Abstract
Background
Home-based rehabilitation is a cost-effective means of making services available for patients. The aim of this study is to determine the evidence in the literature on the effects of home-based neurostimulation in patients with stroke.
Method
We searched PubMED, Embase, Web of Science, Scopus, and CENTRAL for randomized controlled trials on the subject matter using keywords such as stroke, electrical stimulation and transcranial direct current stimulation. Information on participants’ characteristics and mean scores on the outcomes of interest were extracted. Risks of bias and methodological quality of the included studies were assessed using Cochrane Risks of bias tool and PEDro scale respectively. The data was analyzed using both narrative and quantitative syntheses. In the quantitative synthesis, meta-analysis was carried out using random effect model analysis.
Result
The results showed that, home-based neurostimulation is superior to the control at improving upper limb muscle strength (SMD = 0.72, 95% CI = 0.08 to 1.32, p = 0.03), functional mobility (SMD = -0.39, 95% CI = -0.65 to 0.14, p = 0.003) and walking endurance (SMD = 0.33, 95% CI = 0.08 to 0.59, p = 0.01) post intervention; and upper limb motor function (SMD = 0.9, 95% CI = 0.10 to 1.70, p = 0.03), functional mobility (SMD = -0.30, 95% CI = -0.56 to -0.05, p = 0.02) and walking endurance (SMD = 0.33, 95% CI = 0.08 to 0.59, p = 0.01) at follow-up.
Conclusions
Home-based neurostimulation can be used to improve upper and lower limb function after stroke.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10072-024-07633-2.
Keywords: Stroke, Neurostimulation, Telerehabilitation, Patient-centered care, Activities of daily living, Quality of life
Introduction
Neurostimulation is fast growing in the field of neurological rehabilitation, where many types of patients such as those with stroke, Parkinson’s disease and multiple sclerosis are benefitting from it [1–10]. It is defined as the use of electric, electromagnetic, chemical or optogenetic methods to stimulate or block the flow of action potential through the central nervous system (CNS) [11–15]. In patients with stroke, it is used to help with recovery of brain functions such as sensory, motor and cognitive functions [1, 16].
There are basically two methods of application of neurostimulation, invasive (where the stimulation is achieved by surgically implanting electrodes in the stimulation sites) and non-invasive (where the stimulation is achieved by connecting electrodes to the external parts of the stimulation sites such as the skin) techniques. The invasive type of neurostimulation includes techniques such as the invasive vagus nerve stimulation (VNS) and deep brain stimulation [17, 18]; whereas, the non-invasive type of neurostimulation includes techniques such as the transcutaneous electrical nerve stimulation (TENS), neuromuscular electrical stimulation, orthosis-supported neuromuscular electrical stimulation, transcranial direct current stimulation (tDCS), transcranial alternating current simulation (tACS), transcranial pulse simulation (tPS), transcranial random noise stimulation (tRNS), transcranial magnetic stimulation (TMS), radio-electric asymmetry conveyer (REAC) and non-invasive VNS [19–26]. However, functional electrical stimulation can be used as either non-invasive or invasive type of neurostimulation [10].
Neurostimulation techniques can be delivered in the clinic or at home [27–29]. A home-based mode of rehabilitation is a healthcare delivery model employed to enhance easy access of rehabilitation services for patients with various conditions [30–33]. Its sole aims are to help reduce the cost of healthcare, and improve patients’ confidence and motivation, and compliance with the rehabilitation [34, 35]. This is because aside from the effectiveness of an intervention based on behavioural and neurophysiological outcomes, its cost is equally important; and a recent suggestion seeks for the use of the most cost-effective interventions [36]. In addition, home-based rehabilitation seems to offer more opportunity for increased intensity of rehabilitation, which is an important factor for recovery of function after stroke [37]. Similarly, it affords the patients with the opportunity to save money on transport, and reduce or prevent the risk of hospital-acquired infections and other communicable diseases, especially during epidemics or pandemics [38–40].
Furthermore, what is very interesting in stroke rehabilitation is that, home-based rehabilitation using exercises produces similar positive results as clinic-based rehabilitation [41]. The aim of this study is to carry out a systematic review and meta-analysis to determine from the literature, the effects of home-based neurostimulation in patients with stroke. In addition, the study is aimed at investigating its reported feasibility by summarizing reports of serious adverse events, and participants’ compliance with the protocols.
Materials and methods
We conducted a systematic review and meta-analysis, which was registered in PROSPERO database (registration number, CRD42023401257) using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.
Inclusion and exclusion criteria
In the study, only randomized controlled trials (RCT) that compared the effects of home-based neurostimulation with sham neurostimulation or a control intervention on outcomes such as upper limb function, lower limb function, neurophysiological changes, spasticity and adverse events after stroke were included. The studies must also include participants who were 18 years old or more. However, studies that were not published in English language were excluded.
To make effective syntheses of the included studies, they were grouped based on the body part treated (upper and lower limbs) and the outcomes they assessed.
Procedure for literature search
The following databases: PubMED, Embase, Web of Science, Scopus, and CENTRAL were searched from their earliest dates until July, 2023. In addition, manual search of the references of the included studies and relevant systematic reviews was also carried out [29, 42]. The search was carried out using strategies adapted to the particular database by one of the researchers (AA); however, it was independently verified by another researcher (TWLW). The search terms used include stroke, brain infarction, cerebrovascular accident, electrical stimulation, transcutaneous electrical nerve stimulation, transcranial direct current stimulation, transcranial magnetic stimulation, deep brain stimulation, transcranial alternating current stimulation, transcranial random noise stimulation, telerehabilitation, virtual rehabilitation and remote rehabilitation. See Appendix 1 for the details of the search strategy used.
Selection of studies and extraction of data
Eligible studies were selected manually and by using Endnote software. The selection was carried out independently by two of the researchers (AA & TWLW).
At first, some of the studies that were ineligible based on the information from their titles and abstract were excluded. However, when the information in their titles and abstract was not sufficient to decide on their eligibility, their full texts were read to decide for their inclusion or otherwise. Moreover, in case of disagreement on the selection decisions between the two researchers (AA & TWLW), a third researcher (SSMN) was consulted for consensus.
Similarly, the data was extracted by one of the researchers (AA); however, it was verified by the other two researchers (TWLW & SSMN). The data extracted include information on the sociodemographic and clinical characteristics of the study participants such as the study authors, participants mean age, time since stroke, sample size, type of stroke and side affected; and the mean scores on the outcomes of interest (primary and secondary outcomes).
The primary outcomes are upper limb function (level of motor impairment, motor function, real world arm use and manual dexterity), lower limb function (walking speed, walking endurance, number of steps, cadence and functional mobility), neurophysiological changes such cortical activation or electrical activity of the muscles, muscle strength, trunk impairment, muscle thickness, spasticity, balance, range of motion, disability and cognitive function. The secondary outcomes are adverse events and caregiver stress.
Since we extracted sufficient information from the studies, no additional information was sought from the authors of the included studies. However, in case of any missing or unreported data, it was designated as ‘not reported.’
Risks of bias and methodological quality assessments
We used Cochrane Risks of Bias Assessment tool and PEDro scale to assess the risks of bias and methodological quality of the included studies. Both the tool and the scale are known to be valid and reliable [43, 44].
The Cochrane Risk of Bias Assessment tool assesses bias due to the selection of participants, blinding of participants and personnel and outcome assessors, attrition and reporting. The result of the assessment is presented in a risk of bias graph.
The PEDro scale consists of 11 items that assess external and internal validity of a study. The external validity is assessed using the first item; whereas, the internal validity is assessed using the remaining 10 items [44]. In addition, a two-point scale, 0 (no) to 1 (yes) is used to rate the responses to the items that assess the internal validity. In this regard, since the scale has 10 items, the possible scores for methodological quality of a study will range between 0 and 10. When the total score ranges between zero and three; or four and five; or six and ten, the methodological quality is said to be low, moderate or high respectively. [45–47] The result of the assessment is presented in a table.
All assessments were carried out independently by two of the researchers (AA & TWLW); however, any disagreements arising from the assessments were managed by consulting the other researcher (SSMN).
Qualitative and quantitative syntheses of the extracted data
In the qualitative synthesis, a summary of the characteristics, risk of bias and methodological quality, and findings of the included studies was carried out. In the quantitative synthesis, a random effect model meta-analysis was carried out.
In the meta-analysis, the data used were the study sample size, the group mean and standard deviation of the scores on the outcomes of interest at post intervention and follow-up. However, when a study provided median scores and interquartile range on the outcomes of interest, the formulae, mean = [where a = the smallest value (minimum), b = the largest value (maximum), and m = median]; and standard deviation = [where IQR = interquartile range] were used to determine the mean and the standard deviation respectively [48]. Furthermore, percentage of variation across the studies due to heterogeneity (I2) was deemed significant when the value is between 50 and 90% at p < 0.05.
Eligibility for inclusion in the syntheses was determined using a table of characteristics of the included studies to check which studies assessed similar outcomes. The meta-analysis was carried out using RevMan version 5.4.1; [49] and all the results of the meta-analyses were visually displayed using forest plots. In addition, sensitivity analyses of the findings of the included studies were carried out based on the period of the outcomes’ assessments (post intervention and follow-up). In addition, an adapted body of evidence matrix of the Australian National Health and Medical Research Council's (NHMRC) was used to interpret the findings of the study [50].
Result
The qualitative synthesis
Selection of the studies
The search provided a total of 11,380 studies. Following screening of the studies, only 14 studies were eligible for inclusion [51–64]. However, two other studies seemed to be eligible for inclusion, but they were excluded following careful scrutiny. [65, 66]
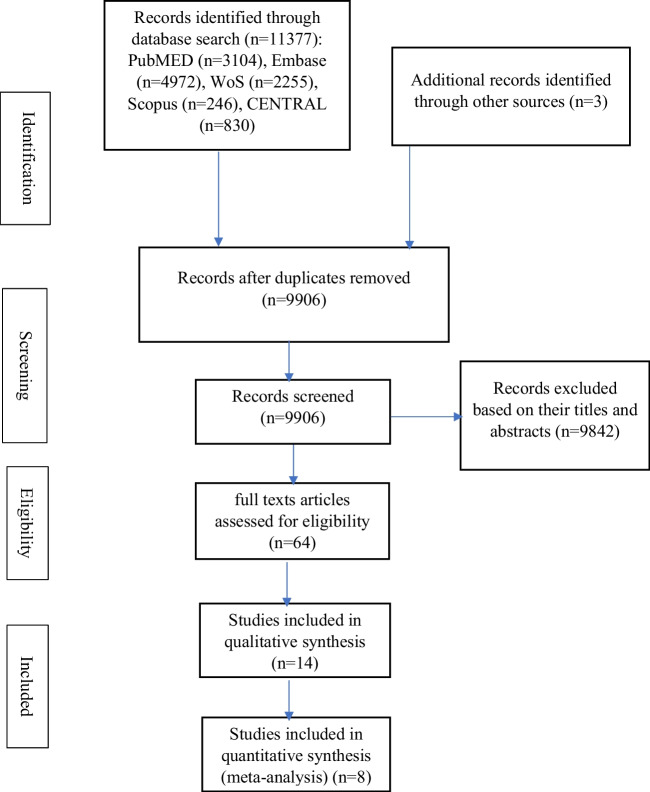
Among the included studies, two of them contain two experimental and two control groups each [54, 55]; whereas, in one study, there were two experimental groups and one control group [52]. Figure 1 provides the details of the literature search and the process of selection of the eligible studies.
Fig. 1.
The study flowchart showing the process of selection of the included studies
Characteristics of the included studies
In total, the number of participants in the included studies was 558. In addition, although two studies did not provide information on sex [51, 55]; 291 and 139 of the participants in the included studies are men and women respectively. Furthermore, only one study included participants in the acute and subacute stages of stroke [59]; all the other studies included participants who were in the chronic stage.
In addition, information on types of stroke was provided in only seven studies [51, 52, 57, 59–61, 63]. In these studies, 212 and 63 participants had ischaemic and haemorraghic stroke respectively. Furthermore, only nine studies provided information on the side affected [53–55, 57, 58, 60, 61, 63, 64]. In these studies, there were 204 and 136 participants who had left and right sided hemiplegia respectively. Similarly, only two studies provided information on handedness before the stroke, wherein one and 43 participants were left and right handed respectively [57, 61].
The inclusion criteria used in the studies include mild to moderate impairment in motor ability [51–53, 55–59, 61, 62, 64]; ability to walk several meters independently [51, 54, 57]; no joint deformity [51]; tolerance for electrical stimulation [51]; impaired sitting balance [60]; no significant cognitive impairment [54–57, 59, 60, 62, 64]; and no significant spasticity [54, 57].
The exclusion criteria used in the studies include presence of severe joint deformity [57, 62, 64]; a debilitating medical or any chronic condition [51, 52, 54–61, 64]; use of chemotherapy, use of anti-spasticity medication or a medication that can impair neuromuscular performance [51, 58, 64]; pregnancy or lactation [59, 64]; having a pacemaker or other implants [51, 56, 57, 59, 60, 62, 64, 67]; excessive pain [57, 58, 61, 62, 64]; presence of aphasia or dysphasia [52, 54]; having severe sensory deficit or neglect [60]; skin infection [57, 59]; hearing or visual impairment [52]; left-handedness before the stroke [61]; and contraindication to stimulation [58].
The result showed that, home-based neurostimulation is feasible and improves outcomes such as level of motor impairment, motor function, real-world arm use, manual dexterity, walking speed and endurance, functional mobility, joint range of motion, cortical activity, cognitive function and spasticity. Further details on the study participants, intervention protocols including intensity for the experimental and the control groups, and the outcomes assessed are presented in Table 1.
Table 1.
Characteristics of the included studies
| References | N | Stroke duration | Mean age (years) | Intervention | Outcomes | Findings | Adverse events |
|---|---|---|---|---|---|---|---|
| Alon et al. [51] | N=19; experimental (n=10, females=no information); control (n=9, females=no information) | (experimental = 4.05±2.9; control =4.3±3.3) years | 67 ± 6.8years; (experimental = 62.7±11.3; control =62.6±8.2) |
FES Experimental= received self-administered stimulation using multi-segment hybrid orthosis-stimulation system . Upper limb- electrodes were placed to ensure accurate stimulation of extensor digitorum, extensor pollicis brevis, flexor digitorum superficialis, flexor pollicis longus, and thenar muscles groups . Lower limb -3 electrodes were positioned over the peroneus longus and tibialis anterior; while 2 electrodes were placed over the 2 heads of gastrocnemius muscle. An alternating current (AC) was delivered at a carrier frequency of 11 KHz for 60 mins two times per day. Control= performed exercise for the distal and upper limb, and foot-leg starting with 10 mins and progressing to 60 mins. Both groups received functional exercises for 60 mins two times per day Interventions in both groups lasted for 3 months in both groups |
Gross motor function (BBT), manual dexterity (JHFT), walking speed (10MWT), cadence (10MWT) and number of steps (10MWT) | All outcomes improved better in the experimental group post intervention. | Two participants in the experimental group experienced a temporary and minor skin irritation that resolved after 2 to 3 days |
| Kimberly et al. [53] | N=16; experimental (n=8, females=3); control (n=8, females=2) | 35.5±25.1 months; (experimental = 68.13±39.62; control =22.00±16.06) | 60.1±14.5 years years; (experimental = 62.75±7.36; control =53.75±8.53) |
NMES Experimental=subjects were instructed to use the electrical stimulator, which delivers 200 μs rectangular biphasic, constant current at 50 Hz, 6 hours per day, for 10 days over the course of 3 weeks. Control= Sham electrical stimulation for the same period |
Gross motor function (BBT), quality and quantity of use of the limb, manual dexterity (JHFT), muscle strength (load cell), finger movement control (tracking), and changes in cortical activation (fMRI). |
All functional performance outcomes improved in the experimental group. On the other hand, in the control group, only muscle strength improved. However, there was no significant improvement in finger tracking performance and cortical activation in either group. |
Fatigue in one patient |
| Gabr et al. [64] | N=12; experimental (n=8, females=4); control (n=4, females=1) | 52.75 ± 39.82 months; (experimental = 68.13±39.62; control =22.00±16.06) | 59.7 ± 8.53 years; (experimental = 62.75±7.36; control =53.75±8.53) |
NMES Experiment: Participants used electromyography-triggered neuromuscular stimulation (ETMS) at a pulse width, between 100 and 400 μs to stimulate forearm muscles in addition to home exercise, twice every weekday in 35-min increments during an eight-week period Control: performed home exercises comprising of supination/pronation exercises; flexion and extension of the individual fingers; wrist extension and flexion exercises; elbow flexion and extension exercises; and shoulder adduction and abduction exercises. Participants switched groups after 8 weeks. |
Compliance (home-use diary), range of motion (goniometry) level of motor impairment (UEFMA), motor function (ARAT) | Range of motion improved in both groups. However, only stimulation used in the experimental group improved level of motor impairment | Not reported |
| Ng et al. [54] | N=80; Control (n=20, females=3); TENS (n=19, females=2); Placebo+TRT (n=20, females=3); TENS+TRT (n=21, females=5); | Control (5.2±2.9 years); TENS 6.2±4.1); Placebo+TRT (4.7±4.1); TENS+TRT (5.0±3.0); | Control (57.3±8.6 years); TENS 56.4±9.1); Placebo+TRT (57.1±7.8); TENS+TRT (58.4±7.1); |
TENS There are 4 groups in this study. Two of the groups are considered as experimental groups (TENS and TENS+TRT), and they received 60 minutes of TENS (100 Hz, 0.2-ms square pulses, 2 to 3 times sensory threshold) alone, or followed by 60 minutes of TRT comprising of 4 weightbearing and stepping exercises using wooden blocks of 2.5 or 5 cm in height respectively, 5 days a week for 4 weeks. The other two groups are considered as control (placebo+ TENS and control), which received sham TENS and TRT or no treatment at all respectively for the same period as in the experimental group |
Ankle flexor spasticity (composite spasticity scale), peak torques of maximum isometric voluntary contraction of ankle dorsiflexors and plantar flexors (load cell) and gait velocity (GAITRite) Compliance- phone call and logbook |
Significant increase in gait velocity, functional mobility and walking endurance in the TENS and TENS+TRT group compared to the other groups | Not reported |
| Hara et al. [62] | N=20; experimental (n=10, females=2); control (n=10, females=4) | (experimental = 13 months±; control =13 months±) | (experimental = 56.0 years; control =60.5 years) |
FES Experimental=received a train of biphasic rectangular electric impulses with a pulse width of 50 ms of functional electrical stimulation (FES) to finger and wrist extensors in addition to standard therapy for 1 hour (starting from 30 ins in the first 5 days) per day for 5 months to finger and wrist extensors. Control= received standard therapy alone for the same period. |
Motor impairment (SIAS), ROM (goniometer), spasticity (MAS), gross motor function (10-CMT)), manual dexterity (9-HPT)) and muscle activity (EMG) |
Significant improvement in active wrist and finger extension and shoulder flexion in the experimental group compared to the control Spasticity decreased in wrist and finger flexors in the experimental group Significant improvement in level of motor impairment, muscle activity, gross motor function and manual dexterity in the experimental group |
Not reported |
| Ng et al. [55] | N=109; Control (n=29, females=no information); TENS (n=28, females==no information); Placebo+TRT (n=27, females==no information); TENS+TRT (n=25, females==no information); | 4.7±3.4 years | 56.6±7.9 years |
TENS There are 4 groups in this study. Two of the groups are considered as experimental groups (TENS and TENS+TRT), and they received 60 minutes of TENS (100 Hz, 0.2ms square pulses, at 4 lower limb acupuncture sites recommended in Chinses Medicine Literature alone, or followed by 60 minutes of TRT, respectively, 5 days a week for 4 weeks. The other two groups are considered as control (placebo+ TENS and control), which received sham TENS and TRT or no treatment at all respectively for the same period as in the experimental group |
Ankle flexor spasticity (Composite Spasticity Scale), peak torques of maximum isometric voluntary contraction of ankle dorsiflexors and plantarflexors (load cell), gait velocity (GAITRite), walking endurance (6MWT)) and functional mobility (TUG) compliance- phone call and logbook |
Significant iincrease in gait velocity, functional mobility and walking endurance in the TENS and TENS+TRT group compared to the other groups. | Not reported |
| Sullivan et al. [58] | N=38; experimental (n=18, females=13); control (n=20, females=4) |
7.2 ± SD (1–29) (experimental = 7.7 ± SD (1–29) years±; control =6.6 ± SD (3–14) years |
60.6 ± SD (37–88) (experimental = 61.6 ± SD (37–88); control =59.5 ± SD (41–85) |
NMES All subjects were instructed to exercise twice daily for 30 minutes, 5 days/week for four weeks. During practice, subjects in the experimental group received electrical stimulation with the following current parameters: symmetrical biphasic waveform, pulse duration 250 microseconds, amplitude at sensory threshold, frequency 35 Hz, and a duty cycle of 10 seconds ON: 10 seconds OFF The control group received sham electrical stimulation. |
Level of motor impairment (UEFMA), motor function (AMAT), real world arm use (MAL), tolerance for Eeectrical stimulation (PTTES), stereognosis (Nottingham stereognosis assessment), quality of life (SIS) and spasticity (Tardieu scale) | There was no significant difference between groups in the outcomes of interest. | Not reported |
| dos Santos-Fontes et al [61] | N=20; experimental (n=10, females=5); control (n=10, females=4) | (experimental = 3.8 ± 4.5; control =3.3 ± 2.1) years | (experimental = 52.2 ± 11.1; control =59.1 ± 11.1) |
NMES Experimental=2 hours, daily biphasic square-wave electrical nerve stimulation at a frequency of 31 Hz, immediately before motor training for 4 weeks Control= sham stimulation, 2 hours daily before motor training for 4 weeks. Both groups performed two blocks of the following tasks: writing, turning cards, picking small objects, picking beans with a spoon, and stacking checkers daily for four weeks |
Feasibility-compliance (weekly phone call) and safety (presence of adverse events), and manual dexterity (JHFT) |
Participants in both groups reported good compliance with the stimulation. However, the participants in the experimental had significantly better compliance. Only 1 participant in the control group reported nocebo effect In addition, manual dexterity improved more significantly in the experimental than the control group |
Only 1 participant in the control group reported nocebo effect |
| Chan [60] | N=37; TENS+TRTT (n=12, females=4); Placebo + TRTT (n=13, females=3); control (n=12, females=2) |
44.2 ± 28.3 months TENS+TRT (43.9 ± 28.4); Placebo + TRT (41.8 ± 28.7); control (47.3 ± 29.8) |
57.8 ± 9.4 years TENS+TRT (58.2 ± 10.7); Placebo + TRT (56.3 ± 7.4); control (59.3 ± 10.4) |
TENS The experimental group is the TENS+TRTT; while the control groups are two (Placebo + TRTT and control). The experimental group received high-frequency TENS (frequency 100 Hz; pulse width 0.2 ms) to the abdominal muscles simultaneously with the TRTT at home for 60 mins per day, 5 times a week for 6 weeks. under the instruction of a physical therapist. For the two control groups, the placebo-TENS + TRTT received sham TENS+ TRTT; while the control group did receive any active treatment except health education. |
The isometric peak trunk flexion torque and extension torque was measured using a Cybex NORM isokinetic dynamometer, dynamic balance (functional reach test), trunk control (trunk impairment scale) |
The experimental groups improved significantly better the control in all outcomes of interest at all periods post intervention and at follow-up. However, TENS+TRT group demonstrated greater and earlier improvement than placebo TENS+TRTT group. | Not reported |
| Chen [59] | N=54; experimental (n=27, females=9); control (n=27, females=12) | Days; (experimental = 24.96 ± 5.62; control =26.85 ± 4.68) | (experimental = 66.52 ± 12.08; control =66.15 ± 12.33) |
NMES Experimental (at home- tele-supervised) and control (conventional in the clinic) Both groups received electromyography-triggered neuromuscular stimulation of ECRL and tibialis anterior muscle of hemiplegic side limbs the for 20 minutes, twice in a working day for 12 weeks, a total of 60 sessions. The stimulation parameters used were: stimulus duration for 5 seconds, intermittent time for 2 seconds, pulse width of 0.2 seconds, frequency of 50 Hz, stimulus intensity of between 8 to 45 mA. Both groups also performed physical exercises comprising of Bobath and Neuromuscular facilitation concepts for 1 hour, twice in a working day for 12 weeks, a total of 60 sessions |
Disability and ADL (MBI), balance (BBS), caregiver stress (CSI), muscle contraction condition (EMG), | There was no significant difference between groups in all outcomes of interest post intervention and at follow-up. | Not reported |
| Minami [56] | N=8; experimental (n=5, females=2); control (n=3, females=1) | 8.8±5.6 years | 63.1±10.9 years (experimental = 64.0± 13.4; control = 61.7± 10.4) |
FES All participants received occupational therapy consisting of 40-min sessions that include range of motion training, strength training, and exercise involving occupational activities with the aim of maintaining and recovering daily life Experimental=10–20 min per session, twice per day, at least 3 time a week of purposeful activity-based electrical stimulation therapy (PA-EST) at 36 Hz for 3 months. Control= stretching/ exercise for the same period Cross over took place after the intervention period |
Level of motor impairment (UEFMA), real world arm use (MAL), goal attainment (GAS-light), and muscle thickness of the upper limb and abdominal muscles. | Level of motor impairment, motor function, real world arm use and goal attainment improved significantly in the experimental group compared to the control. | None |
| Choudhury [52] | N=95; experimental paired (n=32, females=8); ; experimental random (n=32, females=7); control (n=31, females=13) | months; (experimental paired= 55 (142); experimental random= 43 (94); control =30 (29)) | (experimental paired= 51 (12.1); experimental random= 53 (9.9); control =53 (10.6)) |
TENS There are to experimental groups (paired and random stimulation groups). In the paired stimulation group, each shock was given 12 ms before the click. For the random stimulation group, the click and shock occurred independently at random, with the same interval distribution as in the paired stimulation group. For the experimental groups, electrodes were placed over the forearm extensor muscles for the transcutaneous electrical stimulation using a single 0.15-ms pulse, over 4 weeks for at least 4 h/d at home from the first day of assessment. Control= received standard care |
Motor function (ARAT), ROM (goniometer), muscle torque/ strength, spasticity (MAS), grip strength (dynamometer), maximum muscle contraction (a custom device) | Only experimental paired improved motor function post intervention | Not reported |
| Prathum et al. [57] | N=24; experimental (n=12, females=4); control (n=12, females=4) | 15.92 ± 2.06 months (experimental = 16.33 ± 3.30; control = 15.50 ± 2.60) | 57.75 ± 2.45 years; (experimental = 56.83 ± 3.58; control = 58.67 ± 3.70) |
tDCs Experimental= 1-h home-based exercise after 20-min dual-tDCS at 2-mA, thrice a week for 4 weeks Control= sham 1-h home-based exercise after 20-min dual-tDCS at 2-mA, thrice a week for 4 weeks The exercise comprises of (1) stretching of the elbow flexor, wrist flexor, and shoulder flexor muscles (hold for 2 min/muscle group); (2) active exercise involving elbow extension, shoulder flexion, forearm pronation, and supination (10 times/ set/ direction, 3 sets/direction/session); (3) reach-to-grasp exercise in different directions (50 times/direction, 3 directions/session) |
Level of motor impairment (UEFMA & LEFMA), motor function (WMFT), functional mobility (TUG), walking speed (6MWT), lower-limb functional muscle strength (five times sit to stand test), muscle strength (handheld dynamometer), and grip strength (handgrip dynamometer) |
Experimental group had significantly better improvement in level of motor impairment and motor function than the control. | Mild tingling, itching, headache and burning sensation in the experimental group. |
| Ko et al. [63] | N=26; experimental (n=12, females=8); control (n=14, females=6) | (≥6 months after onset) | 59.42±11.32 years; (experimental = 61.25±12.85; control = 57.86±10.04) |
tDCs Experimental=tDCS (constant current of 2 mA) self -application, 5 d/wk for 4 weeks for 30 minutes per session. Control= sham tDCS (constant current of 2 mA) self-application, 5 d/wk for 4 weeks for 30 minutes per session. Both groups received 30 mins cognitive therapy comprising of various tasks based on memory and attention areas. |
Cognitive function (K-MoCA) Dementia (Korean version of the Dementia Rating Scale-2), lexical retrieval abilities and aphasia (Korean-Boston Naming Test), visual attention and task switching (Trail Making Test), determining the ability of an individual to inhibit a response deemed inappropriate (Go/No Go), and verbal fluency (Controlled Oral Word Association Test) Feasibility (completion rate and protocol adherence) |
No significant difference between groups in any of the outcomes post intervention and at follow-up. Feasibility: Adherence rate was 98.4%. |
No serious adverse effects were detected |
Key: BBT=box and block test, JHFT=Jebsen Taylor hand function test, 10MWT= ten-meter walk test, fMRI =function magnetic resonance imaging, NMES=Neuromuscular electrical stimulation, FES=Functional electrical stimulation, Key: UEFMA=upper extremity Fugl Meyer motor assessment, ARAT=Action research arm test, SIAS= stroke impairment assessment set, ROM=range of motion, MAS =modified Ashworth scale, 10-CMT = Ten-Cup-Moving Test, 9-HPT= Nine-Hole-Peg Test, EMG=Electromyography, TRT=tasks-related training, NMES=Neuromuscular electrical stimulation, TENS=Transcutaneus electrical stimulation, Key: TENS=transcutaneous electrical nerve stimulation, TRT=tasks-related training, 6MWT =six-minute walk test, TUG = timed-up and go test, UEFMA = upper extremity Fugl Meyer motor assessment, AMAT = arm mobility test, MAL=motor activity log, PTTES= Perceptual Threshold Test – Electrical Stimulation, ARAT=Action research arm test, SIS= stroke impact scale, JHFT= Jebsen Taylor hand function test, NMES=Neuromuscular electrical stimulation, TENS=Transcutaneus electrical stimulation. Key: TRTT =task-related trunk training, ECRL= extensor carpi radialis longus, MBI=modified Barthel index, BBS=Berg balance scale, MRS= modified Rankin scale, CSI=caregiver strain index, EMG=electromyography, UEFMA = upper extremity Fugl Meyer motor assessment, MAL=motor activity log, GAS-light=goal attainment scale-light, NMES=Neuromuscular electrical stimulation, TENS=Transcutaneus electrical stimulation, FES=functional electrical stimulation. Key: ARAT=Action research arm test, ROM=range of motion, MAS =modified Ashworth scale tDCss=transcortical direct current stimulation, UEFMA= upper extremity Fugl Meyer motor assessment, LEFMA= lower extremity Fugl Meyer motor assessment, WMFT=Wolf motor function test, TUG = timed-up and go test, 6MWT =six-minute walk test, TUG = timed-up and go test, K-MoCA=Korean-Montreal cognitive assessment, TENS=Transcutaneus electrical stimulation
Risks of bias and methodological quality of the included studies
Risks of bias
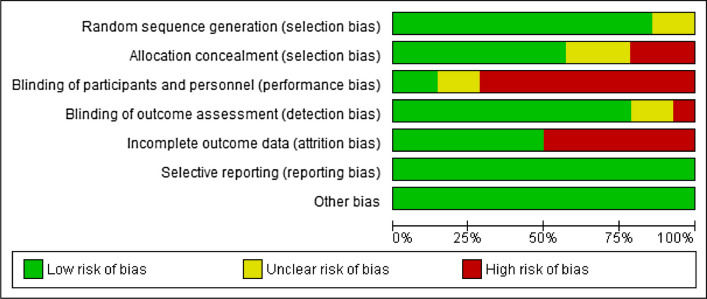
Some of the studies have high risk of bias in allocation concealment (selection bias) [51, 53, 61]; blinding of participants and personnel (performance bias) [51–55, 58–62]; blinding of outcome assessment (detection bias) [51]; and incomplete outcome data (attrition bias) [54–58, 62, 63].
Similarly, some of the studies have unclear risks of bias in random sequence generation (selection) [51, 57]; allocation concealment (selection bias [54–56]; blinding of participants and personnel (performance bias) [56, 63]]; and blinding of outcome assessment (detection bias) [56, 63]. See Fig. 2 and Supplementary File 1 for the risk of bias graph and summary table of the included studies respectively.
Fig. 2.
Risks of bias graph of the included studies
Methodological quality
The methodological quality of the included studies is either moderate [56, 63, 64]; or high [51–55, 57–62]. See Table 2 for the met.
Table 2.
Methodological quality of the included studies
| Study | Eligibility criteria specified | Random allocation | Concealed allocation | Comparable subjects | Blind subjects | Blind therapists | Blind assessors | Adequate follow-up | Intention to treat analysis | Between group comparison | Point estimation and variability | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alon et al. [51] | Yes | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6/10 |
| Gabr et al. [64] | Yes | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 4/10 |
| Kimberly et al. [53] | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 8/10 |
| Hara et al. [62] | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7/10 |
| Ng et al. [54] | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6/10 |
| Sullivan al. [58] | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6/110 |
| dos Santos-Fontes [61] | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8/10 |
| Ng et al. [55] | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6/10 |
| Chan [60] | ||||||||||||
| Chen [59] | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8/10 |
| Minami [56] | Yes | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 4/10 |
| Choudry [52] | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 7/10 |
| Prathum [57] | Yes | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 8/10 |
| Ko [63] | Yes | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 5/10 |
The quantitative synthesis
Only eight studies were used in the meta-analysis for the post intervention outcomes [51–58]. Out of this number, five studies were used for the meta-analysis of upper limb function [52–54, 56–58]; and four studies were used for the meta-analysis of lower limb function [51, 54, 55, 57]. However, for the upper limb, only two studies were included for the meta-analysis at follow-up [52, 57].
For one of the studies, the scores for the outcome of interest were given in median and interquartile range [52]. Consequently, the formulae already explained in the method sections were used to convert them to mean and standard deviation respectively [48].
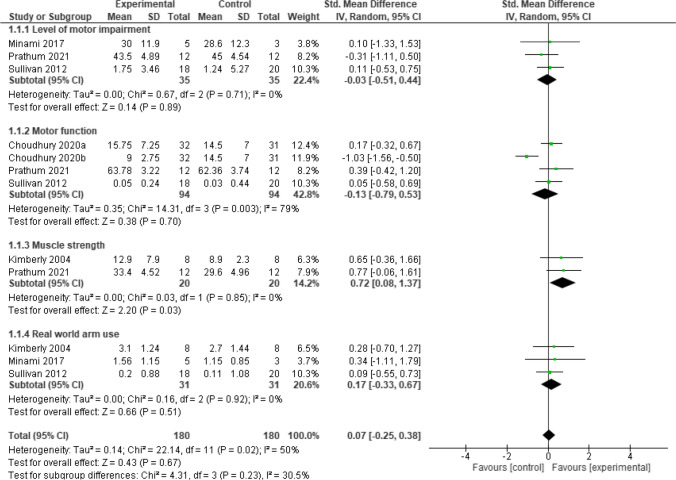
Upper limb function
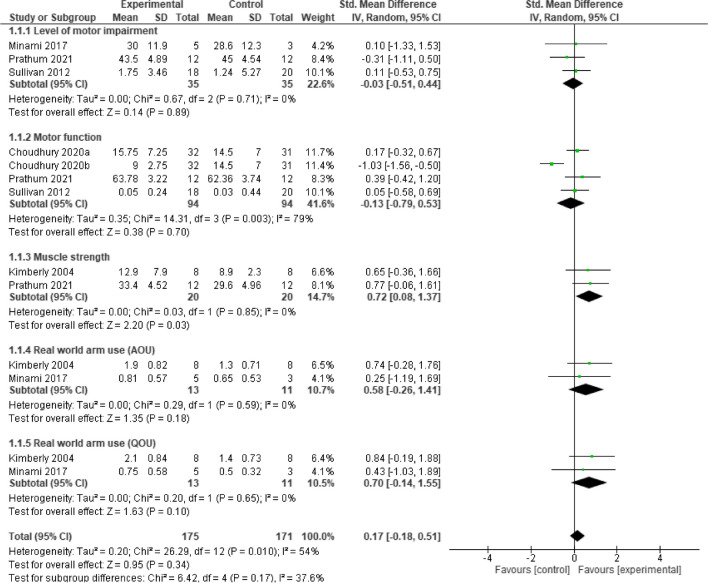
Post intervention, the result showed that, home-based neurostimulation compared to the control, was only superior at improving muscle strength (SMD = 0.72, 95% CI = 0.08 to 1.32, p = 0.03). In addition, there was no significant heterogeneity between the included studies (I2 = 0%, p = 0.85). See Fig. 3 for the forest plot detailing the result. Furthermore, sensitivity analysis carried out by considering motor activity log (MAL) amount of use (AOU) subscale and MAL quality of movement (QOU) separately, did not reveal any significant difference between groups for the two subscales respectively, (SMD = 0.58, 95% CI = -0.26 to 1.41, p = 0.18) and (SMD = 0.70, 95% CI = -0.14 to 1.55, p = 0.10). See Fig. 4 for the details of the result.
Fig. 3.
A forest plot showing effects of neuromodulation on upper limb function post intervention
Fig. 4.
A forest plot showing effects of neuromodulation on upper limb function post intervention (sensitivity analyses)
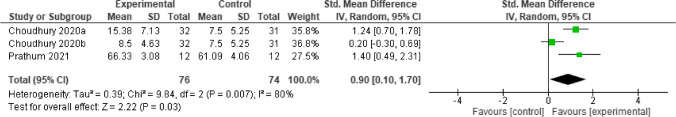
At follow-up, only two studies assessed one outcome, motor function [52, 57]. The result showed that, home-based neurostimulation was superior to the control at improving motor function (SMD = 0.9, 95% CI = 0.10 to 1.70, p = 0.03). However, there was significant heterogeneity between the included studies (I2 = 80%, p = 0.007). See Fig. 5 for the forest plot detailing the result.
Fig. 5.
A forest plot showing effects of neuromodulation on upper limb function at follow-up
Lower limb function
Post intervention, the result showed that, home-based neurostimulation compared to the control, was only superior at improving functional mobility (SMD = -0.39, 95% CI = -0.65 to 0.14, p = 0.003), and walking endurance (SMD = 0.33, 95% CI = 0.08 to 0.59, p = 0.01). In addition, there was no significant heterogeneity between the included studies (I2 = 0%, p = 0.49) and (I2 = 0%, p = 0.92), respectively. See Fig. 6 for the forest plot detailing the result.
Fig. 6.
A forest plot showing effects of neuromodulation on lower limb function post intervention
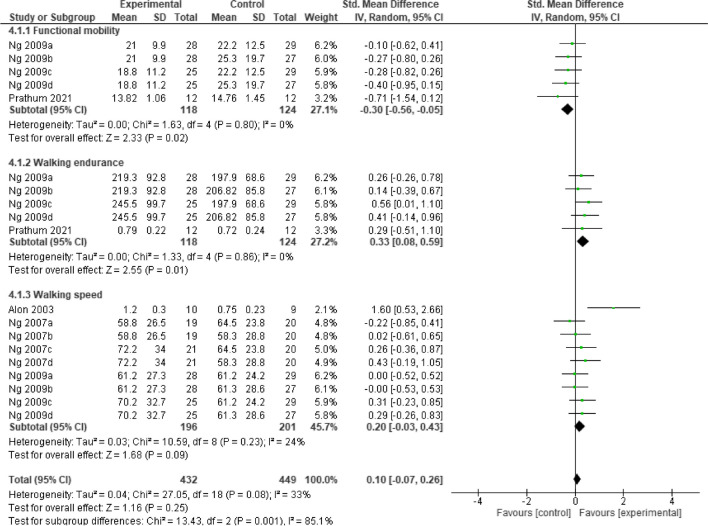
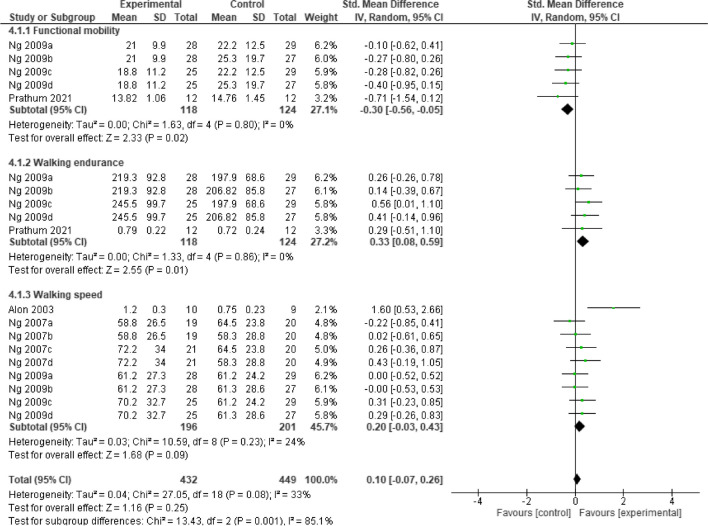
At follow-up, the result showed that, still home-based neurostimulation compared to the control, maintained its superiority at improving functional mobility (SMD = -0.30, 95% CI = -0.56 to -0.05, p = 0.02), and walking endurance (SMD = 0.33, 95% CI = 0.08 to 0.59, p = 0.01). In addition, there was no significant heterogeneity between the included studies (I2 = 0%, p = 0.80) and (I2 = 0%, p = 0.86), respectively. See Fig. 7 for the forest plot detailing the result.
Fig. 7.
A forest plot showing effects of neuromodulation on lower limb function at follow-up
Interpretation of the evidence
It is difficult to be very sure of the evidence since there is variation between studies especially in the use of outcome measures, intensity of rehabilitation used and the types of neurostimulation and devices used. However, the evidence seems excellent, appreciably consistent, with satisfactory clinical impact and excellent generalizability and applicability, and as such, it may be used in clinical practice. See Table 3 for more details.
Table 3.
Body of evidence matrix
| Component | Grade | Comments |
|---|---|---|
| 1. Evidence |
A-Excellent Several Level II evidence |
Quantity: a total of 14 studies Participants: 558 patients with stroke Level II studies: 14 |
| 2. Consistency | C-satisfactory | There is significant heterogeneity between studies, especially in terms of the outcomes assessed and the outcome measures used, devices used for the stimulation and the sample size used |
| 3. Clinical impact | C-Satisfactory | Only one study reported effect size (Prathum et al. [57] |
| 4. Generalizability | A-Excellent | The studied population is the same as the target population (patients with stroke) |
| 5. Applicability | A-Excellent | The evidence is applicable globally since the studies were carried out in 9 different countries (Brazil, China, Japan, Hong Kong (China), Israel, Japan, South Korea, Thailand, and USA) in four different continents |
| Recommendation | Home-based neuroelectric modulation may be used in practice |
Discussion
The aim of this study is to determine the effects of home-based neurostimulation on outcomes after stroke. The result showed that, home-based neurostimulation is feasible and is superior to the control at improving upper limb muscle strength post intervention, and motor function at follow-up. In addition, it is also superior to the control at improving functional mobility and walking endurance both post intervention and at follow-up. This is not surprising since home-based rehabilitation has been reported to be feasible and effective at improving outcomes such as motor function following the use of various interventions such as the constraint induced movement, mirror therapy and therapeutic exercise [29, 68].
Concerning the findings of this study, improvement in muscle strength (an important aspect of motor function), motor function, walking endurance and functional mobility is important for independence in carrying out ADL [69, 70]. For instance, the upper limb is used for eating, washing and grooming oneself. In addition, independence in carrying out ADL is important for overall well-being and good quality of life [71, 72]. Furthermore, it is important for return to work, and by extension economic opportunities and sustainable development [73].
Similarly, impairment in lower limb function may result in sedentary lifestyle and its attendant muscle weakness [74, 75]. Sedentary life is a risk factor for various non-communicable diseases such diabetes, heart disease and depression [76, 77]. Moreover, impaired limb motor function is a significant risk factor for not returning to work after stroke [78]. Thus, finding an intervention such as home-based neurostimulation that will help improve the above outcomes and eventually the patients’ quality of life is important. In particular, home-based neurostimulation, being a home-based intervention may be more cost-effective and acceptable to patients.
However, home-based neurostimulation also has its own limitations like any other home-based rehabilitation. These include problems with the ability of patients and/ or their caregivers to operate the devices and frustration with the use of the devices [79]. In addition, it may be difficult to administer some neurostimulation techniques such as TMS without medical supervision. Furthermore, the cost and size of devices can limit the home-based procedure. However, to help solve some of those problems, we suggest maintaining a regular communication between patients, their caregivers and the clinicians. This can be achieved by using tele-supervision such as via video conference, where the clinicians can observe what the patients are doing [80, 81]. Similarly, community-based rehabilitation can also be used where the clinicians supervise the sessions in person at the patient’s home [82]. In addition, a hybrid model of rehabilitation can be adopted, where in-clinic and home-based sessions are combined to help supplement each other.
Furthermore, the types of neurostimulation and the devices used differ between studies. In particular, five out of the eight studies included in the meta-analysis used neuromuscular electrical stimulation [53, 58, 59, 61, 64]; four studies used TENS which is a weaker form of neuromuscular electrical stimulation [52, 54, 55, 60]; three studies used FES [51, 56, 62]; and two used tDCS [57, 63]. These techniques of neurostimulation have different mechanisms through which they modulate the nervous system. The neuromuscular electrical stimulation is used to stimulate the peripheral nervous which will indirectly help to modulate the central nervous system (CNS) [16, 83]. The tDCS works to directly modulate the CNS [84, 85]. Thus, the findings of this study may only be limited to the effects of neuromuscular electrical stimulation. However, the findings are still very significant since neuromuscular electrical stimulation is easier to administer compared to other forms of neurostimulation such as the tDCS and TMS.
Similarly, in most of the studies, neurostimulation was combined with other rehabilitation techniques such as functional exercises. Thus, it is difficult to confidently say the effects were exclusively due to the neurostimulation. However, the findings are still a significant milestone since providing rehabilitation at home has so may merits such as the opportunity to increase the intensity of rehabilitation [37]. Therefore, further well controlled studies should be carried out to determine the effects of different forms of home-based neurostimulation on outcomes after stroke. In addition, the process of our review is limited in terms of the language in which the included studies were published. Therefore, the findings of the review should be interpreted bearing all the above discussed limitations in mind.
Conclusion
Home-based neuromuscular electrical stimulation, TENS, FES, and tDCS are feasible and effective at improving many outcomes after stroke. These findings represent a significant milestone since providing rehabilitation at home has so many merits such as the opportunity to increase the intensity of rehabilitation. However, further well controlled studies should be carried out to determine the effects of home-based neurostimulation on outcomes after stroke.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix 1
The Search strategy used in PUBMED, Embase, Web of Science (WoS) and Scopus:
((((((((((((((((((((((((((((Stroke) OR (Ischaemic stroke) OR (Haemorrhagic stroke)) OR (Brain infarction)) OR (Cerebrovascular accident)) AND (Electrical stimulation)) OR (Transcutaneous electrical nerve stimulation)) OR (deep brain stimulation)) OR (Transcranial direct current stimulation)) OR (Anodal Transcranial direct current stimulation)) OR (Cathodal Transcranial direct current stimulation)) OR (Repetitive Transcranial electrical stimulation)) OR (Transcranial electrostimulation)) OR (Transcranial random noise stimulation)) OR (Transcranial alternating current stimulation)) OR (Percutaneous electric nerve stimulation)) OR (Percutaneous electrical nerve stimulation)) OR (Percutaneous electrical neuromodulation)) OR (Percutaneous neuromodulation therapy)) OR (Transcranial magnetic stimulation)) OR (Transcranial magnetic stimulation, repetitive)) OR (Transcranial magnetic stimulation, paired pulse)) OR (Transcranial magnetic stimulation, single pulse)) AND (Telerehabilitation)) OR (Tele-rehabilitation)) OR (Virtual rehabilitation)) OR (Remote rehabilitation)) OR (Telemedicine).
The Search strategy used in CENTRAL:
Stroke AND Electrical stimulation OR Transcranial direct current stimulation OR Transcranial magnetic stimulation AND Telerehabilitation.
Funding
Open access funding provided by The Hong Kong Polytechnic University This work was supported by the research funding of the Research Centre for Chinese Medicine Innovation of The Hong Kong Polytechnic University (Ref. No. P0041139) awarded to Prof Shamay Ng and her team; and PolyU Distinguished Postdoctoral Fellowship Scheme (P0035217).
Data Availability
All the data used in this study are included in the manuscript.
Declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Ethical approval and Informed consent
Not applicable
Footnotes
The original online version of this article was revised: The original version of this article unfortunately contained a mistake in Table 1 alignment. The errors pertain to the information under the column 'Findings' in page 3 of Table 1.The studies involved are Ng et al. [2009] and Sullivan et al. [2012].
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/6/2024
A Correction to this paper has been published: 10.1007/s10072-024-07711-5
References
- 1.Ting WK, Fadul FA, Fecteau S, Ethier C (2021) Neurostimulation for Stroke Rehabilitation. Front Neurosci 15:649459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cury RG, Pavese N, Aziz TZ, Krauss JK, Moro E (2022) Gaps and roadmap of novel neuromodulation targets for treatment of gait in Parkinson’s disease. NPJ Parkinsons Dis 8(1):8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abboud H, Hill E, Siddiqui J, Serra A, Walter B (2017) Neuromodulation in multiple sclerosis. Mult Scler 23(13):1663–1676 [DOI] [PubMed] [Google Scholar]
- 4.Rahnama’i MS (2020) Neuromodulation for functional bladder disorders in patients with multiple sclerosis. Mult Scler 26(11):1274–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson J, Abdul-Rahim AH, Kimberley TJ (2024) Neurostimulation for treatment of post-stroke impairments. Nat Rev Neurol 20(5):259–268 [DOI] [PubMed] [Google Scholar]
- 6.Hodics T, Dromerick A, Pezzullo J, Kowalske K, Cohen L (2022) Transcranial Direct Current Stimulation (tDCS) Enhanced Stroke Recovery and Cortical Reorganization (S10.004). Neurology 98(18_supplement):2892 [Google Scholar]
- 7.Hong Z, Sui M, Zhuang Z et al (2018) Effectiveness of Neuromuscular Electrical Stimulation on Lower Limbs of Patients With Hemiplegia After Chronic Stroke: A Systematic Review. Arch Phys Med Rehabil 99(5):1011-1022.e1011 [DOI] [PubMed] [Google Scholar]
- 8.Osman H, Siu R, Makowski NS, Knutson JS, Cunningham DA (2024) Neurostimulation After Stroke. Phys Med Rehabil Clin N Am 35(2):369–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen QR, Hu MT, Feng W, Li KP, Wang W (2022) Narrative Review of Noninvasive Brain Stimulation in Stroke Rehabilitation. Med Sci Monit 28:e938298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin HE, Kim M, Lee D et al (2022) Therapeutic Effects of Functional Electrical Stimulation on Physical Performance and Muscle Strength in Post-stroke Older Adults: A Review. Ann Geriatr Med Res 26(1):16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yavari F, Nitsche MA, Ekhtiari H (2017) Transcranial Electric Stimulation for Precision Medicine: A Spatiomechanistic Framework. Front Hum Neurosci 11:159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao S, Mehta AS, Zhao M (2020) Biomedical applications of electrical stimulation. Cell Mol Life Sci 77(14):2681–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klomjai W, Katz R, Lackmy-Vallée A (2015) Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med 58(4):208–213 [DOI] [PubMed] [Google Scholar]
- 14.Luan S, Williams I, Nikolic K, Constandinou TG (2014) Neuromodulation: present and emerging methods. Front Neuroeng 7:27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krames ES, Peckham PH, Rezai A, Aboelsaad F (2009) What is neuromodulation? Neuromodulation 1:3–8. 10.1016/B978-0-12-374248-3.00002-1 [Google Scholar]
- 16.Bao SC, Khan A, Song R, Kai-Yu TR (2020) Rewiring the Lesioned Brain: Electrical Stimulation for Post-Stroke Motor Restoration. J Stroke 22(1):47–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson J, Liu CY, Francisco GE et al (2021) Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet 397(10284):1545–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias GJB, Namasivayam AA, Lozano AM (2018) Deep brain stimulation for stroke: Current uses and future directions. Brain Stimul Jan-Feb 11(1):3–28 [DOI] [PubMed] [Google Scholar]
- 19.Takeda K, Tanino G, Miyasaka H (2017) Review of devices used in neuromuscular electrical stimulation for stroke rehabilitation. Med Devices (Auckl) 10:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pipatsrisawat S, Klaphajone J, Kitisak K, Sungkarat S, Wivatvongvana P (2022) Effects of combining two techniques of non-invasive brain stimulation in subacute stroke patients: a pilot study. BMC Neurol 22(1):98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuhmann T, Duecker F, Middag-van Spanje M et al (2022) Transcranial alternating brain stimulation at alpha frequency reduces hemispatial neglect symptoms in stroke patients. Int J Clin Health Psychol Sep-Dec 22(3):100326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matt E, Kaindl L, Tenk S et al (2022) First evidence of long-term effects of transcranial pulse stimulation (TPS) on the human brain. J Transl Med 20(1):26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnao V, Riolo M, Carduccio F et al (2019) Effects of transcranial random noise stimulation combined with Graded Repetitive Arm Supplementary Program (GRASP) on motor rehabilitation of the upper limb in sub-acute ischemic stroke patients: a randomized pilot study. J Neural Transm 126(12):1701–1706 [DOI] [PubMed] [Google Scholar]
- 24.Herrera-Murillo MA, Treviño M, Manjarrez E (2022) Random noise stimulation in the treatment of patients with neurological disorders. Neural Regen Res 17(12):2557–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machado VG, Brun ABS, Manffra EF (2023) Effects of the radio electric asymmetric conveyer (REAC) on motor disorders: An integrative review. Front Med Technol 5:1122245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Wang D, Pan H et al (2022) Non-invasive Vagus Nerve Stimulation in Cerebral Stroke: Current Status and Future Perspectives. Front Neurosci 16:820665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everaert DG, Stein RB, Abrams GM et al (2013) Effect of a foot-drop stimulator and ankle-foot orthosis on walking performance after stroke: a multicenter randomized controlled trial. Neurorehabil Neural Repair 27(7):579–591 [DOI] [PubMed] [Google Scholar]
- 28.Eraifej J, Clark W, France B, Desando S, Moore D (2017) Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: a systematic review and meta-analysis. Syst Rev 6(1):40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toh SFM, Chia PF, Fong KNK (2022) Effectiveness of home-based upper limb rehabilitation in stroke survivors: A systematic review and meta-analysis. Front Neurol 13:964196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rezaei M, Sharifi A, Vaccaro AR, Rahimi-Movaghar V (2019) Home-Based Rehabilitation Programs: Promising Field to Maximize Function of Patients with Traumatic Spinal Cord Injury. Asian J Neurosurg Jul-Sep 14(3):634–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim JH, Lee HS, Song CS (2021) Home-based rehabilitation programs on postural balance, walking, and quality of life in patients with stroke: A single-blind, randomized controlled trial. Med (Baltimore) 100(35):e27154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelaw AY, Janakiraman B, Gebremeskel BF, Ravichandran H (2020) Effectiveness of Home-based rehabilitation in improving physical function of persons with Stroke and other physical disability: A systematic review of randomized controlled trials. J Stroke Cerebrovasc Dis 29(6):104800 [DOI] [PubMed] [Google Scholar]
- 33.Truijen S, Abdullahi A, Bijsterbosch D et al (2022) Effect of home-based virtual reality training and telerehabilitation on balance in individuals with Parkinson disease, multiple sclerosis, and stroke: a systematic review and meta-analysis. Neurol Sci 43(5):2995–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zampolini M, Todeschini E, Bernabeu Guitart M et al (2008) Tele-rehabilitation: present and future. Ann Ist Super Sanita 44(2):125–134 [PubMed] [Google Scholar]
- 35.Azhari A, Parsa A (2020) Covid-19 Outbreak Highlights: Importance of Home-Based Rehabilitation in Orthopedic Surgery. Arch Bone Jt Surg 8(Suppl1):317–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vega-Ramírez FA, López-Liria R, Granados-Gámez G, Aguilar-Parra JM, Padilla-Góngora D (2017) Analysis of home-based rehabilitation in patients with motor impairment in primary care: a prospective observational study. BMC Geriatr 17(1):145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballester BR, Ward NS, Brander F, Maier M, Kelly K, Verschure P (2022) Relationship between intensity and recovery in post-stroke rehabilitation: a retrospective analysis. J Neurol Neurosurg Psychiatry 93(2):226–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Candio P, Violato M, Luengo-Fernandez R, Leal J (2022) Cost-effectiveness of home-based stroke rehabilitation across Europe: A modelling study. Health Policy 126(3):183–189 [DOI] [PubMed] [Google Scholar]
- 39.Allen L, John-Baptiste A, Meyer M et al (2019) Assessing the impact of a home-based stroke rehabilitation programme: a cost-effectiveness study. Disabil Rehabil 41(17):2060–2065 [DOI] [PubMed] [Google Scholar]
- 40.Briones-Claudett KH, Mónica HB, Briones-Zamora KH, Briones-Márquez DC, Icaza-Freire A, Grunauer M (2021) Telemedicine and Home-Based Treatment of COVID-19 in Resource-Limited Countries. Report of 3 Cases. Eurasian J Med 53(2):155–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nascimento LR, Gaviorno LF, de Souza BM, Gonçalves JV, Arêas F (2022) Home-based is as effective as centre-based rehabilitation for improving upper limb motor recovery and activity limitations after stroke: A systematic review with meta-analysis. Clin Rehabil 36(12):1565–1577 [DOI] [PubMed] [Google Scholar]
- 42.Yang JD, Liao CD, Huang SW et al (2019) Effectiveness of electrical stimulation therapy in improving arm function after stroke: a systematic review and a meta-analysis of randomised controlled trials. Clin Rehabil 33(8):1286–1297 [DOI] [PubMed] [Google Scholar]
- 43.Higgins JP, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 343:d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M (2003) Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 83(8):713–721 [PubMed] [Google Scholar]
- 45.Moseley AM, Herbert RD, Maher CG, Sherrington C, Elkins MR (2011) Reported quality of randomized controlled trials of physiotherapy interventions has improved over time. J Clin Epidemiol 64(6):594–601 [DOI] [PubMed] [Google Scholar]
- 46.Herbert R, Moseley A, Sherrington C (1998) PEDro: a database of randomised controlled trials in physiotherapy. Health Inf Manag 28(4):186–188 [DOI] [PubMed] [Google Scholar]
- 47.da Costa BR, Hilfiker R, Egger M (2013) PEDro’s bias: summary quality scores should not be used in meta-analysis. J Clin Epidemiol 66(1):75–77 [DOI] [PubMed] [Google Scholar]
- 48.Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Review Manager Web (RevMan Web) [computer program]. Version 5.4.1: The Cochrane Collaboration; 2020
- 50.Hillier S, Grimmer-Somers K, Merlin T et al (2011) FORM: an Australian method for formulating and grading recommendations in evidence-based clinical guidelines. BMC Med Res Methodol 11:23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alon G, Ring H (2003) Gait and Hand Function Enhancement Following Training with a Multi-Segment Hybrid-Orthosis Stimulation System in Stroke Patients. J Stroke Cerebrovasc Dis 12(5):209–216 [DOI] [PubMed] [Google Scholar]
- 52.Choudhury S, Singh R, Shobhana A et al (2020) A Novel Wearable Device for Motor Recovery of Hand Function in Chronic Stroke Survivors. Neurorehabil Neural Repair 34(7):600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey LL, Lojovich JM, Carey JR (2004) Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp Brain Res 154(4):450–460 [DOI] [PubMed] [Google Scholar]
- 54.Ng SS, Hui-Chan CW (2007) Transcutaneous electrical nerve stimulation combined with task-related training improves lower limb functions in subjects with chronic stroke. Stroke 38(11):2953–2959 [DOI] [PubMed] [Google Scholar]
- 55.Ng SS, Hui-Chan CW (2009) Does the use of TENS increase the effectiveness of exercise for improving walking after stroke? A randomized controlled clinical trial. Clin Rehabil 23(12):1093–1103 [DOI] [PubMed] [Google Scholar]
- 56.Minami S, Fukumoto Y, Kobayashi R, Aoki H, Aoyama T (2021) Effect of home-based rehabilitation of purposeful activity-based electrical stimulation therapy for chronic stroke survivors: a crossover randomized controlled trial. Restor Neurol Neurosci 39(3):173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prathum T, Piriyaprasarth P, Aneksan B et al (2022) Effects of home-based dual-hemispheric transcranial direct current stimulation combined with exercise on upper and lower limb motor performance in patients with chronic stroke. Disabil Rehabil 44(15):3868–3879 [DOI] [PubMed] [Google Scholar]
- 58.Sullivan JE, Hurley D, Hedman LD (2012) Afferent stimulation provided by glove electrode during task-specific arm exercise following stroke. Clin Rehabil 26(11):1010–1020 [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Jin W, Dong WS et al (2017) Effects of Home-based Telesupervising Rehabilitation on Physical Function for Stroke Survivors with Hemiplegia: A Randomized Controlled Trial. Am J Phys Med Rehabil 96(3):152–160 [DOI] [PubMed] [Google Scholar]
- 60.Chan BK, Ng SS, Ng GY (2015) A home-based program of transcutaneous electrical nerve stimulation and task-related trunk training improves trunk control in patients with stroke: a randomized controlled clinical trial. Neurorehabil Neural Repair 29(1):70–79 [DOI] [PubMed] [Google Scholar]
- 61.Dos Santos-Fontes RL, Ferreiro de Andrade KN, Sterr A, Conforto AB (2013) Home-based nerve stimulation to enhance effects of motor training in patients in the chronic phase after stroke: a proof-of-principle study. Neurorehabil Neural Repair 27(6):483–490 [DOI] [PubMed] [Google Scholar]
- 62.Hara Y, Ogawa S, Tsujiuchi K, Muraoka Y (2008) A home-based rehabilitation program for the hemiplegic upper extremity by power-assisted functional electrical stimulation. Disabil Rehabil 30(4):296–304 [DOI] [PubMed] [Google Scholar]
- 63.Ko MH, Yoon JY, Jo YJ et al (2022) Home-Based Transcranial Direct Current Stimulation to Enhance Cognition in Stroke: Randomized Controlled Trial. Stroke 53(10):2992–3001 [DOI] [PubMed] [Google Scholar]
- 64.Gabr U, Levine P, Page SJ (2005) Home-based electromyography-triggered stimulation in chronic stroke. Clin Rehabil 19(7):737–745 [DOI] [PubMed] [Google Scholar]
- 65.Kutlu M, Freeman C, Spraggs M (2017) Functional electrical stimulation for home-based upper-limb stroke rehabilitation. Curr Dir Biomed Eng 3(1):25–29 [Google Scholar]
- 66.Kutlu M, Freeman C, Hughes A-M, Spraggs M (2017) A Home-based FES System for Upper-limb Stroke Rehabilitation with Iterative Learning Control. IFAC-PapersOnLine 50(1):12089–12094 [Google Scholar]
- 67.Ko Y, Satoshi H, Masatoshi N et al (2018) Electrical Stimulation to the Infraspinatus on Hypertrophy and Strength of the Shoulder. Int J Sports Med 39(11):828–834 [DOI] [PubMed] [Google Scholar]
- 68.Barzel A, Ketels G, Stark A et al (2015) Home-based constraint-induced movement therapy for patients with upper limb dysfunction after stroke (HOMECIMT): a cluster-randomised, controlled trial. Lancet Neurol 14(9):893–902 [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto H, Takeda K, Koyama S et al (2020) Relationship between upper limb motor function and activities of daily living after removing the influence of lower limb motor function in subacute patients with stroke: A cross-sectional study. Hong Kong J Occup Ther 33(1):12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hiraga Y, Hayashi T (2022) Mediating Effect of Upper Limb Use on the Relationship Between Upper Limb Performance and Activities of Daily Living: A Longitudinal Mediation Analysis. Cureus 14(10):e30849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fatema Z, Sigamani A, Vikneswaran G, Manuel D (2022) Quality of life at 90 days after stroke and its correlation to activities of daily living’: A prospective cohort study. J Stroke Cerebrovasc Dis 31(11):106806 [DOI] [PubMed] [Google Scholar]
- 72.Kim K, Kim YM, Kim EK (2014) Correlation between the Activities of Daily Living of Stroke Patients in a Community Setting and Their Quality of Life. J Phys Ther Sci 26(3):417–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.GhanbariGhoshchi S, De Angelis S, Morone G et al (2020) Return to Work and Quality of Life after Stroke in Italy: A Study on the Efficacy of Technologically Assisted Neurorehabilitation. Int J Environ Res Public Health 17:14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azzollini V, Dalise S, Chisari C (2021) How Does Stroke Affect Skeletal Muscle? State of the Art and Rehabilitation Perspective. Front Neurol 12:797559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohammed M, Li J (2022) Stroke-Related Sarcopenia among Two Different Developing Countries with Diverse Ethnic Backgrounds (Cross-National Study in Egypt and China). Healthc (Basel). 10:11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ezeugwu VE, Garga N, Manns PJ (2017) Reducing sedentary behaviour after stroke: perspectives of ambulatory individuals with stroke. Disabil Rehabil 39(25):2551–2558 [DOI] [PubMed] [Google Scholar]
- 77.Park JH, Moon JH, Kim HJ, Kong MH, Oh YH (2020) Sedentary Lifestyle: Overview of Updated Evidence of Potential Health Risks. Korean J Fam Med 41(6):365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matérne M, Strandberg T, Lundqvist LO (2019) Risk Markers for Not Returning to Work Among Patients with Acquired Brain Injury: A Population-Based Register Study. J Occup Rehabil 29(4):728–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Velez M, Lugo-Agudelo LH, Patiño Lugo DF et al (2023) Factors that influence the provision of home-based rehabilitation services for people needing rehabilitation: a qualitative evidence synthesis. Cochrane Database Syst Rev 2(2):Cd014823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buick AR, Kowalczewski J, Carson RG, Prochazka A (2016) Tele-Supervised FES-Assisted Exercise for Hemiplegic Upper Limb. IEEE Trans Neural Syst Rehabil Eng 24(1):79–87 [DOI] [PubMed] [Google Scholar]
- 81.Nam C, Zhang B, Chow T et al (2021) Home-based self-help telerehabilitation of the upper limb assisted by an electromyography-driven wrist/hand exoneuromusculoskeleton after stroke. J Neuroeng Rehabil 18(1):137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park YJ, Lee CY (2016) Effects of community-based rehabilitation program on activities of daily living and cognition in elderly chronic stroke survivors. J Phys Ther Sci 28(11):3264–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsai SY, Schreiber JA, Adamczyk NS et al (2021) Improved Functional Outcome After Peripheral Nerve Stimulation of the Impaired Forelimb Post-stroke. Front Neurol 12:610434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G (2010) Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 75(24):2176–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsu S-P, Lu C-F, Lin B-F et al (2023) Effects of bihemispheric transcranial direct current stimulation on motor recovery in subacute stroke patients: a double-blind, randomized sham-controlled trial. J NeuroEngineering Rehabil 20(1):27 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data used in this study are included in the manuscript.