 | 28,981 | 0.48 | 0.97 | 0.52 | |||
| T2D | Before | 21 | 16,146 | 4817 | 22,073 | 6099 | 26,193 | |||
| After | 21 | 16,412 | 4837 | 22,168 | 6322 | 26,503 | ||||
| Difference | − 266 | 6826 | − 14,063 | 13,531 | 0.49 | 0.99 | 0.51 | |||
Difference = Before–After
95% CI 95% confidence interval, SD standard deviation, SE standard error, T1D type 1 diabetes, T2D type 2 diabetes
Incremental Cost-Effectiveness Ratios (ICERs)
While the ICERs of each intervention versus control changed with and without including the unrelated future medical costs (Supplement 5, see ESM), the t-test showed that the changes were insignificant for both T1D and T2D (Table 5).
Table 5.
T-test analysis on the incremental cost-effectiveness ratios (costs per quality-adjusted life-year gained) before and after adding the unrelated future medical costs (£GBP 2017–2018)
| Diabetes type | Variable | Observation | Mean | SE | SD | 95% CI | p-Value (difference <0) | p-Value (difference != 0) | p-Value (difference >0) | |
|---|---|---|---|---|---|---|---|---|---|---|
| T1D | Before | 12 | 35,008 | 15,485 | 53,640 | 927 | 69,090 | |||
| After | 12 | 35,849 | 15,550 | 53,868 | 1623 | 70,075 | ||||
| Difference | − 841 | 21,945 | − 46,352 | 44,670 | 0.48 | 0.97 | 0.52 | |||
| T2D | Before | 21 | 51,027 | 28,582 | 130,977 | − 8593 | 110,647 | |||
| After | 21 | 51,760 | 29,336 | 134,435 | − 9434 | 112,954 | ||||
| Difference | − 733 | 40,957 | − 83,511 | 82,045 | 0.49 | 0.99 | 0.51 | |||
Difference = Before–After
95% CI 95% confidence interval, SD standard deviation, SE standard error, T1D type 1 diabetes, T2D type 2 diabetes
Model Conclusions
The incremental cost-effectiveness diagram showed no change in model conclusions under the threshold of £20,000 per QALY gained for both T1D and T2D (Figs 1 and 2).
Fig. 1.
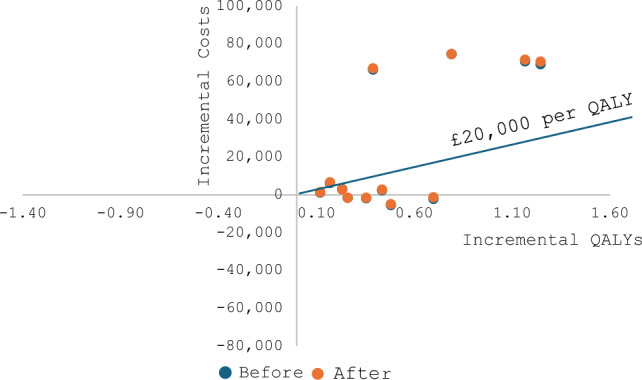
Incremental cost-effectiveness diagram for type 1 diabetes before and after adding unrelated future medical costs. The ICERs for type 1 diabetes interventions before and after adding the unrelated future medical costs were nearly overlapping in the plane, which means there were only minor changes to ICERs after adding the unrelated future medical costs. As shown in the diagram, adding the unrelated future medical costs did not change any conclusions of the model outcome under the £20,000 per QALY gained threshold. Costs were presented in UK currency (£GBP 2017–2018). ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year
Fig. 2.
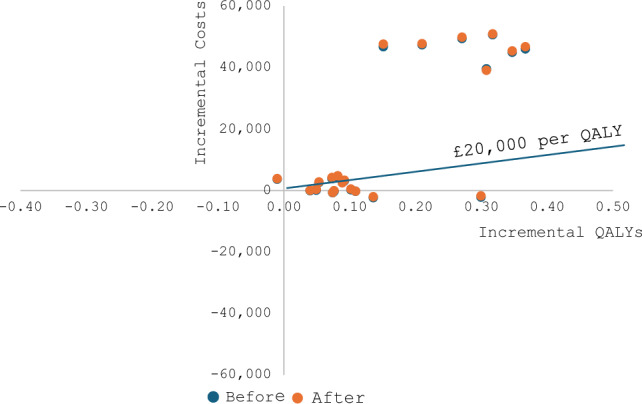
Incremental cost-effectiveness diagram for type 2 diabetes before and after adding unrelated future medical costs. The ICERs for type 2 diabetes interventions before and after adding the unrelated future medical costs were nearly overlapping in the plane, which means there were only minor changes to ICERs after adding the unrelated future medical costs. As shown in the diagram, adding the unrelated future medical costs did not change any conclusions of the model outcome under the £20,000 per QALY gained threshold. Costs were presented in UK currency (£GBP 2017-2018). ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year
Regression on ICERs With and Without Unrelated Future Medical Costs
Regression result showed that the post-inclusion ICERs were only significantly associated with the pre-inclusion ICERs, with a coefficient 1.05 (Table 6).
Table 6.
The regression of post-ICERs on pre-ICERs and added unrelated future medical costs
| ICER_After | Coefficient | SE | t | p > t | 95% CI | |
|---|---|---|---|---|---|---|
| ICER_Before | 1.05 | 0.03 | 36.87 | 0.00 | 0.99 | 1.11 |
| FutureCost | − 0.04 | 0.06 | − 0.59 | 0.56 | − 0.16 | 0.09 |
| ICER_Before#FUTURECOST | 0.00 | 0.00 | − 0.89 | 0.38 | 0.00 | 0.00 |
| Constant | 575.44 | 1743.57 | 0.33 | 0.74 | − 2990.55 | 4141.43 |
The added unrelated future medical costs to each model in each intervention equal to the total lifetime costs of each intervention from each model after adding the unrelated future medical costs minus the corresponding total lifetime costs before adding the unrelated future medical costs
ICER incremental cost-effectiveness ratio
Discussion
This study leveraged unique simulation results from a large number of independent models to assess whether unrelated future medical costs should be routinely considered in economic analysis for diabetes interventions. While, by definition, including unrelated future costs increased total lifetime costs in absolute terms, we found that there was no significant change in incremental costs (and thus ICERs) for both T1D and T2D, in line with the relatively small increases in life expectancy provided by these interventions (relative to the control arm).
Three features of diabetes and this analysis may explain the limited impact. First, the three interventions were associated with relatively small improvements (and in one case a worsening) in life expectancy versus the control arm, with mean discounted incremental life-years across the models ranging from − 0.03 to 0.52 years. Therefore, compared with the control arm, the additional unrelated future medical costs occurring in those incremental life-years in the intervention groups were relatively small. Second, this life extension and the associated unrelated medical costs occurred primarily far into the future, which when discounted at 3.5% annually, deflates much of the impact. Third, we assumed the same annual expenditure on unrelated medical costs by age and sex for all groups and assumed these costs remain unchanged by the occurrence of diabetes-related complications. This may not hold in a real-world situation as these costs can vary both between individuals (based on their sociodemographic and clinical profiles) and over time due to complications or other factors. For example, in a cancer cost-effectiveness analysis where cost inputs used in the economic model accounted for cancer type and phase of the disease, a notable impact on the ICER was observed [31].
The regression result indicated that the post-inclusion ICER was only significantly associated with the pre-inclusion ICER, with a coefficient close to 1. This suggests that the inclusion of unrelated future medical costs does not dramatically alter the ICERs, as the relationship remains strong and consistent. The direct effect of unrelated future medical costs and its interaction with pre-inclusion ICER was not significant, indicating that the amount of future costs added does not significantly impact the shifts in ICERs. This implies that the overall influence of unrelated future medical costs on cost-effectiveness outcomes is minimal within the context of this study. The minimal impact on the ICERs can be explained theoretically considering the following approximation of an ICER equation [8, 32], where diabetes interventions led to a smaller increase in life expectancy than in QALYs (Supplement 6, see ESM).
where C is the annual expenditure on unrelated medical costs.
Two studies have investigated the impact of unrelated future medical costs on the cost effectiveness of diabetes interventions [7, 8]. Our results align well with the study conducted by Roberts et al. using a UK-based sample of adults with diabetes (aged 50–59 years). Their study also observed no change in the cost effectiveness of the considered diabetes intervention after the incorporation of unrelated future medical costs in their analyses [7]. Another study by Meltzer et al. examined the effect of future costs on the cost effectiveness of intensive therapy for T1D among young adults aged 13–39 years [8]. This study found a 57% decrease in ICER, which fell from $22,576/QALY gained to $9626/QALY gained after the incorporation of unrelated future medical costs, future consumption, and loss of productivity. Inclusion of these factors could potentially change the original model conclusions, and hence, the decision-making process. Meltzer et al. focused on a much younger population and an intervention that leads to a gain in life expectancy in preretirement years when net resource use is negative, which could be one of the key differentiators to our study.
The inclusion of unrelated future medical costs could be more applicable to particular therapeutic areas and public health interventions. For example, in rare diseases with high mortality rates at a young age like cystic fibrosis, patients often face significant immediate healthcare costs due to the severity of their condition, but their shorter lifespans result in lower future healthcare costs. The economic evaluation in such scenarios might prioritize the high immediate costs over long-term costs. In contrast, a new gene therapy transforming a previous fatal condition into a chronic manageable one would need to consider patients incurring unrelated future medical costs over their extended lifespans in the economic evaluations. Such unrelated future medical costs could be highly relevant for life-extending interventions like vaccination and early screening, that increase unrelated future medical costs, as people live longer and develop other health conditions unrelated to the initial interventions.
The key strength of our study is the systematic analysis of the results from eleven independent diabetes models, each applied to the same standardized scenario, thus allowing for consistent comparisons across models and improving the reliability of our conclusions. Our study also has limitations. First, most of the model parameters were obtained from UK-based literature and/or rely on a set of assumptions that may or may not hold true in real-life situations and are unlikely to be generalizable across other countries. For instance, unrelated future medical costs from UK sources [30] are low in comparison to some other countries with similar levels of wealth such as the Netherlands and Germany [33, 34]. Second, our study is reporting the results of a past challenge, for which we were not able to conduct any further analyses including updates with newly available input parameters or subgroup analyses. Furthermore, we relied on the assumption of constant unrelated future medical costs irrespective of the occurrence of complications, which increased the risk of double counting the costs of complications. With respect to future research, we believe it may be helpful to explore the impact of different approaches of incorporating such costs, particularly for chronic diseases that have compounding health implications. We also suggest that future researchers are encouraged to provide more accurate estimates of unrelated future medical costs that could be used in economic evaluation studies, especially when interventions have an effect on life expectancy.
Conclusions
An analysis of results from the multiple independent models in the 2022 Mount Hood Diabetes Challenge found that incorporating constant unrelated future medical costs had limited impact on the cost effectiveness of some diabetes interventions, at least those whose benefits include QALY gains and cost offsets, but do not include significant changes in life expectancy. This will need to be revisited when treatments that extend life substantially for diabetes patients are developed.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
Andrew J. Palmer and Ting Zhao conceptualized the study and led the design and implementation of the study. Philip M. Clarke, Talitha Feenstra, Pieter van Baal and Michelle Tew collaborated in refining the study design. Talitha Feenstra, Pieter van Baal, Michael Willis, William J. Valentine, Philip M. Clarke, Barnaby Hunt, James Altunkaya, An Tran-Duy, Richard F. Pollock, Samuel J.P. Malkin, Andreas Nilsson, Phil McEwan, Volker Foos, Jose Leal, Elbert S. Huang, Neda Laiteerapong, Mark Lamotte, Harry Smolen, Jianchao Quan, Luís Martins, Mafalda Ramos and Andrew J. Palmer were diabetes modelling group members participating in the 2022 Mount Hood Diabetes Challenge and generated the data. All authors reviewed and edited the paper and approved the final version.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The author(s) received no financial support for the research, authorship and/or publication of this article.
Data Availability
Data sets generated during the current study are available from the corresponding author on reasonable request.
Code Availability
Available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication (from Patients/Participants)
Not applicable.
References
- 1.Kearns B. The relevance of future, unrelated health costs in economic evaluation in NICE appraisals. In: NICE dsu report. 2020.
- 2.Jiang S, Wang Y, Zhou J, Jiang Y, Liu GG, Wu J. Incorporating future unrelated medical costs in cost-effectiveness analysis in China. BMJ Glob Health. 2021;6(10):1-11. [DOI] [PMC free article] [PubMed]
- 3.Sittimart M, Rattanavipapong W, Mirelman AJ, Hung TM, Dabak S, Downey LE, et al. An overview of the perspectives used in health economic evaluations. Cost Effect Resour Allocat. 2024;22(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Vries LM, van Baal PHM, Brouwer WBF. Future costs in cost-effectiveness analyses: past, present future. Pharm Econ. 2019;37(2):119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Baal PHM, Feenstra TL, Hoogenveen RT, Ardine De Wit G, Brouwer WBF. Unrelated medical care in life years gained and the cost utility of primary prevention: in search of a ‘perfect’ cost–utility ratio. Health Econ. 2007;16(4):421–33. [DOI] [PubMed] [Google Scholar]
- 6.de Vries LM, Kellerborg KM, Brouwer WBF, van Baal PHM. Don’t forget about the future: the impact of including future costs on the cost-effectiveness of adult pneumococcal conjugate vaccination with PCV13 in the Netherlands. Vaccine. 2021;39(29):3834–43. [DOI] [PubMed] [Google Scholar]
- 7.Roberts S, Craig D, Adler A, McPherson K, Greenhalgh T. Economic evaluation of type 2 diabetes prevention programmes: Markov model of low- and high-intensity lifestyle programmes and metformin in participants with different categories of intermediate hyperglycaemia. BMC Med. 2018;16(1):1-12. [DOI] [PMC free article] [PubMed]
- 8.Meltzer D, Egleston B, Stoffel D, Dasbach E. Effect of future costs on cost-effectiveness of medical interventions among young adults: the example of intensive therapy for type 1 diabetes mellitus. Med Care. 2000;38(6):679–85. [DOI] [PubMed] [Google Scholar]
- 9.Institute for Clinical and Economic Review. ICER’s reference case for economic evaluations: principles and rationale.
- 10.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103. [DOI] [PubMed] [Google Scholar]
- 11.Nederland Z. Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg. Diemen, Zorginstituut Nederland. 2015.
- 12.Bosmans JE, De Bruijne MC, Van Hout HPJ, Hermens MLM, Adèr HJ, Van Tulder MW. Practical guidelines for economic evaluations alongside equivalence trials. Value Health. 2008;11(2):251–8. [DOI] [PubMed] [Google Scholar]
- 13.Sharma D, Aggarwal AK, Downey LE, Prinja S. National healthcare economic evaluation guidelines: a cross-country comparison. Pharm Econ Open. 2021;5(3):349–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Läkemedelsförmånsverket T-O. Läkemedelsförmånsnämndens allmänna råd om ekonomiska utvärderingar. LFNAR. 2003;2003:2. [Google Scholar]
- 15.Gæde P, Valentine WJ, Palmer AJ, Tucker DMD, Lammert M, Parving H-H, et al. Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes. Diabetes Care. 2008;31(8):1510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steg PG, Bhatt DL, James SK, Darlington O, Hoskin L, Simon T, et al. Cost–effectiveness of ticagrelor in patients with type 2 diabetes and coronary artery disease: a European economic evaluation of the THEMIS trial. Eur Heart J Cardiovasc Pharmacother. 2022;8(8):777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarride J-E, Hopkins R, Blackhouse G, Bowen JM, Bischof M, Von Keyserlingk C, et al. A review of methods used in long-term cost-effectiveness models of diabetes mellitus treatment. Pharmacoeconomics. 2010;28(4):255–77. [DOI] [PubMed] [Google Scholar]
- 18.Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. The CORE diabetes model: projecting long-term clinical outcomes, costs and costeffectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(sup1):S5–26. [DOI] [PubMed] [Google Scholar]
- 19.Tran-Duy A, Knight J, Palmer AJ, Petrie D, Lung TWC, Herman WH, et al. A patient-level model to estimate lifetime health outcomes of patients with type 1 diabetes. Diabetes Care. 2020;43(8):1741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valentine WJ, Pollock RF, Saunders R, Bae J, Norrbacka K, Boye K. The prime diabetes model: novel methods for estimating long-term clinical and cost outcomes in type 1 diabetes mellitus. Value Health. 2017;20(7):985–91. [DOI] [PubMed] [Google Scholar]
- 21.McEwan P, Ward T, Bennett H, Bergenheim K. Validation of the UKPDS 82 risk equations within the cardiff diabetes model. Cost Effect Resour Allocat. 2015;13(1): 1-7. [DOI] [PMC free article] [PubMed]
- 22.Quan J, Ng CS, Kwok HHY, Zhang A, Yuen YH, Choi C-H, et al. Development and validation of the CHIME simulation model to assess lifetime health outcomes of prediabetes and type 2 diabetes in Chinese populations: a modeling study. PLoS Med. 2021;18(6): e1003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollock RF, Norrbacka K, Boye KS, Osumili B, Valentine WJ. The PRIME Type 2 diabetes model: a novel, patient-level model for estimating long-term clinical and cost outcomes in patients with type 2 diabetes mellitus. J Med Econ. 2022;25(1):393–402. [DOI] [PubMed] [Google Scholar]
- 24.Willis M, Johansen P, Nilsson A, Asseburg C. Validation of the economic and health outcomes model of type 2 diabetes mellitus (ECHO-T2DM). Pharmacoeconomics. 2017;35(3):375–96. [DOI] [PubMed] [Google Scholar]
- 25.Van Der Heijden AAWA, Feenstra TL, Hoogenveen RT, Niessen LW, De Bruijne MC, Dekker JM, et al. Policy evaluation in diabetes prevention and treatment using a population-based macro simulation model: the MICADO model. Diabet Med. 2015;32(12):1580–7. [DOI] [PubMed] [Google Scholar]
- 26.Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, et al. A model to estimate the lifetime health outcomes of patients with Type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747–59. [DOI] [PubMed] [Google Scholar]
- 27.ADVANCE-Action in Diabetes and Vascular Disease. patient recruitment and characteristics of the study population at baseline. Diabet Med. 2005;22(7):882–8. [DOI] [PubMed] [Google Scholar]
- 28.Beaudet A, Clegg J, Thuresson P-O, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–70. [DOI] [PubMed] [Google Scholar]
- 29.Mount Hood 2022 Diabetes Challenge. Available from: https://www.mthooddiabeteschallenge.com/challenge-sessions.
- 30.Briggs ADM, Scarborough P, Wolstenholme J. Estimating comparable English healthcare costs for multiple diseases and unrelated future costs for use in health and public health economic modelling. PLoS One. 2018;13(5): e0197257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tew M, Clarke P, Thursky K, Dalziel K. Incorporating future medical costs: impact on cost-effectiveness analysis in cancer patients. Pharmacoeconomics. 2019;37(7):931–41. [DOI] [PubMed] [Google Scholar]
- 32.Meltzer D. Accounting for future costs in medical cost-effectiveness analysis. J Health Econ. 1997;16(1):33–64. [DOI] [PubMed] [Google Scholar]
- 33.Perry-Duxbury M, Asaria M, Lomas J, Van Baal P. Cured today, ill tomorrow: a method for including future unrelated medical costs in economic evaluation in England and wales. Value Health. 2020;23(8):1027–33. [DOI] [PubMed] [Google Scholar]
- 34.Mokri H, Kvamme I, De Vries L, Versteegh M, Van Baal P. Future medical and non-medical costs and their impact on the cost-effectiveness of life-prolonging interventions: a comparison of five European countries. Eur J Health Econ. 2023;24(5):701–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sets generated during the current study are available from the corresponding author on reasonable request.
Available from the corresponding author on reasonable request.


