Abstract
Bletilla striata has been used in traditional Chinese medicine for thousands of years to treat a variety of health diseases. Currently, metabolic causes of differences in medicinal values are unknown, due to the lack of a large-scale and comprehensive investigation of metabolites in Bletilla species. In order to gain a better understanding of the major chemical constituents responsible for the medicinal value, this study aimed to explore the metabolomic differences among three Bletilla species (Bletilla striata: Bs, Bletilla ochracea: Bo and Bletilla formosana: Bf). There were 258 different metabolites between ‘Bo’ and ‘Bf’, the contents of 109 metabolites had higher abundance, while 149 metabolites showed less accumulation. There were 165 different metabolites between the ‘Bs’ and ‘Bf’, content of 72 metabolites was increased and content of 93 metabolites was decreased. There were 239 different metabolites between the ‘Bs’ and ‘Bo’, content of 145 metabolites was increased and content of 94 metabolites was decreased. In the Bo_vs_Bf, Bs_vs_Bf and Bs_vs_Bo groups, the major differential categories were flavonoids, phenolic acids, organic acids and alkaloids. Moreover, the differential metabolites were clustered into clear and distinct profiles via K-means analysis. In addition, the major differential categories were flavonoids, phenolic acids, organic acids and alkaloids. The ‘Flavonoid biosynthesis’ (ko00941) and ‘Phenylalanine metabolism’ (ko00360) pathways were significantly enriched in Bo_vs_Bf, Bs_vs_Bf and Bs_vs_Bo comparisons. These results clarify the metabolomics in different Bletilla species, as well as providing basis for the phamaceutical value of novel species of Bletilla.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74204-y.
Keywords: Bletilla striata, Bletilla ochracea, Bletilla formosana, Metabolites, Chinese traditional medicine
Subject terms: Plant molecular biology, Secondary metabolism
Introduction
Orchidaceae is a large flora with important research value, not only because their ornamental characteristics, but also due to their rich pharmacological activities of phytochemicals1. The genus Bletilla pertain to the Orchidaceae family, which comprise Bletilla striata, Bletilla ochracea, Bletilla formosana, Bletilla foliosa, and Bletilla chartacea (http://www.theplantlist.org/). Bletilla were mainly planted in Asia, North America and Europe2,3. Among the five species of Bletilla, B. striata (‘Bai Ji’ in Chinese), a perennial herb, has been widely used as a traditional Chinese medicine for thousands of years, which is an officially recognized medicinal herb in Traditional Chinese Medicine (TCM) (Chinese Pharmacopeia Commission, 2015)4,5. In the past 40 years, natural product chemists has explored the active ingredients of B. striata due to the application of B. striata in TCM6. The medicinal values of B. striata may be related to its multiple functional components. So far, hundreds of compounds have been identified from B. striata and classified into several categories, such as glycosides, biphenyls, phenanthrene, quinones, biphenyls, dihydrophenanthrene, anthocyanins, steroids, triterpenes, and phenolic acids7–9. In addition, it is reported that the chemicals extracted from pseudobulb of B. striata have a variety of therapeutic effects, including antibacterial, anti-inflammatory, immune regulation, hemostasis maintenance, wound healing, antiulcer, anti-oxidation, anti-fibrosis, anti-aging and anti-allergy7,10. China Food and Drug Administration has ratified four patent medicines including “Bai ji” Pill, “Bai ji” Capsule, “Bai ji” Syrup, and “Bai ji” Granule, with B. striata as a unique medicinal ingredient7. Zhang et al.11 described a type of B. striata polysaccharide (BSP) sponge, which forms layered porous channels through directional freezing technology, has high biocompatibility and degradation ability, and excellent hemostatic ability. Wang et al.12 pointed out that B. striata could exert a good therapeutic effect by reversing the levels of some biomarkers in the rats with lung injury caused by PM2.5. Although the bioactive substances extracted from B. striata have multiple functions, there is little research on the metabolites, especially the differences in B. striata and other species, which hinders further utilization of novel species of Bletilla. In folk culture, B. ochracea and B. formosana are often used as substitutes for B. striata, so a comparative metabolomics study of the three species is necessary.
Metabolomics aims to comprehensively analyze all metabolites in biological samples, and has great potential in elucidating plant metabolic processes13. In recent years, due to many improvements in instrumentation and analysis, the application and sensitivity of metabolomics have rapidly increased. Liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based widely targeted metabolome analysis is a rapid and reliable approach for detection of a wide range of plant metabolites. The development of this technology has made large-scale metabonomic studies of plant species that are rarely studied more common and accurate. For thousands of years, humans have relied on plant secondary metabolites as food, raw materials, and drugs. Many traditional Chinese medicine preparations are based on plant secondary metabolites to treat diseases14–16. There are a large number of secondary metabolites in B. striata including phenypropanoids, flavonoids, terpenoids, alkaloids, and phenolic acids17–19. However, most previous studies on Bletilla metabolites have focused on B. striata with very little comparative work to other medicinal or culinary species like B. ochracea, B. formosana. Therefore, in order to understand the contributions of different metabolite categories, coherent research is still needed to report large-scale identification and quantification of metabolites.
B. striata is representative medicinal plants in Bletilla. However, so far, research on the metabolomics of B. striata has been limited, and there has been no description of the metabolic process of different Bletilla species. In this study, the metabolomics of three Bletilla species were analyzed using LC-MS/MS, aiming at clarifying the metabolomics in different Bletilla species, as well as providing basis for the phamaceutical value of novel species of Bletilla (Fig. 1).
Fig. 1.

Phenotype of Bletilla striata, Bletilla ochracea and Bletilla formosana. (A) Bletilla striata, (B) Bletilla formosana, (C) Bletilla ochracea.
Results
Metabolic differences in the three Bletilla species
The three Bletilla species of ‘Bs’, ‘Bo’ and ‘Bf’ are representative plants in Bletilla, which possess flowers with different colors, suggesting possible differences in accumulated metabolites. The total ion current (TIC) chromatograms of one quality control sample (QC) were shown in Fig. 2, which illustrated the summed intensity of all ions in the mass spectrum at different time points. Furthermore, a total of 887 metabolites were included in the LC-MS untargeted metabolomics analysis between Bletilla species, of which 400 were detected in positive ion mode and 487 were detected in negative ion mode.
Fig. 2.
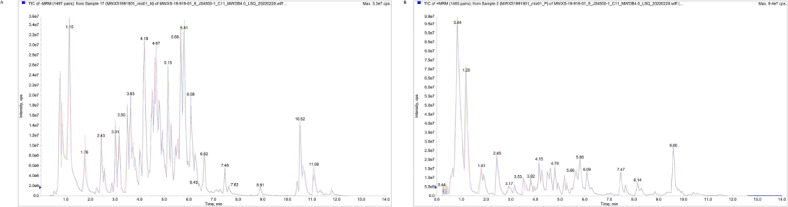
Total ion current (TIC) chromatograms and multiple reaction monitoring (MRM) metabolite detection multi-peak diagram (XIC of multi-substance extraction), abscess is Retention time (Rt) of metabolite detection, ordinate is ion current intensity of ion detection (cps, count per second) obtained from Bletilla samples. (A. sample in negative mode, B. sample in positive mode).
Widely targeted metabolome analyses from three Bletilla species
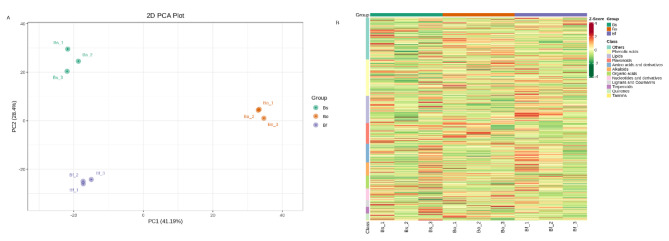
PCA analysis was utilized on the 887 metabolites. The plots of PCA scores showed obvious separation between the three Bletilla species (Fig. 3A). This analysis revealed three distinct groups associated with ‘Bs’, ‘Bo’ and ‘Bf’, respectively. Thus, together PCA and cluster analysis suggested that these three cultivars had distinct metabolite profiles. Three distinct groups of ‘Bs’, ‘Bo’ and ‘Bf’ were identified using hierarchical clustering analysis and heatmap based on differences in metabolite (Fig. 3B). The results of principal component analysis (PCA) and hierarchical clustering analysis clearly showed that each species had different identifiable metabolite profiles.
Fig. 3.
The PCA and heatmap clustering between ‘Bs’, ‘Bo’ and ‘Bf’. (A) PCA analysis of all metabolites identified from ‘Bs’, ‘Bo’ and ‘Bf’. (B) Heatmap clustering showing correlation among ‘Bs’, ‘Bo’ and ‘Bf’. The color indicates the level of accumulation of each metabolite, from low (green) to high (red).
Differentially accumulated metabolites among three Bletilla species
To characterize differences between three Bletilla species, metabolites were screened using Fold Change ≥ 2 or Fold Change ≤ 0.5, and VIP ≥ 1. The results from these comparisons were illustrated using Venn diagrams (Fig. 4A–C) and volcano plots (Fig. 4D–F). A Venn diagram and volcano plots were utilized to depict the shared differently accumulated metabolites among Bo vs. Bf, Bs vs. Bf, and Bs vs. Bo. There were 258 different metabolites between ‘Bo’ and ‘Bf’, the contents of 109 metabolites had higher abundance, while 149 metabolites showed less accumulation. There were165 different metabolites between the ‘Bs’ and ‘Bf’, content of 72 metabolites was increased and content of 93 metabolites was decreased. There were 239 different metabolites between the ‘Bs’ and ‘Bo’, content of 145 metabolites was increased and content of 94 metabolites was decreased (Supplementary Table 1). The differential levels of secondary metabolites in Bo vs. Bf, Bs vs. Bf, and Bs vs. Bo groups of Bletilla were shown in Fig. 4G–I. As the color changes, there are significant differences in the levels and types of metabolites between different Bletilla species. For example, the category of phenolic acids and flavonoid were significantly different among Bo vs. Bf, Bs vs. Bf, and Bs vs. Bo.
Fig. 4.
Differential metabolite analyses in Bo_vs_Bf, Bs_vs_Bf and Bs_vs_Bo comparisons (A–C): Venn diagram showing the overlapping and species-specific metabolites in Bo_vs_Bf, Bs_vs_Bf and Bs_vs_Bo comparisons. (D–F): Volcano plots for differentially accumulated metabolite levels between Bo_vs_Bf, Bs_vs_Bf and Bs_vs_Bo comparisons, red dots represent upregulated differentially accumulated metabolites, green dots represent downregulated differentially accumulated metabolites. (G–I): The heatmap of differentially accumulated metabolite between Bo_vs_Bf, Bs_vs_Bf and Bs_vs_Bo comparisons. Red color represented upregulated differentially accumulated metabolites, green color represented downregulated differentially accumulated metabolites.
In this study, compounds such as polysaccharides, benzenes, phenols, flavonoids, amino acids, alkaloids, terpenes were detected in all three species. There were similar metabolites categories in the three species. Polysaccharides have been regarded as the principal active components responsible for the various biological functions in Bletilla. Polysaccharides with similar structural features have been reported from Bo and Bs20. We compared the polysaccharides including D-Mannose and D-Glucose etc. among these three Bletilla species. As shown in supplementary Table 2, there were 65 polysaccharides in the Bo vs. Bf, in which 51 polysaccharides were not significantly accumulated, 14 polysaccharides were significantly accumulated. Likewise, there were 65 polysaccharides in the Bo vs. Bs, in which 58 polysaccharides were not significantly accumulated, 7 polysaccharides were significantly accumulated. Furthermore, there were 65 polysaccharides in the Bo vs. Bs, in which 63 polysaccharides were not significantly accumulated, 2 polysaccharides were significantly accumulated. Among which, D-Mannose and D-Glucose was increased in Bo vs. Bf, and not significantly accumulated in Bs vs. Bo and Bs vs. Bf.
Metabolic pathway analysis
We further analyzed the functional involvement of the differential metabolites in different pathways by mapping them to the KEGG database. In Supplementary figure 1A–C, the results showed that ‘Flavonoid biosynthesis’(ko00941) and ‘Phenylalanine metabolism’(ko00360) pathways were significantly enriched in Bo_vs_Bf, Bs_vs_Bf and Bs_vs_Bo comparisons. Meanwhile, Butanoate metabolism (ko00650), Caffeine metabolism (ko00232), Pentose phosphate pathway(ko00030), and Arginine and proline metabolism (ko00330) pathways were highly enriched in Bo_vs_Bf comparison. Plant hormone signal transduction (ko04075), Flavone and flavonol biosynthesis (ko00944), Biotin metabolism(ko00780) and Carotenoid biosynthesis pathways were dominant in Bs_vs_Bf comparison. Tryptophan metabolism (ko00380), Phenylpropanoid biosynthesis (ko00940), Vitamin B6 metabolism (ko00750) and Ubiquinone and other terpenoid-quinone biosynthesis (ko00130) were enriched in Bs_vs_Bo comparison. Based on KEGG database, the ‘Flavonoid biosynthesis’ (ko00941) and ‘Phenylalanine metabolism’(ko00360) pathways were further mapped (Supplementary Fig. 1D–E).
The most significantly different metabolites in three Bletilla species
The twenty compounds that were the most significantly different (VIP > 1, and top 20) in all comparisons were obtained (Supplementary Fig. 2). In the Bo_vs_Bf, Bs_vs_Bf and Bs_vs_Bo groups, the major differential categories were flavonoids, phenolic acids, organic acids and alkaloids. In Table 1, in the Bo_vs_Bf group, the category of flavonoids includes 3-Hydroxy-3′-methoxyflavone, Isorhamnetin-3,7-O-diglucoside, Luteolin-3′-O-glucoside, 6-Hydroxykaempferol-7,6-O-Diglucoside, Okanin-4′-O-glucosyl-O-glucoside, Phellamurin and Phellodendroside. The category of phenolic acids 3-O-p-Coumaroylquinic acid, Gallic acid-4-O-(6′′′-feruloyl)sophoroside and Phenyl acetate. In the Bs_vs_Bf group, the category of flavonoids includes Phellodendroside, Orientin-2′′-O-galactoside, Isorhamnetin-3,7-O-diglucoside, Luteolin-3′-O-glucoside, 6,7,8-Tetrahydroxy-5-methoxyflavone and 3-Hydroxy-3′-methoxyflavone. The category of phenolic acids includes α-Hydroxycinnamic Acid*, 2-Amino-3-methoxybenzoic acid, Plantamajoside, 1,2-O-Diferuloylglycerol* and 3-O-p-Coumaroylquinic acid. In the Bs_vs_Bo group, the category of flavonoids includes Orientin-2′′-O-galactoside, 6-Hydroxykaempferol-7,6-O-Diglucoside, Okanin-4′-O-glucosyl-O-glucoside and Phellamurin. The category of phenolic acids includes α-Hydroxycinnamic Acid*, 2-Amino-3-methoxybenzoic acid, parishin J, Tyrosol; 4-Hydroxyphenylethanol and Phenyl acetate.
Table 1.
Top 20 identification of differentially abundant metabolites in the three comparisons.
| Group | Compounds | VIP | Fold_Change | Log2 FC | Regulation |
|---|---|---|---|---|---|
| Phellodendroside | 1.48 | 1.09E+04 | 13.41 | Up | |
| Erianthridin | 1.48 | 9.55E +03 | 13.22 | Up | |
| 3-Methylxanthine | 1.48 | 8.66E +03 | 13.08 | Up | |
| 4,8-Dihydroxyquinoline-2-carboxylic acid | 1.45 | 8.37E +03 | 13.03 | Up | |
| 3-hydroxy-1,2-dimethoxy-anthraquinone | 1.48 | 6.34E +03 | 12.63 | Up | |
| Phenyl acetate | 1.47 | 4.59E +03 | 12.16 | Up | |
| D-Threose | 1.47 | 3.96E +03 | 11.95 | Up | |
| Phellamurin | 1.48 | 3.36E +03 | 11.71 | Up | |
| Gallic acid-4-O-(6′′′-feruloyl) sophoroside | 1.48 | 3.33E +03 | 11.70 | Up | |
| Bo vs. Bf | 1-Oleoyl-Sn-Glycerol | 1.48 | 3.29E +03 | 11.68 | Up |
| Okanin-4′-O-glucosyl-O-glucoside | 1.48 | 2.35E-04 | -12.06 | Down | |
| Scopine | 1.48 | 2.32E-04 | -12.07 | Down | |
| 6-Hydroxykaempferol-7,6-O-Diglucoside | 1.47 | 1.88E-04 | -12.38 | Down | |
| 10,16-Dihydroxypalmitic acid | 1.48 | 1.51E-04 | -12.69 | Down | |
| 3-O-p-Coumaroylquinic acid | 1.46 | 1.47E-04 | -12.73 | Down | |
| Luteolin-3′-O-glucoside | 1.48 | 1.06E-04 | -13.20 | Down | |
| Isorhamnetin-3,7-O-diglucoside | 1.48 | 1.00E-04 | -13.28 | Down | |
| 3-Hydroxy-3′-methoxyflavone | 1.48 | 5.96E-05 | -14.03 | Down | |
| 4-Acetamidobutyric acid | 1.48 | 3.12E-05 | -14.97 | Down | |
| L-Histidine | 1.48 | 3.19E-06 | -18.26 | Down | |
| 4-Phenylbutyric acid | – | 1.52E +04 | 13.89 | Up | |
| α-Hydroxycinnamic Acid* | – | 1.44E +04 | 13.81 | Up | |
| Phellodendroside | – | 1.09E +04 | 13.41 | Up | |
| 1,3-O-Dicaffeoylglycerol | – | 6.54E +03 | 12.67 | Up | |
| Nobilomethylene | – | 4.98E +03 | 12.28 | Up | |
| 2-Amino-3-methoxybenzoic acid | – | 4.88E +03 | 12.25 | Up | |
| Plantamajoside | – | 4.59E +03 | 12.16 | Up | |
| Fraxetin (7,8-Dihydroxy-6-methoxycoumarin) | – | 4.42E +03 | 12.11 | Up | |
| Orientin-2′′-O-galactoside | – | 3.80E +03 | 11.89 | Up | |
| Bs vs. Bf | 1,2-O-Diferuloylglycerol* | – | 3.73E +03 | 11.86 | Up |
| Isorhamnetin-3,7-O-diglucoside | – | 6.33E-04 | −10.62 | Down | |
| 3-Epiursolic acid | – | 5.26E-04 | −10.62 | Down | |
| 3-O-p-Coumaroylquinic acid | – | 3.66E-04 | −11.41 | Down | |
| Luteolin-3′-O-glucoside | – | 3.19E-04 | −11.61 | Down | |
| Xanthosine | – | 2.24E-04 | −12.12 | Down | |
| 6,7,8-Tetrahydroxy-5-methoxyflavone | – | 1.69E-04 | −12.53 | Down | |
| 3-Hydroxy-3′-methoxyflavone | – | 1.65E-04 | −12.56 | Down | |
| Scopine | – | 1.41E-04 | −12.79 | Down | |
| 4-Acetamidobutyric acid | – | 6.28E-05 | −13.96 | Down | |
| L-Histidine | – | 1.14E-06 | −19.74 | Down | |
| Orientin-2′′-O-galactoside | – | 6.03E +04 | 15.88 | Up | |
| α-Hydroxycinnamic Acid* | – | 3.88E +04 | 15.24 | Up | |
| 4-Phenylbutyric acid | – | 2.45E +04 | 14.58 | Up | |
| 2-Amino-3-methoxybenzoic acid | – | 8.46E +03 | 13.04 | Up | |
| parishin J | – | 8.02E +03 | 12.97 | Up | |
| 1,3-O-Dicaffeoylglycerol | – | 6.20E +03 | 12.60 | Up | |
| 6-Hydroxykaempferol-7,6-O-Diglucoside | – | 5.32E +03 | 12.38 | Up | |
| Tyrosol; 4-Hydroxyphenylethanol | – | 5.11E +03 | 12.32 | Up | |
| Okanin-4′-O-glucosyl-O-glucoside | – | 4.26E +03 | 12.06 | Up | |
| N7-Methylguanosine | – | 3.79E +03 | 11.89 | Up | |
| Bs vs. Bo | Phellamurin | – | 3.07E-04 | −11.67 | Down |
| Glycyl-tryptophan | – | 2.82E-04 | −11.79 | Down | |
| 1-Oleoyl-Sn-Glycerol | – | 2.54E-04 | −11.94 | Down | |
| D-Threose | – | 2.48E-04 | −11.97 | Down | |
| 4,8-Dihydroxyquinoline-2-carboxylic acid | – | 2.09E-04 | −12.23 | Down | |
| Phenyl acetate | – | 1.99E-04 | −12.29 | Down | |
| Erianthridin | – | 1.63E-04 | −12.58 | Down | |
| Feruloylcholine glucoside | – | 1.34E-04 | −12.86 | Down | |
| 3-hydroxy-1,2-dimethoxy-anthraquinone | – | 1.29E-04 | −12.92 | Down | |
| 3-Methylxanthine | – | 8.65E-05 | −13.50 | Down |
Analysis of the changing trends of differential metabolites and pathway enrichment of the clusters
To explore the variation trends of differential metabolites in each species, K-means clustering analysis, which enabled us to observe metabolites clusters, was performed among ‘Bs’, ‘Bo’ and ‘Bf’. The different metabolites were screened out in the three comparison groups by pairwise comparison and the standardized and centralized data were listed in Supplementary Table 1. As was shown in Fig. 5A, these differential metabolites were divided into 6 subclasses, of which, cluster 1 and 4 included 52 and 125 differential metabolites, respectively, which all showed high expression in ‘Bo’. Cluster 3 and 5 included 29 and 57 differential metabolites, espectively, which all showed high expression in ‘Bf’. Cluster 2 and 6 included 59 and 58 differential metabolites, respectively, which all showed high expression in ‘Bs’.
Fig. 5.
K-means analysis of all differential metabolites and pathway enrichment of cluster 1, 2, 3, 4, 5 and 6. (A) K-means clustering analysis based on differential metabolites from all comparison groups. (B) Subclass 1, 2, 3, 4, 5 and 6 were enriched by KEGG.
In Fig. 5B, the KEGG analysis of cluster 1, 2, 3, 4, 5 and 6 was further conducted. We observed that cluster 1 was highly enriched in carotenoid biosynthesis, flavonoid biosynthesis. Cluster 2 was highly enriched in phenylpropanoid biosynthesis, biosynthesis of amino acids, arginine biosynthesis and D-amino acid metabolism. Meanwhile, plant hormone signal transduction, penicillin and cephalosporin biosynthesis and flavone and flavonol biosynthesis were prominent in clusters 3. Additionally, cluster 4 was highly enriched in phenylpropanoid biosynthesis, flavonoid biosynthesis and biosynthesis of various plant secondary metabolites. Cluster 5 was highly enriched in sulfur metabolism, propanoate metabolism and oxidative phosphorylation. Cluster 6 was highly enriched in tryptophan metabolism, indole alkaloid biosynthesis, biosynthesis of secondary metabolites and biosynthesis of amino acids.
Discussion
Metabolomics is currently an excellent and advanced discipline for studying endogenous metabolites in biological systems. Metabolomics has a global metabolic spectrum analysis that closely matches the holistic view of traditional Chinese medicine. The use of omics techniques is crucial for understanding and interpreting TCM’s efficacy21. Bletilla species has been used in traditional Chinese medicine for thousands of years to treat a variety of diseases, such as gastrointestinal diseases, peptic ulcers, lung diseases, and traumatic bleeding22. The study and chemical composition of Bletilla can be traced back to the late 19th century. Research has shown that the chemical components of Bletilla are mainly glycosides, biphenyls, phenanthrenes, quinones, biphenyls, dihydrophenanthrenes, anthocyanins, steroids, triterpenes, phenolic acids, etc10. Among the three species, B. striata was more comprehensively explored in terms of the phytochemistry and biological activities as compared to the other species. The medicinal value of B. striata has been well documented10,23–25 while the lack of metabolomic information for other species has restricted its potential in phamaceutical value. Previous work using metabolome analyses have reported that 109 differential metabolites were identified between ‘MBS’ and ‘BS’ (two B. striata varieties, ‘MBS’ is from Zhangjiajie, China and ‘BS’ is from Zhuzhou, China). The contents of melittin, salicin, leukoside, 5-deoxyakiwikone, rutin, hesperidin, anthocyanin 3-galactoside, anthocyanin 7-glucoside and astragaloside in ‘BS’ and ‘MBS’ varieties were significantly different26.
Based on the metabolic fingerprints from LC-MS in either ES1 + or ESI- mode, Bo, Bf, and Bs were clearly separated by PCA analysis and hierarchical clustering analysis (Fig. 2). Furthermore, a total of 662 differential metabolites were identified in the comparison groups. There were 258 different metabolites between ‘Bo’ and ‘Bf’, the contents of 109 metabolites had higher abundance, while 149 metabolites showed less accumulation. There were 165 different metabolites between the ‘Bs’ and ‘Bf’, content of 72 metabolites was increased and content of 93 metabolites was decreased. There were 239 different metabolites between the ‘Bs’ and ‘Bo’, content of 145 metabolites was increased and content of 94 metabolites was decreased. In the Bo_vs_Bf, Bs_vs_Bf and Bs_vs_Bo groups, the major differential categories were flavonoids, phenolic acids, organic acids and alkaloids. In the Bo_vs_Bf group, the category of flavonoids includes downregulated 3-Hydroxy-3′-methoxyflavone, Isorhamnetin-3,7-O-diglucoside, Luteolin-3′-O-glucoside, 6-Hydroxykaempferol-7,6-O-Diglucoside, Okanin-4′-O-glucosyl-O-glucoside, upregulated Phellamurin and Phellodendroside. In the Bs_vs_Bf group, the category of flavonoids includes upregulated Phellodendroside, Orientin-2′′-O-galactoside, downregulated Isorhamnetin-3,7-O-diglucoside, Luteolin-3′-O-glucoside, 6,7,8-Tetrahydroxy-5-methoxyflavone and 3-Hydroxy-3′-methoxyflavone. In the Bs_vs_Bo group, the category of flavonoids includes upregulated Orientin-2′′-O-galactoside, 6-Hydroxykaempferol-7,6-O-Diglucoside, Okanin-4′-O-glucosyl-O- glucoside, downregulated Phellamurin. Till date, only nine flavonoids have been identified from the genus Bletilla. In 2005, Lin et al.27 obtained six flavonoids from the whole plant of B. formosana for the first time. In 2017, Bae et al.28 isolated three C-methylated flavan-3-ols, including two new compounds, flavonols A and B from the tubers of B. striata. Flavonoids are the most abundant polyphenols in plants and are an important type of plant secondary metabolites. Plant flavonoids have many pharmacological effects such as antioxidant29, anti-inflammatory30, and anticancer31. The biosynthetic pathway of flavonoids in Arabidopsis32 seeds and Ginkgo biloba33 leaves was explored. However, the biosynthetic process of flavonoids in B. striata is poorly understood.
In order to better understand the regulatory patterns of metabolites identified in each cultivar, K-means analysis was used to cluster the metabolites. We identified that cluster 1, 3 and 4 were associated with ‘Flavonoid biosynthesis’. The ‘Flavonoid biosynthesis’ pathway is relatively conserved and the most sufficiently explored biosynthetic pathway for plant secondary metabolites34. Our results showed that ‘Flavonoid biosynthesis’ (ko00941) and ‘Phenylalanine metabolism’ (ko00360) pathways were significantly enriched in Bo_vs_Bf, Bs_vs_Bf and Bs_vs_Bo comparisons. The synthesis of flavonoids originates from the phenylpropane metabolic pathway, and the production of the intermediate of this pathway, phenylalanine, is catalyzed by phenylalanine lyase (PAL), cinnamic acid-4-hydroxylase (C4H), etc35. The biosynthetic pathways of flavonoids have been well studied in many plants36–38. In the Bs_vs_Bo group, the category of flavonoids includes upregulated Orientin-2′′-O-galactoside, 6-Hydroxykaempferol-7,6-O-Diglucoside, Okanin-4′-O-glucosyl-O- glucoside, which may be the reason for medicinal differences between B. striata and B. ochracea. In the Bs_vs_Bf group, the category of flavonoids includes upregulated Phellodendroside and Orientin-2′′-O-galactoside, which may be the reason for medicinal differences between B. striata and B. foliosa.
Phenolic compounds are a well-known class of secondary metabolites with extensive pharmacological activities39. According to reports, phenolic acids have different biological activities. Increasing bile secretion, lowering blood cholesterol and lipid levels, and antibacterial activity against certain bacteria such as Staphylococcus aureus are some of the biological activities of phenolic acids40. Several phenolic acids in B. striata are thought to promote wound healing41. Yang and colleagues reported that the esterification products of caffeic acid and phenylethyl alcohol can inhibit the transforming growth factor-β1/Mothers against the decapentaplegic homolog 3 (TGF-β1/Smad3) signaling pathway42. There is evidence that smad3 can bind the DNA sequences of target genes at the transcriptional level, and for pathologic skin conditions, assumes important roles in tissue repair and fibrosis43. Phenolic and flavonoid compounds have various biological activities, such as anti-ulcer, anti-inflammatory, antioxidant, cytotoxic and anti-tumor, anti spastic, and antidepressant effects44. Phenolic acid is an organic acid containing a phenolic ring, hydroxybenzoic acid, and hydroxycinnamic acid45. They are important secondary metabolites in plants because they provide protection against insects, viruses, and bacteria. Eating foods rich in phenolic acids can help accelerate the elimination of oxygen free radicals in the body, thereby protecting cells28. In the Bo_vs_Bf group, the category of phenolic acids includes downregulated 3-O-p-Coumaroylquinic acid, upregulated Gallic acid-4-O-(6′′′-feruloyl)sophoroside and Phenyl acetate. In the Bs_vs_Bf group, the category of phenolic acids includes upregulated α-Hydroxycinnamic Acid*, 2-Amino-3-methoxybenzoic acid, Plantamajoside, 1,2-O-Diferuloylglycerol* and downregulated 3-O-p-Coumaroylquinic acid. In the Bs_vs_Bo group, the category of phenolic acids includes upregulated α-Hydroxycinnamic Acid*, 2-Amino-3-methoxybenzoic acid, parishin J, Tyrosol; 4-Hydroxyphenylethanol and downregulated Phenyl acetate. B.striata are rich in α-Hydroxycinnamic Acid*, 2-Amino-3-methoxybenzoic acid, Plantamajoside, 1,2-O-Diferuloylglycerol* compared with B. formosana, which may cause the medicinal differences between B.striata and B. foliosa. B.striata are rich in α-Hydroxycinnamic Acid*, 2-Amino-3-methoxybenzoic acid, parishin J, Tyrosol; 4-Hydroxyphenylethanol compared with B. ochracea, which may cause the medicinal differences between B.striata and B.ochracea.
The basic research of traditional Chinese medicine mainly focuses on two aspects: chemical composition and pharmacology, with pharmacology being the most important. Pharmacological research on B. striata mainly focuses on pharmacokinetics. The latter has promoted our understanding of the mechanism of the components in B. striata. In recent years, pharmacological research on B. striata has mainly focused on its hemostasis, wound healing, antioxidant, anti-cancer, antiviral, antibacterial and other aspects9. The hemostatic effect is one of the main pharmacologic effects of B. striata. Interestingly, recent studies have shown that the water-soluble part of B. striata has a positive hemostatic effect, and its function is believed to be related to adenosine diphosphate, which promotes and accelerates platelet aggregation46,47. Hung and Wu found that B.striata polysaccharide (BSP) in this water-soluble component played a key role in hemostatic activity48. In folk culture, B. ochracea and B. formosana are often used as substitutes for B. striata has two main reasons:similar phenotype and composition of metabolite categories. The main metabolites of Bletilla include polysaccharides, amino acids, flavonoids, etc. In this study, compounds such as sugars, benzenes, phenols, flavonoids, amino acids, alkaloids, terpenes, etc. were detected in all three species. Besides, major polysaccharides identified in this study were not significantly accumulated among the Bo_vs_Bf, Bs_vs_Bf and Bs_vs_Bo comparisons. Furthermore, the pharmaceutical substances, including bibenzyls, phenanthrenes, triterpenoids and their saponins, steroids and their saponins were also indistinctively different among the Bo_vs_Bf, Bs_vs_Bf and Bs_vs_Bo comparisons.
Conclusions
This study explored the differential metabolites of three Bletilla species, and that medicinally important compounds differed in accumulation by species. For example, B. striata are rich in α-Hydroxycinnamic Acid*, 2-Amino-3-methoxybenzoic acid, Plantamajoside, 1,2-O-Diferuloylglycerol* compared with B.formosana, which may cause the medicinal differences between B. striata and B.foliosa. B. striata are rich in α-Hydroxycinnamic Acid*, 2-Amino-3-methoxybenzoic acid, parishin J, Tyrosol; 4-Hydroxyphenylethanol compared with B. ochracea, which may cause the medicinal differences between B.striata and B. ochracea. In the future, combining the UPLC/MS-MS data presented here with gene expression data of more Bletilla species will promote the use of different Bletilla species for use in medicine.
Materials and methods
Plant materials
Three B. striata species (B. striata, B. ochracea, B. formosana) with purple, yellowish green and lavender flowers were selected for the study. Phenotype of B. striata, B. ochracea and B. formosana were shown in Fig. 1. The B. striata cultivar is abbreviated as Bs. Similarly, B. ochracea and B. formosana are referred to as Bo and Bf, respectively. Seedlings of the B. striata were planted in the experimental fields of Zhejiang Institute of Subtropical Crops, Zhejiang Academy of Agricultural Sciences (16° 23′ 25″~116° 24′ 41″ E, 39° 55′ 19″ ~39° 55′ 56" N) with a random arrangement. The annual mean temperature is 22 °C and the average annual precipitation is 1700 mm. The experimental plot was one mu, with 3 replicates. Seedlings were planted after aseptic propagation (50% light transmittance) in October 2019 and pseudobulb was obtained for analysis in October 2021. After gentle washing, the pseudobulb were cut into small pieces and immediately frozen in liquid nitrogen and stored in a −80 °C ultra-low freezer. For each cultivar, three biological replicates were used for experimental analysis. The ornamental features (above-ground biomass) were recorded in May-June, 2020. The economic traits (below-ground biomass) were recorded in October, 2021.
Conditions to the analysis of UPLC and MS/MS
Metabolomics analysis was conducted by Wuhan MetWare Biotechnology Co., Ltd. (www.metware.cn) following their standard procedures. Ultra Performance Liquid Chromatography (UPLC) and Tandem mass spectrometry (MS/MS) were used to perform the data acquisition. The liquid phase conditions were listed as follows: UPLC: column, Waters ACQUITY UPLC HSS T3 C18 (1.8 μm, 2.1 mm × 100 mm); solvent system, ultrapure water (0.04% acetic acid): acetonitrile (0.04% acetic acid); gradient program, 95:5 V/V at 0 min, 5:95 V/V at 11.0 min, 5:95 V/V at 12.0 min, 95:5 V/V at 12.1 min, 95:5 V/V at 14.0 min; flow rate, 0.4 mL/min; column temperature, 40 °C; injection volume 2 µL49.
LIT and triple quadrupole (QQQ) scanning are obtained on the triple quadrupole linear ion trap mass spectrometer (QTRAP), AB4500 Q TRAP UPLC/MS/MS system, which is equipped with an ESI Turbo ion spray interface and controlled by Analyst 1.6.3 software (AB Sciex) to operate in both positive and negative ion modes. The operation parameters of ESI source are as follows: ion source, turbine spray; Source temperature 550 °C; Ion spray voltage (IS) 5500 V (positive ion mode)/- 4500 V (negative ion mode); Ion source gas I (GSI), gas II (GSII) and curtain gas (CUR) are set to 50, 60 and 25.0 psi respectively, and the collision-induced ionization parameter is set to high. Use 10 and 100 respectively in QQ and LIT modes µ Mol/L polypropylene glycol solution for instrument tuning and quality calibration. QQ scanning uses MRM mode and sets collision gas (nitrogen) to medium. Through further optimization of DP and CE, DP and CE of each MRM ion pair were completed. A specific set of MRM ion pairs was monitored at each period based on the metabolites eluted at each period.
Data analysis
Using analyst 1.6.3 (AB SCIEX, Foster City, CA, USA) to process mass spectrometry data. Principal component analysis (PCA) was performed using the R (base package) 3.5.1 software package. Heatmap was performed using the R (ComplexHeatmap) 2.8.0. VIP values were extracted from OPLS-DA result, which also contain score plots and permutation plots, was generated using R package MetaboAnalystR. The variable importance values (VIP > 1, FC > 2, or FC < 0.5) of differentially expressed metabolites through projection (VIP) and folding changes (FC).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
W.X. and Z.Z. designed the experiments. J.H., P.W. and S.F. performed all experiments. Y.Y. analysed the data and wrote the manuscript. Y.Y. and Z.Z. revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Wenzhou Agricultural High-tech Park Open Project(grant no. KN20210001).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hossain, M. M. Therapeutic orchids: traditional uses and recent advances—An overview. Fitoterapia82(2), 102–140 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Xu, D. L., Pan, Y. C. & Chen, J. S. Chemical constituents, pharmacologic properties, and clinical applications of Bletilla striata. Front. Pharmacol. 10 (2019). [DOI] [PMC free article] [PubMed]
- 3.Yang, L. et al. A new macrolide and six cycloartane triterpenoids from the tubers of Bletilla striata. Biochem. Syst. Ecol.57, 238–241 (2014). [Google Scholar]
- 4.Hew, C. S. & Yong, J. The physiology of tropical orchids in relation to the industry: The physiology of tropical orchids in relation to the industry (2004).
- 5.Jiang, S., Wang, M., Jiang, L., Xie, Q. & Wang, W. The medicinal uses of the genus Bletilla in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 114263. [DOI] [PubMed]
- 6.Jiang, S. et al. The medicinal uses of the genus Bletilla in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol.280, 114263 (2021). [DOI] [PubMed] [Google Scholar]
- 7.He, X. et al. Bletilla striata: Medicinal uses, phytochemistry and pharmacological activities. J. Ethnopharmacol. (2017). [DOI] [PubMed]
- 8.Nishidono, Y. et al. Effect of heat processing on the chemical constituents and no-suppressing activity of Bletilla tuber. J. Nat. Med.2020(1), 74. [DOI] [PubMed]
- 9.Xu, D. L., Pan, Y. C. & Chen, J. S. Chemical constituents, pharmacologic properties, and clinical applications of Bletilla striata. Front. Pharmacol. 10 (2019). [DOI] [PMC free article] [PubMed]
- 10.He, X. R. et al. Bletilla striata: Medicinal uses, phytochemistry and pharmacological activities. J. Ethnopharmacol.195, 20–38 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Zhang, Q. et al. Biocompatible and degradable Bletilla striata polysaccharide hemostasis sponges constructed from natural medicinal herb Bletilla striata. Carbohydr. Polym. 226 (2019). [DOI] [PubMed]
- 12.Wang, X. Y. et al. Using UPLC-QTOF/MS and multivariate analysis to explore the mechanism of Bletilla striata improving PM2.5-induced lung impairment. Anal. Biochem. 631 (2021). [DOI] [PubMed]
- 13.Jacobowitz, J. R. & Weng, J. K. Exploring uncharted territories of plant specialized metabolism in the postgenomic era. In Annual Review of Plant Biology, 71, 631–658 (2020). [DOI] [PubMed]
- 14.Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Med. (Basel Switzerland)2(3), 251–286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao, H. X., Zhang, A. H., Zhang, H. M., Sun, H. & Wang, X. J. The application of metabolomics in traditional Chinese medicine opens up a dialogue between Chinese and western medicine. Phytother. Res.29(2), 159–166 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Jain, C., Khatana, S. & Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res.10(2), 494–504 (2019). [Google Scholar]
- 17.Afzal, A., Oriqat, G., Khan, M. A., Jose, J. & Afzal, M. Chemistry and Biochemistry of terpenoids from Curcuma and related species. J. Biol. Act. Prod. Nat.3(1), 1–55 (2013). [Google Scholar]
- 18.Singh, N. & Sharma, A. Turmeric (Curcuma longa): miRNAs and their regulating targets are involved in development and secondary metabolite pathways. C.R. Biol.340(11–12), 481–491 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Sheeja, T. E., Deepa, K., Santhi, R. & Sasikumar, B. Comparative transcriptome analysis of two species of Curcuma contrasting in a high-value compound curcumin: Insights into genetic basis and regulation of biosynthesis. Plant. Mol. Biology Rep.33(6), 1825–1836 (2015). [Google Scholar]
- 20.Wang, B. L. et al. Optimizing the extraction of polysaccharides from Bletilla ochracea Schltr. Using response surface methodology (RSM) and evaluating their antioxidant activity. Processes8(3) (2020).
- 21.Guo, J. M. et al. Investigation of < i > in vivo metabolic profile of Abelmoschus manihot based on pattern recognition analysis. J. Ethnopharmacol.148(1), 297–304 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Jiang, S. et al. The medicinal uses of the genus Bletilla in traditional Chinese medicine: a phytochemical and pharmacological review. J. Ethnopharmacol. 280 (2021). [DOI] [PubMed]
- 23.Xu, D. L. et al. Chemical constituents of Bletilla striata. J. Asian Nat. Prod. Res.21(12), 1184–1189 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Dai, O., Yang, L., Zhou, Q. M. & Cheng, P. Chemical constituents from tubers of Bletilla striata. Chin. J. Exp. Tradition. Med. Formulae (2018).
- 25.Guo, Q., Meng, Y., Zhao, Y., Cheng, X. C. & Zhang, Q. L. Inhibitory effect of Bletilla striata extracts on bleomycin-induced pulmonary fibrosis in rats. J. Int. Pharm. Res. (2016).
- 26.Chen, J. et al. Integrative analyses of transcriptome and metabolome shed light on the regulation of secondary metabolites in pseudobulbs of two Bletilla striata (Thunb.) Reichb.f. Varieties. J. Appl. Res. Med. Aromatic Plants 20 (2021).
- 27.Lin, Y. L., Chen, W. P. & Macabalang, A. D. Dihydrophenanthrenes from Bletilla formosanaChem. Pharm. Bull.53(9), 1111–1113 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Bae, J. Y. et al. Chemical constituents isolated from Bletilla striata and their Inhibitory effects on nitric oxide production in RAW 264.7 cells. Chem. Biodivers.14(2) (2017). [DOI] [PubMed]
- 29.Prochazkova, D., Bousova, I. & Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia82(4), 513–523 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Maleki, S. J., Crespo, J. F. & Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 299 (2019). [DOI] [PubMed]
- 31.Raffa, D., Maggio, B., Raimondi, M. V., Plescia, F. & Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem.142, 213–228 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Lepiniec, L. et al. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol.57, 405–430 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Meng, J. et al. Metabolomics Integrated with transcriptomics reveals redirection of the phenylpropanoids metabolic flux in Ginkgo biloba. J. Agric. Food Chem.67(11), 3284–3291 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Schijlen, E. G. W., de Vos, C. H. R., van Tunen, A. J. & Bovy, A. G. Modification of flavonoid biosynthesis in crop plants. Phytochemistry65(19), 2631–2648 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Fowler, Z. L. & Koffas, M. A. G. Biosynthesis and biotechnological production of flavanones: Current state and perspectives. Appl. Microbiol. Biotechnol.83(5), 799–808 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Gao, J. et al. Comparative metabolomic analysis reveals distinct flavonoid biosynthesis regulation for leaf color development of cymbidium sinense ‘red sun’. Int. J. Mol. Sci.21(5) (2020). [DOI] [PMC free article] [PubMed]
- 37.Li, H. Y. et al. Metabolite profiling and transcriptome analyses provide insights into the Flavonoid biosynthesis in the developing seed of Tartary Buckwheat (Fagopyrum tataricum). J. Agric. Food Chem.67(40), 11262–11276 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Wu, Y. Q. et al. Integrative analysis of the metabolome and transcriptome provides insights into the mechanisms of flavonoid biosynthesis in blackberry. Food Res. Int. 153 (2022). [DOI] [PubMed]
- 39.Ghasemzadeh, A. & Ghasemzadeh, N. Flavonoids and phenolic acids: role and biochemical activity in plants and human. J. Med. Plants Res.5(31), 6697–6703 (2011). [Google Scholar]
- 40.Mandal, S. M., Chakraborty, D. & Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav.5(4), 359–368 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song, Y. et al. <i > in vivo wound healing and invitro antioxidant activities of Bletilla striata phenolic extracts. Biomed. Pharmacother.93, 451–461 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Yang, N. et al. Caffeic acid phenethyl ester attenuates liver fibrosis via inhibition of TGF-β1/Smad3 pathway and induction of autophagy pathway. Biochem. Biophys. Res. Commun.486(1), 22–28 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Ashcroft, G. S. et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol.1(5), 260–266 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Dai, J. & Mumper, R. J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules15(10), 7313–7352 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heleno, S. A., Martins, A., Queiroz, M. & Ferreira, I. Bioactivity of phenolic acids: metabolites versus parent compounds: A review. Food Chem.173, 501–513 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Lu, B., Xu, Y. M., Zhang, H. M., Li, T. J., & Qiu, Y. Effects of different extracts from bletilla colloid on rabbit platelet aggregation (2005).
- 47.Gachet, C. Regulation of platelet functions by P2 receptors. Annu. Rev. Pharmacol. Toxicol.46, 277–300 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Hung, H. Y. & Wu, T. S. Recent progress on the traditional Chinese medicines that regulate the blood. J. Food Drug Anal.24 (2), 221–238 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bujak, R., Struck-Lewicka, W., Markuszewski, M. J. & Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal.113, 108–120 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.