Abstract
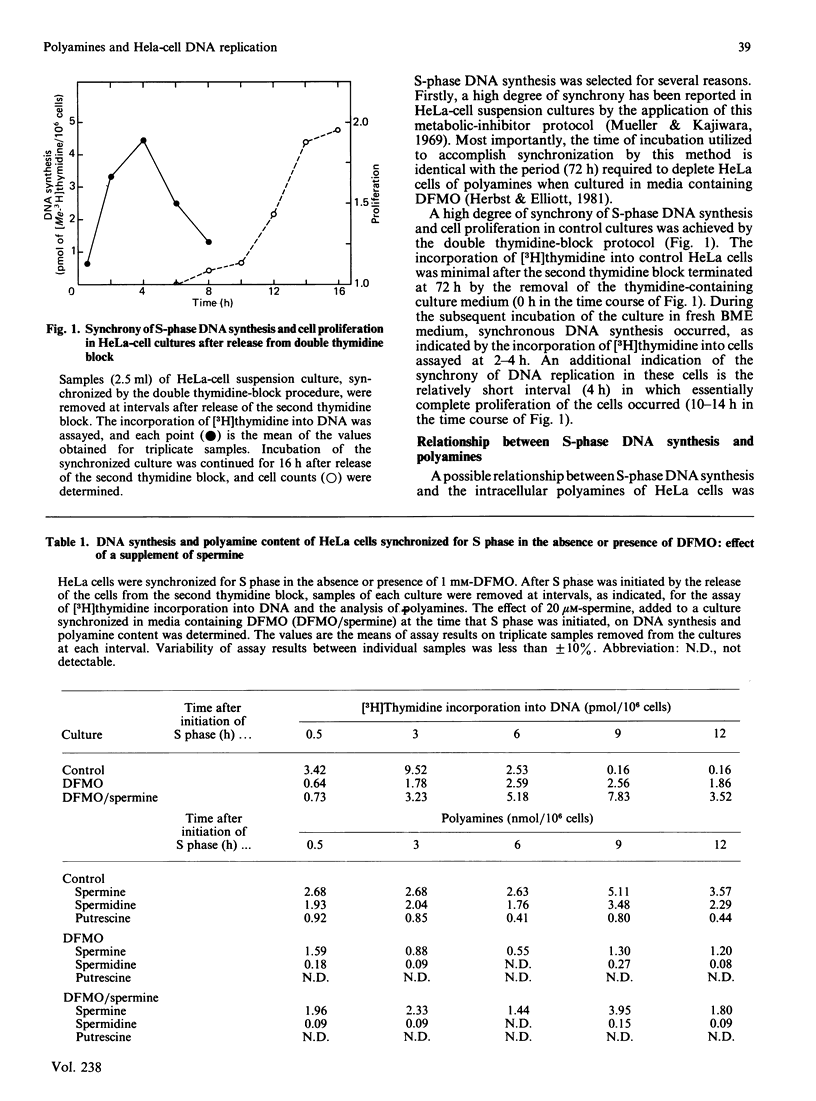
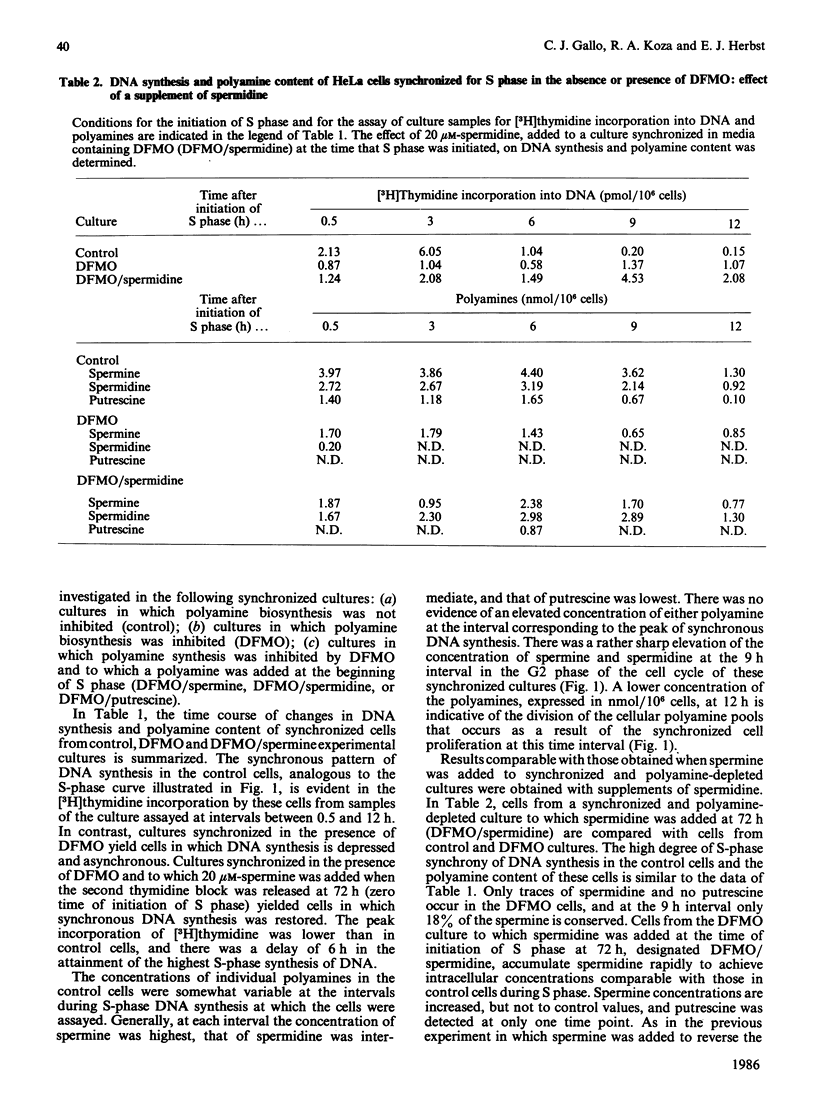
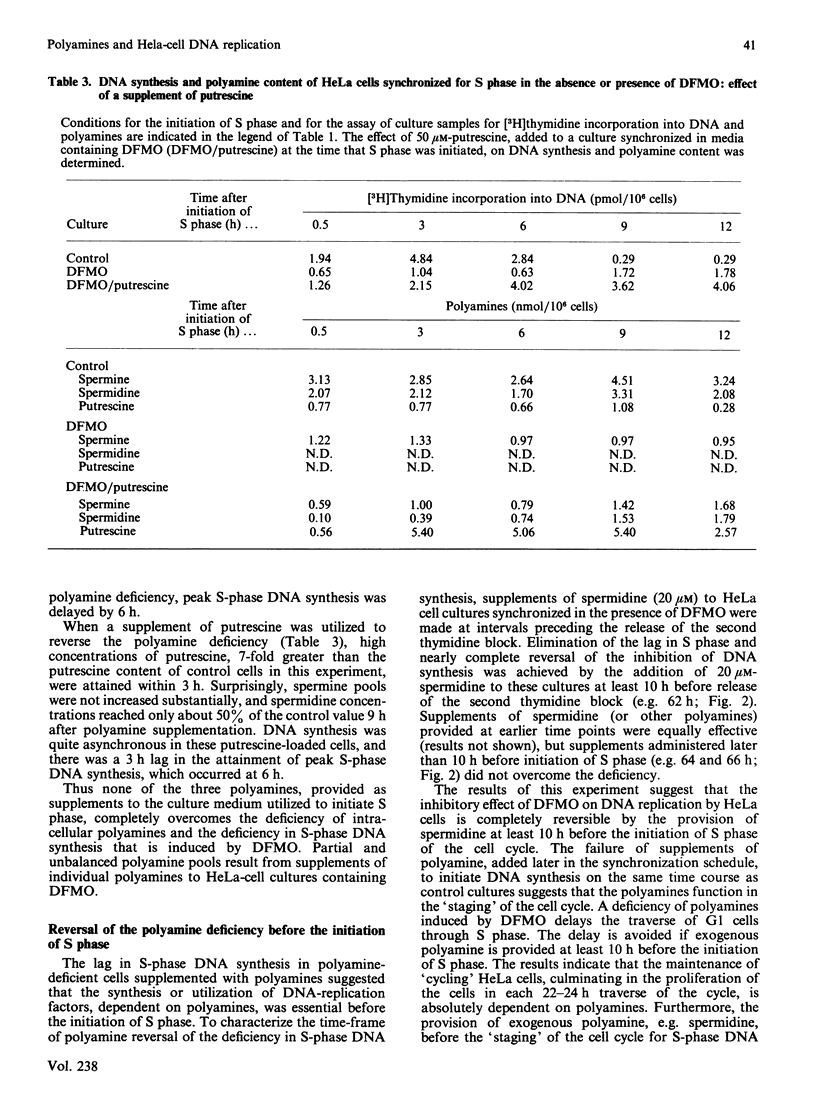
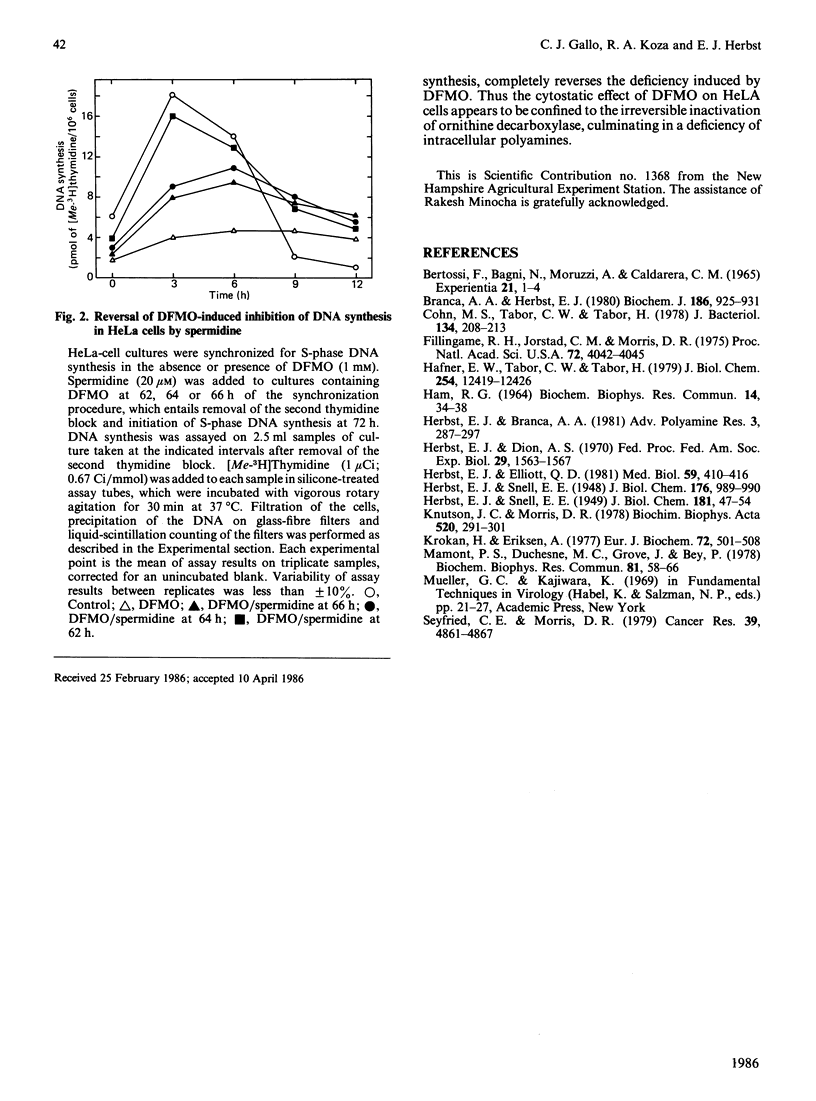
HeLa cells were synchronized for S-phase DNA synthesis by the double thymidine-block procedure. A comparison was made of the polyamine content and S-phase DNA synthesis in cells from control cultures and cultures to which an inhibitor of polyamine biosynthesis, alpha-difluoromethylornithine, was added to the synchronization medium. Control cells showed a peak of synchronous DNA synthesis at 3 h and a maximum concentration of polyamines at 6-9 h after release of the second thymidine block. Cells from cultures containing the inhibitor were severely inhibited in the synthesis of DNA and contained no putrescine and only traces of spermidine while the spermine content was lowered by as much as 80%. Supplementation of cultures containing alpha-difluoromethylornithine with a polyamine, at the time of release of the second thymidine block, replenished the intracellular pool of the administered polyamine and partially restored S-phase DNA synthesis, with a lag of 3-6 h. Almost complete restoration of DNA synthesis in cells depleted of polyamines was achieved by the addition of a polyamine to cultures at least 10 h before release of the second thymidine block. The lag in initiation of synchronous S-phase DNA synthesis was eliminated in these cells. It is concluded that reversal by polyamines of the deficiency in S-phase DNA synthesis, in polyamine-depleted HeLa cells, is a time-dependent process indicative of the necessity for the replenishment of replication factors or their organization into an active replication complex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLAG W., PLETSCHER A. NEW DEVELOPMENTS IN CANCER RESEARCH. CHEMOTHERAPY OF CANCER: REPORT ON THE LUGANO SYMPOSIUM. Experientia. 1965 Jan 15;21:1–4. doi: 10.1007/BF02136347. [DOI] [PubMed] [Google Scholar]
- Branca A. A., Herbst E. J. Inhibition of ornithine decarboxylase of HeLa cells by diamines and polyamines. Effect on cell proliferation. Biochem J. 1980 Mar 15;186(3):925–931. doi: 10.1042/bj1860925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Isolation and characterization of Saccharomyces cerevisiae mutants deficient in S-adenosylmethionine decarboxylase, spermidine, and spermine. J Bacteriol. 1978 Apr;134(1):208–213. doi: 10.1128/jb.134.1.208-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H., Jorstad C. M., Morris D. R. Increased cellular levels of spermidine or spermine are required for optimal DNA synthesis in lymphocytes activated by concanavalin A. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4042–4045. doi: 10.1073/pnas.72.10.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBST E. J., SNELL E. E. Putrescine and related compounds as growth factors for Hemophilus parainfluenzae 7991. J Biol Chem. 1949 Nov;181(1):47–54. [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Mutants of Escherichia coli that do not contain 1,4-diaminobutane (putrescine) or spermidine. J Biol Chem. 1979 Dec 25;254(24):12419–12426. [PubMed] [Google Scholar]
- Ham R. G. Putrescine and related amines as growth factors for a mammalian cell line. Biochem Biophys Res Commun. 1964;14:34–38. doi: 10.1016/0006-291x(63)90206-7. [DOI] [PubMed] [Google Scholar]
- Herbst E. J., Dion A. S. Polyamine changes during development of Drosophila melanogaster. Fed Proc. 1970 Jul-Aug;29(4):1563–1567. [PubMed] [Google Scholar]
- Herbst E. J., Elliot Q. D. Role of polyamines in HeLa cell proliferation. Med Biol. 1981 Dec;59(5-6):410–416. [PubMed] [Google Scholar]
- Knutson J. C., Morris D. R. Cellular polyamine depletion reduces DNA synthesis in isolated lymphocyte nuclei. Biochim Biophys Acta. 1978 Sep 27;520(2):291–301. doi: 10.1016/0005-2787(78)90228-9. [DOI] [PubMed] [Google Scholar]
- Krokan H., Eriksen A. DNA synthesis in HeLa cells and isolated nuclei after treatment with an inhibitor of spermidine synthesis, methyl glyoxal bis(guanylhydrazone). Eur J Biochem. 1977 Feb;72(3):501–508. doi: 10.1111/j.1432-1033.1977.tb11273.x. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- Seyfried C. E., Morris D. R. Relationship between inhibition of polyamine biosynthesis and DNA replication in activated lymphocytes. Cancer Res. 1979 Dec;39(12):4861–4867. [PubMed] [Google Scholar]


