Abstract
Freshwaters play an essential role in providing ecosystem services worldwide, however, the water quality of different water bodies is strongly influenced by human activities such as urbanization, industry and agriculture. In this study, water and biofilm samples were collected from the main channel of the Danube River upstream and downstream of a metropolitan, from a regulated side arm within an urbanized area, and from two differently separated oxbow lakes located in nature conservation areas. The taxonomic diversity of bacterial communities was revealed by 16S rRNA gene-based amplicon sequencing using Illumina MiSeq platform. The results showed that all samples were dominated by phyla Pseudomonadota, Actinobacteriota and Bacteroidota. The bacterial community structures, however, clearly differentiated according to planktonic and epilithic or epiphytic habitats, as well as by riverine body types (main channel, side arm, oxbow lakes). The taxonomic diversity of biofilm communities was higher than that of planktonic ones in all studied habitats. Human impacts were mainly reflected in the slowly changing biofilm composition compared to the planktonic ones. Genera with pollution tolerance and/or degradation potential, such as Acinetobacter, Pseudomonas and Shewanella were mainly detected in biofilm communities of the highly urbanized section of the river side arm.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75863-7.
Keywords: Water quality, Epilithic, Epiphytic, Planktonic, Microbial communities, Metropolitan, Danube River
Subject terms: Ecology, Microbiology, Molecular biology, Ecology, Environmental sciences, Health care
Introduction
In the 21st century, the uneven distribution and limited availability of freshwater resources have led to severe water scarcity and water quality problems due to the increased water use associated with human population growth as well as the effects of climate change1. Water resources are highly sensitive; thus, they can be easily degraded2. Due to the multiple benefits of densely populated areas, rivers worldwide have been heavily impacted by urbanization3. The main anthropogenic factors include industry, municipal water use, agriculture, and construction or alteration of water bodies, furthermore the usage of the river for transportation1. The impact of urbanization and anthropogenic factors on the chemical and microbiological properties of rivers has become the focus of research nowadays4–8.
Human activities, such as pollution of rivers and construction of dams, can lead to deterioration in water quality and to a decrease in water quantity. Thus, the water cannot be used in industrial processes or agricultural activities and cannot participate in the drinking water supply6. In addition, contamination of river waters can significantly affect human health5,9. At a certain pollution level, water bodies are capable of self-purification, whereby physical, chemical and (micro)biological processes occur in complementary and parallel ways1,10. Bacterial communities inhabiting freshwaters play a key role in biogeochemical cycles, by transforming and/or degrading various organic materials and toxic compounds11,12. Aquatic environments provide diverse habitats for different microorganisms. They can populate water bodies as planktonic communities and form biofilms on all underwater biotic and abiotic surfaces. In littoral zones, where the flow rate is low, submerged plant surfaces can be an appropriate habitat for bacterial biofilm formation13. These complex plant-microbial consortia allow highly efficient self-purification of water bodies through the ability of macrophytes and biofilm-forming bacteria to degrade, assimilate and remove contaminants. In heavily polluted water bodies, however, this self-purification is insufficient to restore good water quality1. Most of research so far has focused on planktonic communities, therefore, our knowledge of riverine biofilm-forming (epiphytic or epilithic) communities is rather limited12,14–17.
The Danube River is the second longest river in Europe. Hungary is in the center of the Danube Basin where the river crosses the capital in a north-south direction. In the Budapest metropolitan area, the Danube River is exposed to significant anthropogenic effects. The Danube, due to its outstanding importance and potential or actual environmental pollution events, has long been the subject of various physical-chemical and microbiological studies, along its entire length, in different sections, and in relation to its various water bodies14,18–21. These studies, however, have not addressed the impact of different human activities on the overall bacterial communities by examining various riverine water bodies and habitat types in parallel.
The aim of this study, therefore, was to compare the planktonic, epilithic, and epiphytic bacterial communities of three different riverine water bodies (main river channel, side arm and oxbow lakes) of the Danube River, and to assess the potential anthropogenic effects of the metropolitan area on these bacterial communities.
Results
The physical and chemical characteristics of the water samples
The mean values and standard deviations of the measured water physical-chemical characteristics according to the sampling sites are presented in the Table 1. Neither the upstream and downstream main river channel sites nor the side arm and the downstream main river channel sampling sites differed significantly for most of the environmental parameters. However, in the case of the electric conductivity and pH values a significant difference was detected between the upstream main river channel and the side arm. The two oxbows differed significantly from the main river channel sampling sites. The pH of the side arm and the oxbow lakes was comparable. The Nyéki-Danube oxbow and the side arm did not differ based on the electric conductivity and phosphate content of the water. The Nyéki-Danube and the Riha lake oxbows clearly separated based on the pH values, although further significant differences were not detected (Supplementary Table 1).
Table 1.
Physical and chemical variables of the water samples at the different sampling sites (average ± SD; n = 12 per sample) (notes: means followed by different letters in the same row differ significantly in Dunn’s post hoc test at 95% confidence interval. Different letters (a–d) in the same line indicate significant statistical difference (p < .05, Dunn’s post-hoc)).
| Sampling sites | Temperature (°C) | Conductivity (mS/cm) | pH | Dissolved oxygen (mg/L) | TOC (mg/L) | NO3− (mg/L) | PO43− (µg/L) |
|---|---|---|---|---|---|---|---|
| Upstream Budapest (DNW) | 13.57 ± 0.46 a | 0.29 ± 0.01 a | 7.54 ± 0.17 a | 9.74 ± 0.51 a | 3.35 ± 1.52 a | 6.23 ± 0.9 ab | 105.83 ± 56.8 a |
| Downstream Budapest (DSW) | 13.06 ± 1.14 a | 0.30 ± 0.01 ab | 7.81 ± 0.20 ab | 10.02 ± 0.78 a | 2.67 ± 0.99 a | 6.24 ± 0.86 a | 110.83 ± 74.16 a |
| Soroksári-Danube (SW) | 13.32 ± 0.24 a | 0.37 ± 0.02 bd | 8.07 ± 0.13 bc | 9.69 ± 0.63 a | 2.88 ± 0.59 a | 7.17 ± 0.98 a | 53.69 ± 7.07 a |
| Nyéki- Danube (NW) | 17.35 ± 0.46 b | 0.37 ± 0.00 cd | 7.82 ± 0.07 ab | 7.46 ± 0.59 b | 8.52 ± 0.51 b | 2.15 ± 0.23 b | 45.09 ± 14.00 ab |
| Riha Lake (RW) | 17.63 ± 0.23 b | 0.64 ± 0.00 c | 8.27 ± 0.04 c | 7.94 ± 0.34 b | 19.12 ± 0.41 b | 4.96 ± 0.24 b | 5.44 ± 2.25 b |
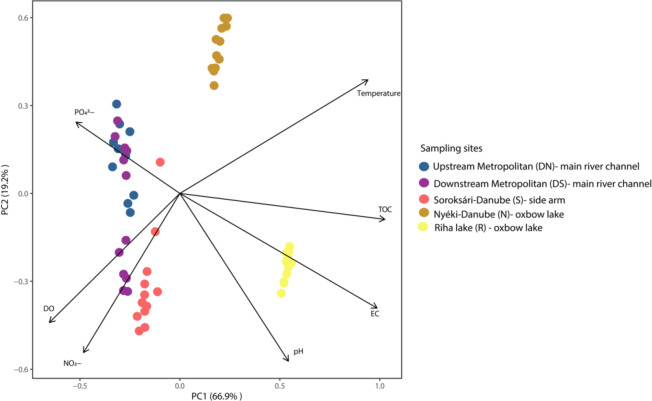
The Principal Component Analysis confirmed the findings discussed above. The environmental variables contributed differently to the separation of the sampling sites (Fig. 1). Water temperature, electrical conductivity, TOC values were higher at the oxbow sampling sites compared to the main river channel and the side arm. Conversely, dissolved oxygen and nitrate concentrations were the highest in the main river channel and the side arm of the river.
Fig. 1.
The principal component analysis (PCA) biplot based on the water physical-chemical variables of the different sampling sites.
The results of the Spearman’s Rank-Order Correlation analysis (Supplementary Table 2.) indicated negative correlations between the dissolved oxygen (DO) concentration in the main river channel and nitrate, phosphate, and total organic carbon (TOC) contents. In the case of the downstream sample a stronger negative correlation was detected in all the three parameters than in the upstream sample. Furthermore, TOC, nitrate and phosphate contents were strongly positively correlated at the two main river channel sampling sites.
The diversity of the planktonic and biofilm bacterial communities
In total 2 755 240 sequences were assigned to 552 OTUs (Operational Taxonomic Unit). Good’s coverage indicated high sequencing depth across all sampling sites and habitats (Table 2). Diversity analysis revealed higher values in the case of the epilithic and epiphytic communities compared to the planktonic ones, based on the predicted number of OTUs (Sobs) and the estimated richness (ACE, Chao1) and diversity (Inverse Simpson) indices. The diversity of the planktonic communities was the highest in the side arm, comparing the five sampling sites. The main river channel sampling sites had a higher diversity compared to the oxbows (Table 2). In contrast, the epiphytic communities of the side arm had the lowest diversity among the biofilm samples.
Table 2.
Observed (Sobs) and estimated (Chao1 and ACE) bacterial species richness, and diversity indices (Inverse Simpson) calculated from the 16S rRNA gene-based amplicon sequencing data of the planktonic and biofilm bacterial communities (sequence numbers were subsampled to the read number of the sample having the lowest sequence count).
| Sampling sites | Community | SEQ. No. | Coverage | Chao | ACE | Sobs | Inverse Simpson |
|---|---|---|---|---|---|---|---|
| Upstream Budapest (DNW) | Planktonic | 3311 | 0.98 ± 0.00 | 283.17 ± 23.02 | 291.49 ± 26.59 | 208.95 ± 5.29 | 23.06 ± 0.58 |
| Downstream Budapest (DSW) | Planktonic | 3311 | 0.98 ± 0.00 | 285.94 ± 22.26 | 302.53 ± 30.34 | 207.00 ± 5.02 | 21.43 ± 0.49 |
| Soroksári-Danube (SW) | Planktonic | 3311 | 0.98 ± 0.00 | 292.25 ± 26.25 | 313.86 ± 40.76 | 212.78 ± 6.73 | 21.99 ± 0.68 |
| Nyéki- Danube (NW) | Planktonic | 3311 | 0.99 ± 0.00 | 187.54 ± 25.13 | 217.04 ± 39.80 | 129.49 ± 5.53 | 17.14 ± 0.43 |
| Riha Lake (RW) | Planktonic | 3311 | 0.99 ± 0.00 | 196.58 ± 21.40 | 206.26 ± 28.78 | 146.03 ± 5.37 | 14.24 ± 0.50 |
| Upstream Budapest (DNB) | Biofilm | 22689 | 1.00 ± 0.00 | 403.04 ± 13.28 | 396.03 ± 8.14 | 351.02 ± 3.46 | 13.16 ± 0.07 |
| Downstream Budapest (DSB) | Biofilm | 22689 | 1.00 ± 0.00 | 368.62 ± 11.83 | 364.04 ± 7.50 | 318.06 ± 2.90 | 15.04 ± 0.09 |
| Soroksári-Danube (SB) | Biofilm | 22689 | 1.00 ± 0.00 | 329.57 ± 5.17 | 329.69 ± 3.54 | 313.17 ± 1.81 | 16.07 ± 0.09 |
| Nyéki- Danube (NB) | Biofilm | 22689 | 1.00 ± 0.00 | 349.18 ± 11.33 | 348.63 ± 8.25 | 321.83 ± 4.08 | 13.33 ± 0.16 |
| Riha Lake (RB) | Biofilm | 22689 | 1.00 ± 0.00 | 362.84 ± 9.70 | 360.84 ± 6.68 | 339.83 ± 3.50 | 13.85 ± 0.16 |
The taxonomic composition of the planktonic bacterial communities
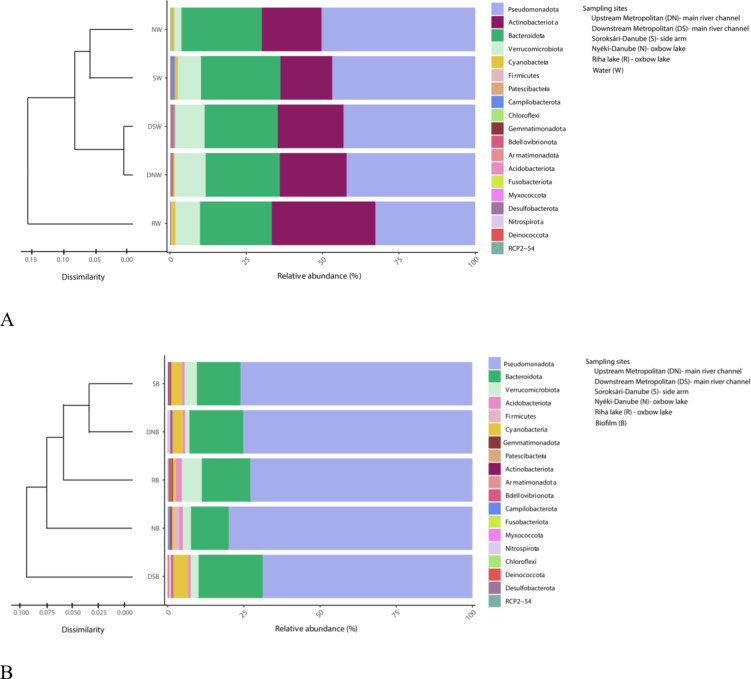
The dissimilarity of the sampling locations is shown in the Fig. 2A based on the relative abundance of the dominant planktonic bacterial phyla. Among the identified bacterial taxa, the phyla Pseudomonadota, Actinobacteriota and Bacteroidota were the predominant across all sampling sites with relative abundance values above 10%. The relative abundance values of the phyla Pseudomonadota and Actinobacteriota showed opposite trends. The Pseudomonadota had the lowest, and the Actinobacteriota had the highest relative abundance in the separated Riha lake oxbow. The phylum Verrucomicrobiota exhibited the highest abundance (> 1%) in the at the upstream and downstream of the main river channel sampling sites, while the lowest values in the Nyéki-Danube oxbow lake.
Fig. 2.
Percentage distribution of 16S rRNA gene amplicon sequences among the phyla in the planktonic (A) and biofilm (B) bacterial communities together with the results of Bray-Curtis similarity index-based cluster analysis.
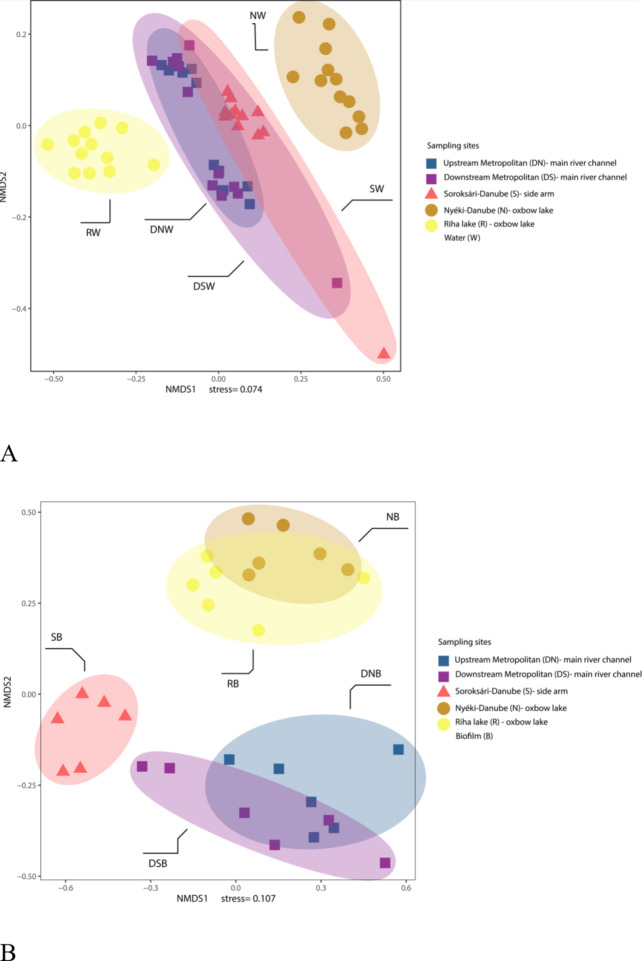
The separation of sampling sites based on planktonic bacterial orders is shown in Fig. 3A. The water samples from the main river channel and the Soroksári-Danube side arm formed an overlapping subgroup, while the two oxbow lakes were clearly separated. The orders Burkholderiales (Pseudomonadota), Frankiales (Actinobacteriota) and Flavobacteriales (Bacteroidota) were predominant within the planktonic bacterial communities at each sampling site. Furthermore, within the phylum Bacteroidota, the orders Chitinophagales, Cytophagales, Kapabacteriales and Sphingobacteriales all had a relative abundance higher than 1% at least one sampling site. Other abundant orders were identified as Chthoniobacterales (Verrucomicrobiota), Microtrichales, and Corynebacteriales (Actinobacteriota) and SAR11_clade (Pseudomonadota) (Supplementary Table 3).
Fig. 3.
The non-metric multidimensional scaling (NMDS) ordination of planktonic (A) and biofilm (B) bacterial communities based on the relative abundance of orders above 1%.
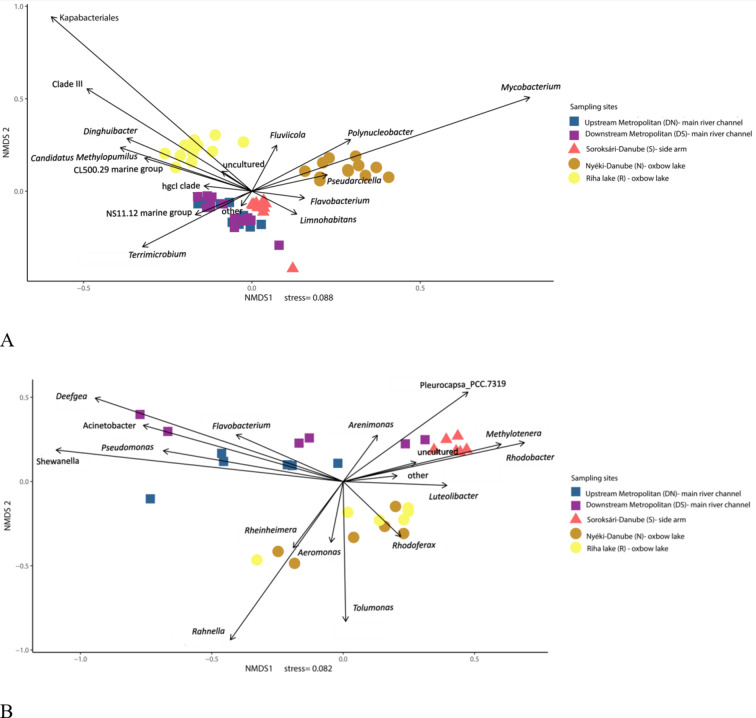
Across all sampling sites, several bacterial genera were abundant, including Flavobacterium (Flavobacteriales), Fluviicola (Flavobacteriales), hgcI_clade (Frankiales), NS11-12_marine_group (Sphingobacteriales), Limnohabitans (Burkholderiales) and Polynucleobacter (Burkholderiales). Notably, genera Limnohabitans (Burkholderiales) and Polynucleobacer (Burkholderiales) exceeded 10% relative abundance at least one sampling site (Fig. 4A, Supplementary Table 3).
Fig. 4.
Non-metric MultiDimensional Scaling (NMDS) ordination of the planktonic (A) and biofilm (B) bacterial communities based on the relative abundance of genera above 2%.
In addition, genera representative of the planktonic community of the sampling sites were Terrimicrobium (Chthoniobacterales) for the main river channel, Dinghuibacter (Chitinophagales), Kapabacteriales (Kapabacteriales) and the Clade_III (SAR11_clade) for the Riha oxbow lake, and Mycobacterium (Corynebacteriales) and Pseudarcicella (Cytophagales) for the Nyéki-Danube oxbow lake (Supplementary Table 3).
The taxonomic composition of the biofilm bacterial communities
The dissimilarity of the sampling sites is represented in the Fig. 2B based on the relative abundance of the dominant biofilm bacterial phyla. The relative abundance of phyla Pseudomonadota, Bacteroidota, Verrucomicrobiota, Cyanobacteria, Firmicutes, Acidobacteriota and Nitrospirota were above 1% at least one biofilm sample. The phyla Pseudomonadota, Bacteroidota and Verrucomicrobiota were the most abundant at each sampling site. The phylum Cyanobacteria was characteristic of the main river channels and the side arm. The phylum Firmicutes was the most abundant in the Nyéki-Danube oxbow lake, while the representatives of the phylum Acidobacteriota showed the highest abundance in the oxbow lakes. The relative abundance of the phylum Nitrospirota reached 1% only in the main river channel samples.
The sampling sites also had a clear separation according to the bacterial orders of the biofilms (Fig. 3B). The two epilithic samples from the main river channel and the two epiphytic samples from the oxbow lakes formed separated subgroups. The side arm epiphytic samples clearly separated from both subgroups. They were closer to the main river channel epilithic than the oxbow epiphytic samples.
The orders Aeromonadales, Enterobacterales and Pseudomonadales (Pseudomonadota), and Flavobacteriales (Bacteroidota) had a relative abundance above 10% at least one sampling site (Supplementary Table 3). The order Enterobacterales (Pseudomonadota) was characteristic of the oxbow lakes (Supplementary Table 3).
At genus level, various taxa were detected above 2% relative abundance at least at one sampling site (Supplementary Table 3). Within the identified genera (Fig. 4B), Pseudomonas (Pseudomonadales) was predominant in the epilithic communities of the main river channel. Other genera typical of this habitat were Acinetobacter (Pseudomonadales), Shewanella (Alteromonadales), Deefgea (Burkholderiales) and Flavobacterium (Flavobacteriales). The genera Rhodobacter (Rhodobacterales) and Methylotenera (Methylophilales) were present in the highest relative abundance in the epiphytic biofilm of the side arm compared to the other samples. The genera Aeromonas (Aeromonadales), Rahnella (Enterobacterales) and Rhodoferax (Burkholderiales) were characteristic of the epiphytic communities in the oxbow lakes.
Discussion
This study focused on a detailed comparative analysis of planktonic and biofilm (both epilithic and epiphytic) samples, from different sections of the same river system, affected to varying degrees by human activity.
The 16S rRNA gene-based amplicon sequencing data indicated significant differences in the composition of planktonic and biofilm bacterial communities across all taxonomic levels (from phylum to genus) and habitat types (main river channel, side arm and oxbow). Although all sample types were dominated by the phylum Pseudomonadota (previously Proteobacteria), like in other freshwater habitats22–26, there was a twofold difference in its relative abundance in favor of biofilm samples compared to planktonic ones. The relative abundance of the phylum Actinobacteriota (previously Actinobacteria), however, was high only in the planktonic communities. Previous studies also reported high relative abundance of the phylum Actinobacteria in freshwater planktonic communities22–26. Among the dominant phyla, the relative abundance of Bacteroidota (previously Bacteroidetes) was higher in the planktonic bacterial communities compared to biofilms. The presence of this phylum could be a possible indication of faecal contamination18,27. In 2007, the Soroksári-Danube side arm was found to be a faecal contamination hot spot, however, for 2013 the extent of the pollution decreased18.
At lower taxonomic levels, hitherto uncultivated, typical freshwater bacteria dominated the planktonic communities e.g. hgcI clade (Actinobacteriota) and NS11.12 marine group (Bacteroidota). Previously, the hgcI clade was a dominant community member, e.g. in pelagic freshwater bacterial communities. Additionally, they play an integral role in the nutrient cycle by fixing carbon dioxide and taking up nitrogen-rich and phosphate containing compounds. Their genome encodes a high variety of degrading enzymes e.g. lysozymes, chitinase28. The occurrence of the NS11-12 marine group was reported from coastal, urbanized and/or polluted environments29–31. Despite its typical association with marine and brackish environments, the NS11-12 marine group has been increasingly detected in freshwater systems, such as Lake Balaton, Zala River, and Lake Fertő in Hungary29,32. This unexpected presence suggests a broader ecological tolerance and potential role in diverse aquatic habitats. While its detection in freshwater is less common, it may be indicative of higher concentrations of organic matter from algal blooms or other external sources29,33. The genus Limnohabitans (Pseudomonadota) was abundant in all studied planktonic communities. It is known as a ubiquitous member of neutral and alkaline planktonic communities, by degrading autochthonous organic matter from algae (e.g. monosaccharides and some amino acids)34–36. The growth rate might be positively influenced by the high nutrient content of the water37,38. In the Nyéki Danube oxbow lake water, the genera Mycobacterium (Actinobacteriota) and Pseudarcicella (Bacteroidota) were abundant. Members of the genus Mycobacterium are highly resistant and could live in many different environments, due to their high variation in degrading enzymes and the ability to metabolize many different compounds. Fast growing, non-tuberculotic mycobacteria were previously frequently isolated from various environmental (e.g. soil and sediment) samples39. Growth of mycobacteria is possible even at low nutrient levels; therefore, these bacteria can survive in oligotrophic environments e.g. in the biofilms of drinking water systems40–42. A recently published metagenome-based correlation network analysis pointed to the possibility of Pseudarcicella being an indicator bacterium of good water quality in freshwater lakes43. In both oxbow lakes, other typical freshwater bacterial genera, e.g. Fluviicola (Bacteroidota)15,44 and Polynucleobacter (Pseudomonadota)45 were also detected. The Polynucleobacter genus inhabits environments with a high variation in physical-chemical parameters, e.g. pH46,47. The genus Candidatus Methylopumilus (Pseudomonadota) is commonly found in freshwaters, especially where the water body has a connection with plants or is surrounded by different plants48, similarly to the Riha-lake sampling site.
The biofilm bacterial communities, both epilithic and epiphytic ones, showed higher taxonomic diversity than the planktonic ones in all Danube riverine water bodies. Similar results were also found previously49–53. Both species richness and taxonomic diversity were the lowest in the biofilm of the Soroksári-Duna side arm compared to the other sampling sites. Of the sampling sites, this side arm is the most affected by the negative impact of urbanization. In the Danube metropolitan area, the sampling sites separated according to the differentiation of the phosphate concentration in the main river channel and the nitrate concentration in the regulated side arm. Both the nitrogen and phosphorus compounds are plant nutrients known as the main non-point pollutants in rivers53,54. The water quality and the contamination of the side arm have already caused serious problems in the past decades. Several studies have reported the eutrophication of the water body and the increased nutrient content (mainly N and P) since the 1990s55–57. In this densely populated region, several diffuse pollutants of anthropogenic origin, such as nitrogen, phosphorus, sulphate, chloride, potassium, and vanadium, were also identified through a detailed physical-chemical analysis of the Danube water20. The measured physical-chemical parameters can also be related to differences observed in the sequence data.
Bacterial genera (e.g. Acinetobacter, Pseudomonas and Shewanella) capable of degrading pollutants and toxic compounds58–60 were found with higher relative abundance in the epilithic than planktonic communities in the main river channel. Members of all three genera isolated from a wastewater treatment system showed high adhesion during biofilm formation61. Recently, Acinetobacter and Pseudomonas was found to be the most abundant genera in microplastic-associated biofilms in the Pearl River Delta (China), as well62. In the epiphytic biofilm of the Soroksári-Danube side arm, the genera Methylotenera and Rhodobacter (Pseudomonadota) was abundant. Members of the Methylotenera genus can play a role in the nitrogen reduction. Furthermore, the genus was identified as a major oil degrading group in Nigeria63. The Rhodobacter species can be used for bioremediation and wastewater pollutant removal due to their metabolic versatility64–66. Members of the genus is highly abundant in eutrophic freshwaters near the shore; they are not common in a low nutrient content environment37. Bacterial genera, e.g. Rhodobacter, Acinetobacter and Pseudomonas are known for their storage and production capacity of polyhydroxy-alkanoates (PHA)58,67. The PHA molecule is used for energy storing; however, it can also improve the stress resistance of the microbes.
In the epiphytic biofilms of the oxbow lakes, the high relative abundance of genera Aeromonas and Rheinheimera (Pseudomonadota) could be a good water quality indicator43. Representatives of both genera are frequently isolated from reed periphyton in freshwater environment32,68,69. The genus Rahnella (Pseudomonadota), a naturally occurring biofilm forming member of Enterobacteriales70, was also characteristic of epiphytic communities in oxbow lakes. The whole genome sequencing of Rahnella aquatilis strain ZF7 revealed its potential for plant growth promotion, biocontrol and stress tolerance71, which may have implications for water quality.
Conclusions
In this study, differently regulated river sections of the Danube River were analyzed to explore the impact of anthropogenic exposure of different water bodies on the composition of planktonic and biofilm bacterial communities using many replicates. The results showed that different abundances of genera Pseudomonas, Acinetobacter, Shewanella, Aeromonas and Rheinheimera in both the planktonic and biofilm bacterial communities may be indicative of human impacts. The negative effects of anthropogenic activities, however, were more evident in the more stable biofilm communities compared to the rapidly changing planktonic ones. The differences in the relative abundances of Rhodobacter genus by water type and habitat may reflect this effect. The taxonomic composition of biofilm bacterial communities can therefore be useful indicators of long-term changes in water quality.
Methods
Description of the sampling locations
Five sampling locations were selected along the Hungarian section of the Danube River with various types and degrees of human influence (Fig. 5).
Fig. 5.
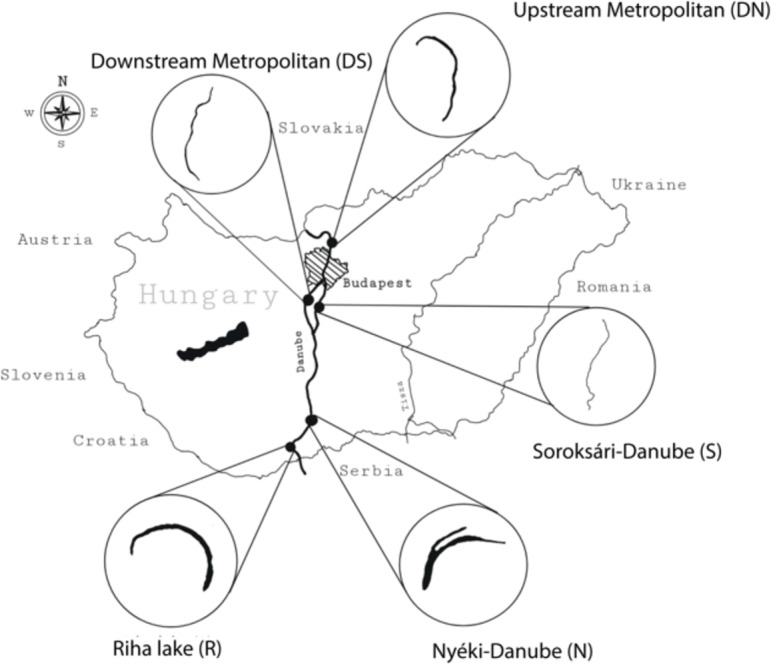
Sampling locations of the different riverine water bodies along the Hungarian section of river Danube (Abbreviations: upstream (north) from Budapest, DN; downstream (south) from Budapest, DS; Soroksári-Danube, S; Nyéki-Danube, N; Riha lake, R).
Two sampling sites were designated in the main river channel, upstream (between 1678 and 1674 river km) and downstream (between 1607 and 1604 river km) from the Hungarian capital14,15,21,72. Here, the river supplies water to about 2.5 million people in the metropolis and its agglomeration area.
Three additional sampling sites were designated in a side arm of the Danube and two backwaters. The Soroksári-Danube (between 1642 and 1586 river km) is the second largest side arm of the Danube in Hungary (Fig. 5). It maintains a continuous connection with the main river channel; although, a sluice system regulates its flow rate and water level. Due to the Kvassay and the Tass sluices, the flow rate of the side arm decreased drastically compared to the main river channel. The riverbed of the Soroksári-Danube becomes wider and deeper downstream, and the riparian reed zone becomes more extensive73. The side arm is heavily exposed to human activities. Surrounded by a densely populated agglomeration area with significant industrial activities, it also offers various recreational activities (e.g. relaxation, water sports, and fishing). Due to the water regulation and the resulting decrease in flow rate, sedimentation and pollution have increased in the side arm compared to the main river channel72,74.
The Nyéki-Danube (at the 1479 river km) is situated in the Gemenc floodplain area of the Duna-Dráva National Park (Fig. 5). This former river sidearm naturally became an oxbow lake more than 200 years ago. Although, it is in contact with the main river channel during floods. Its riverine body is surrounded by a wide reed belt75. Due to the floodings, its water level fluctuates greatly. Based on the properties of the water body, the water renewal is slow, so the high sedimentation results in periodically repeated drying out72,74,76.
Riha lake (at 1447 river km) is a separated oxbow lake (Fig. 5). This water body is also located within the area of the Duna-Dráva National Park, but there is a livestock farm in its vicinity. The oxbow lake has completely lost its connection with the main river channel and is fed only by groundwater and rainwater. The water body is surrounded by a reed belt, the lakebed is uneven and very shallow in some places72,74.
Sample collection and in situ physical-chemical measurements
In the main river channel of the Danube, three transects perpendicular to the shore have been designated upstream (between 1678 and 1674 river km) and downstream (between 1607 and 1604 river km) from the Hungarian capital as described by21. At the Soroksári-Danube side arm (between 1642 and 1586 river km), Nyéki-Danube (at the 1479 river km) and Riha lake (at 1447 river km), 300–500 m long sections parallel to the shore were selected as described by72. Water and biofilm samples were collected in May at the beginning of the vegetation period.
For microbiological analysis of planktonic bacterial communities and ex situ chemical measurements, water samples were collected with twelve replicates from a depth of 50 cm at each of the five sampling sites. The samples were collected into sterile 1 l glass bottles and stored at 4–6 °C until laboratory processing within 24 h.
Biofilms were collected from different substrates with 6 replicates per sampling sites. In the main river channel, where reeds are absent, biofilm communities develop on the pebble surfaces. Therefore, in the upstream and downstream sampling sites, epilithic biofilm samples were taken via from 1, 2, and 5 m water depths at the transects using benthic dredging. Samples from different depths were combined as composite samples per transect. A At the Soroksári-Danube side arm, the Nyéki-Danube and the Riha lake, a reed belt lines the shores and plays a key role in the water self-purification processes. These water bodies have muddy sediment without a gravel bed. Consequently, reed stems were sampled every 25–35 m along the open water edge of the reed stands. The biofilm samples were collected into disposable plastic bags and stored at 6–8 °C until laboratory processing within 24 h. A total of 90 samples (12 replicates of water samples and 6 replicates of biofilm samples from all 5 sampling sites) were used for DNA isolation, sequencing and statistical analysis.
In situ measurements were conducted at each sampling site. Temperature (°C), pH, electrical conductivity (S cm−1), dissolved oxygen (mg L−1), nitrate-N (mg L−1) were analysed by YSI EXO2 Multi-Parameter Water Quality Sonde in situ at the sampling sites. Orthophosphate (mg L−1) was determined by Spekord 210 Plus spectrophotometre (Analytik Jena, Germany), following Eaton et al. (2005)77. Total organic carbon (TOC, mg L−1) was determined by a Multi N/C 2100 S TC-TN analyser (Analytik Jena, Germany) equipped with a nondispersive infrared detector and a chemiluminescent detector, in accordance with the corresponding international standards (MSZ EN 1484:1998).
DNA extraction, Illumina MiSeq amplicon sequencing and bioinformatic analysis
For environmental DNA extraction 500 ml of water samples were concentrated by filtration using 0.22 μm pore-sized polycarbonate filters (Millipore, Billerica, MA, USA). Biofilm from the reed stems and pebbles was washed with saline solution using a sterile paintbrush. The samples were centrifugated (10000 rpm for 2 min). For DNA extraction, biomass from the water on the filter surface, and approximately 100 mg of biofilm compacted by centrifuge were used. DNA extraction was performed using the DNeasy Power Soil Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The DNA concentration of the samples was measured using Qubit 4 fluorometer (Thermo Fisher Scientific, USA).
The V3-V4 region of the 16S rRNA gene was amplified by PCR using the primers Bact 341F (5’-CCT ACG GGN GGC WGC AG-3’) and Bact 805R (5’-GAC TAC NVG GGT ATC TAA TCC-3’) designed for the study of Bacteria and Archaea during next-generation sequencing78. Before sequencing, the amplicon library was assessed using Agilent 2100 Bioanalyzer System (Agilent Technologies, Inc., USA). For sequencing, the Illumina MiSeq platform (Illumina, San Diego, California, USA) pair-end, dual-index sequencing technique was used, via the MiSeq Reagent Kit v3 providing 300 base long reads. The sequences in fastq format are deposited in NCBI as BioProjects PRJNA 838445 and 1119742.
The sequences were analysed using Qiime2 software79. The 500-base long aplicon sequences were achieved via the joining of the read pairs using vsearch module of the software. Using the same module, the quality check of the read was conducted. For the OTU (Operational Taxonomic Unit) picking a 97% identity threshold was used and the OTUs with coverage under 0.005% were filtered out in the vsearch module80,81. The taxonomical classification was conducted using SILVA SSU v.138 database (https://www.arb-silva.de/).
Statistical analysis
Prior to the statistical analysis, subsampling was performed using the Mothur v. 1.48.0 software82. For subsampling, the lowest sequencing number was selected based on the two sample types (water, biofilm). In case of water samples, the lowest read number was 3311 with 498 OTUs, 22,689 read number with 532 OTUs was selected for the biofilm samples. For the bacterial community analyses, the relative abundance of the OTUs was calculated. The Shannon, the Inverse Simpson diversity indexes, the Chao1 species richness and the Good’s coverage were also calculated by Mothur v. 1.48.0 software82. The R 4.2.3 software83 was used for statistical analysis and data visualisation. Shapiro-Wilk test was selected to examine the normality of the data, while the Bartlett test was used to examine the homogeneity of the data. Kruskal-Wallis and Dunn’s post-hoc tests with Bonferroni correction were performed to analyse significant differences between the water samples. principal component analysis (PCA), non-metric multidimensional scaling (NMDS), and UPGMA (unweighted pair group method using arithmetic averages) analysis were performed. The hierarchical cluster analysis and the non-metric multidimensional scaling were based on Bray-Curtis distances, and Euclidean distance was used in the principal component analysis. In the statistical test, the 0.05 p-value was considered significant. The correlation between the environmental parameters and the bacterial taxa was evaluated in R 4.2.3 software83 using envfit function within the vegan package.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was partly financed by the RRF 2.3.1 21 2022 00008 project implemented within the framework of the Széchenyi Terv Plusz program, as well as the National Excellence Project (2018-1.2.1-NKP-2018-00011). The authors would like to thank K. Németh and P. Dobosy for the water chemistry measurements.
Author contributions
K.J.L. and A.K.B. as first authors contributed equally to this work. A.I.E. and A.K.B. contributed to the conceptualization and design the study. D.A., G.K. and A.I.E. participated in the sampling. D.A. and G.K. performed the sample preparation for DNA sequencing. P.B.K. performed NGS data analyses. K.J.L. made statistical analyses with the instruction of A.K.B and A.I.E. K.J.L. and A.K.B. wrote the main part of the manuscript. All authors contributed, revised and approved the final version of the manuscript.
Funding
This work was partly financed by the RRF 2.3.1 21 2022 00008 project implemented within the framework of the Széchenyi Terv Plusz program, as well as the National Excellence Project (2018 − 1.2.1-NKP-2018-00011).
Data availability
The sequences in fastq format are deposited in NCBI as BioProjects PRJNA 838445 and 1119742.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kinga J. Lennert and Andrea K. Borsodi.
References
- 1.Shikiomanov, I. A. Water in Crisis- A Guide to the worlds’s Fresh Water resources (chap. 2). in Water in Crisis- A Guide to the Worlds’s Fresh Water Resources (ed Gleick, P. H.) 13–24 (Oxford University Press, (1993).
- 2.Gleick, P. H. & Shimabuku, M. Water-related conflicts: definitions, data, and trends from the water conflict chronology. Environ. Res. Lett. 1810.1088/1748-9326/acbb8f (2023).
- 3.Ferreira, V. et al. Ecosystem services provided by small streams: an overview. Hydrobiologia. 850, 2501–2535. 10.1007/s10750-022-05095-1 (2023). [Google Scholar]
- 4.Nichol, J. E., Choi, S. Y., Wong, M. S. & Abbas, S. Temperature change and urbanisation in a multi-nucleated megacity: China’s Pearl River delta. Urban Clim. 31, 100592. 10.1016/j.uclim.2020.100592 (2020). [Google Scholar]
- 5.Bowes, M. J. et al. Nutrient and microbial water quality of the upper Ganga River, India: identification of pollution sources. Environ. Monit. Assess. 19210.1007/s10661-020-08456-2 (2020). [DOI] [PubMed]
- 6.Jordaan, K. & Bezuidenhout, C. C. Bacterial community composition of an Urban River in the Northwest Province, South Africa, in relation to physico-chemical water quality. Environ. Sci. Pollut. 23, 5868–5880. 10.1007/s11356-015-5786-7 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Kumar Parida, P. et al. Impact of anthropogenic activity on community structure and function of microbiomes in polluted stretches of River Yamuna at New Delhi, India: insights from shotgun metagenomics. (2022). 10.21203/rs.3.rs-1282532/v1 [DOI] [PubMed]
- 8.Chen, W., Gu, T. & Zeng, J. Urbanisation and ecosystem health in the middle reaches of the Yangtze River urban agglomerations, China: a U-curve relationship. J. Environ. Manage. 318, 115565. 10.1016/j.jenvman.2022.115565 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Jin, L. et al. Assessing the impacts of climate change and socio-economic changes on flow and phosphorus flux in the Ganga River System. Environ. Sci. Process. Impacts. 17, 1098–1110. 10.1039/C5EM00092K (2015). [DOI] [PubMed] [Google Scholar]
- 10.González, S. O., Almeida, C. A., Calderón, M., Mallea, M. A. & González, P. Assessment of the water self-purification capacity on a river affected by organic pollution: application of chemometrics in spatial and temporal variations. Environ. Sci. Pollut. 21, 10583–10593. 10.1007/s11356-014-3098-y (2014). [DOI] [PubMed] [Google Scholar]
- 11.Findlay, S. Stream microbial ecology. J. North. Am. Benthol Soc. 29, 170–181. 10.1899/09-023.1 (2010). [Google Scholar]
- 12.Liu, T. et al. Integrated biogeography of planktonic and sedimentary bacterial communities in the Yangtze River. Microbiome. 610.1186/s40168-017-0388-x (2018). [DOI] [PMC free article] [PubMed]
- 13.Thomaz, S. M. Ecosystem services provided by freshwater macrophytes. Hydrobiologia. 850, 2757–2777. 10.1007/s10750-021-04739-y (2023). [Google Scholar]
- 14.Engloner, A. I., Vargha, M., Kós, P. & Borsodi, A. K. Planktonic and epilithic prokaryota community compositions in a large temperate river reflect climate change related seasonal shifts. PLoS One. 18, e0292057. 10.1371/journal.pone.0292057 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vargha, M. et al. From source to tap: tracking microbial diversity in a riverbank filtration-based drinking water supply system under changing hydrological regimes. Divers. (Basel). 15, 621. 10.3390/d15050621 (2023). [Google Scholar]
- 16.Papale, M. et al. Bacterial diversity in a dynamic and extreme sub-arctic watercourse (Pasvik River, Norwegian Arctic). Water (Basel). 12, 3098. 10.3390/w12113098 (2020). [Google Scholar]
- 17.Wei, G., Li, M., Li, F., Li, H. & Gao, Z. Distinct distribution patterns of prokaryotes between sediment and water in the Yellow River Estuary. Appl. Microbiol. Biotechnol. 100, 9683–9697. 10.1007/s00253-016-7802-3 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Kirschner, A. K. T. et al. Multiparametric monitoring of microbial faecal pollution reveals the dominance of human contamination along the whole Danube River. Water Res. 124, 543–555. 10.1016/j.watres.2017.07.052 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mănoiu, V. M. & Crăciun, A. I. Danube River water quality trends: a qualitative review based on the open access web of science database. Ecohydrol Hydrobiol. 21, 613–628. 10.1016/j.ecohyd.2021.08.002 (2021). [Google Scholar]
- 20.Engloner, A. I., Németh, K., Dobosy, P. & Óvári, M. Exploring the trend effects of diffuse anthropogenic pollution in a large river passing through a densely populated area. Heliyon. 9, e20120. 10.1016/j.heliyon.2023.e20120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engloner, A. I., Németh, K., Kós, P. B., Meglécz, E. & Bereczki, J. Genetic diversity of the submerged macrophyte Ceratophyllum demersum depends on habitat hydrology and habitat fragmentation. Front. Plant. Sci. 14, 1277916. 10.3389/fpls.2023.1277916 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemke, M. J. et al. Description of freshwater bacterial assemblages from the Upper Paraná River floodpulse system, Brazil. Microb. Ecol. 57, 94–103. 10.1007/s00248-008-9398-3 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Liu, Z., Huang, S., Sun, G., Xu, Z. & Xu, M. Phylogenetic diversity, composition and distribution of bacterioplankton community in the Dongjiang River, China. FEMS Microbiol. Ecol. 80, 30–44. 10.1111/j.1574-6941.2011.01268.x (2012). [DOI] [PubMed] [Google Scholar]
- 24.Savio, D. et al. Bacterial diversity along a 2600 km river continuum. Environ. Microbiol. 17, 4994–5007. 10.1111/1462-2920.12886 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, H. et al. Spatial and temporal dynamics of microbial community composition and factors influencing the surface water and sediments of urban rivers. J. Environ. Sci. 124, 187–197. 10.1128/AEM.01849-06 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Winter, C., Hein, T., Kavka, G., Mach, R. L. & Farnleitner, A. H. Longitudinal changes in the bacterial community composition of the Danube River: a whole-river approach. Appl. Environ. Microbiol. 73, 421–431 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kildare, B. J. et al. 16S rRNA-based assays for quantitative detection of universal, human-, cow-, and dog-specific fecal Bacteroidales: a bayesian approach. Water Res. 41, 3701–3715. 10.1016/j.watres.2007.06.037 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Chiriac, M., Haber, M. & Salcher, M. M. Adaptive genetic traits in pelagic freshwater microbes. Environ. Microbiol. 25, 606–641. 10.1111/1462-2920.16313 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Farkas, M. et al. Planktonic and benthic bacterial communities of the largest central European shallow lake, Lake Balaton and its main inflow Zala River. Curr. Microbiol. 77, 4016–4028. 10.1007/s00284-020-02241-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coclet, C. et al. Changes in bacterioplankton communities resulting from direct and indirect interactions with trace metal gradients in an urbanized marine coastal area. Front. Microbiol. 10, 257. 10.3389/fmicb.2019.00257 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, H., Chen, F., Zhang, C., Wang, M. & Kan, J. Estuarine gradients dictate spatiotemporal variations of microbiome networks in the Chesapeake Bay. Environ. Microbiome. 16, 22. 10.1186/s40793-021-00392-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szuróczki, S. et al. Prokaryotic community composition in a great shallow soda lake covered by large reed stands (Neusiedler See/Lake Fertő) as revealed by cultivation-and DNA-based analyses. FEMS Microbiol. Ecol. 96, fiaa159. 10.1093/femsec/fiaa159 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Morency, C., Jacquemot, L., Potvin, M. & Lovejoy, C. A microbial perspective on the local influence of Arctic rivers and estuaries on Hudson Bay (Canada). Elem. Sci. Anth. 10, 00009. 10.1525/elementa.2021.00009 (2022). [Google Scholar]
- 34.Kasalický, V., Jezbera, J., Hahn, M. W. & Šimek, K. The diversity of the Limnohabitans Genus, an important group of freshwater bacterioplankton, by characterization of 35 isolated strains. PLoS One. 8, e58209. 10.1371/journal.pone.0058209 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mentes, A. et al. Differences in planktonic microbial communities associated with three types of macrophyte stands in a shallow lake. FEMS Microbiol. Ecol. 94, 1–12. 10.1093/femsec/fix164 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Šimek, K. et al. Broad habitat range of the phylogenetically narrow r-bt065 cluster, representing a core group of the betaproteobacterial genus Limnohabitans. Appl. Environ. Microbiol. 76, 631–639. 10.1128/AEM.02203-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton, R. J. & McLellan, S. L. A unique assemblage of cosmopolitan freshwater bacteria and higher community diversity differentiate an urbanized estuary from Oligotrophic Lake Michigan. Front. Microbiol. 6, 1028. 10.1128/MMBR.00028-10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newton, R. J., Jones, S. E., Eiler, A., McMahon, K. D. & Bertilsson, S. A guide to the natural history of freshwater lake bacteria. Microbiol. Mol. Biol. Rev. 75, 14–49. 10.3389/fmicb.2015.01028 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukamura, M. The non-pathogenic mycobacteria: their distribution and ecology in non-living reservoirs. In: (eds Kubica, G. P. & Wayne, L. G.) The Mycobacteria: A Source Book, Part B. New York: Marcel Dekker. 1339–1359 (1984). [Google Scholar]
- 40.Honda, J. R., Virdi, R. & Chan, E. D. Global environmental nontuberculous mycobacteria and their contemporaneous man-made and natural niches. Front. Microbiol. 910.3389/fmicb.2018.02029 (2018). [DOI] [PMC free article] [PubMed]
- 41.Falkinham, I. I. I. Surrounded by Mycobacteria: Nontuberculous Mycobacteria in the human environment. J. Appl. Microbiol. 107, 356–367. 10.1111/j.1365-2672.2009.04161.x (2009). [DOI] [PubMed] [Google Scholar]
- 42.Vaerewijck, M. J. M., Huys, G., Palomino, J. C., Swings, J. & Portaels, F. Mycobacteria in drinking water distribution systems: Ecology and significance for human health. FEMS Microbiol. Rev. 29, 911–934. 10.1016/j.femsre.2005.02.001 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Guo, D. et al. Bacterial community analysis of two neighboring freshwater lakes originating from one lake. Pol. J. Environ. Stud. 30, 111–117. 10.15244/pjoes/119094 (2020). [Google Scholar]
- 44.O’Sullivan, L. A., Rinna, J., Humphreys, G., Weightman, A. J. & Fry, J. C. Fluviicola taffensis gen. nov., sp. nov., a novel freshwater bacterium of the family Cryomorphaceae in the phylum ‘Bacteroidetes’. Int. J. Syst. Evol. Microbiol. 55, 2189–2194. 10.1099/ijs.0.63736-0 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Hahn, M. W., Lang, E., Brandt, U., Wu, Q. L. & Scheuerl, T. Emended description of the genus Polynucleobacter and the species Polynucleobacter necessarius and proposal of two subspecies, P. Necessarius subsp. Necessarius subsp. nov. and P. Necessarius subsp. Asymbioticus subsp. Nov. Int. J. Syst. Evol. Microbiol. 59, 2002–2009. 10.1099/ijs.0.005801-0 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hahn, M. W. et al. Polynucleobacter cosmopolitanus sp. nov., free-living planktonic bacteria inhabiting freshwater lakes and rivers. Int. J. Syst. Evol. Microbiol. 60, 166–173. 10.1099/ijs.0.010595-0 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hahn, M. W., Jezberová, J., Koll, U., Saueressig-Beck, T. & Schmidt, J. Complete ecological isolation and cryptic diversity in Polynucleobacter bacteria not resolved by 16S rRNA gene sequences. ISME J. 10, 1642–1655. 10.1038/ismej.2015.237 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salcher, M. M., Neuenschwander, S. M., Posch, T. & Pernthaler, J. The ecology of pelagic freshwater methylotrophs assessed by a high-resolution monitoring and isolation campaign. ISME J. 9, 2442–2453. 10.1038/ismej.2015.55 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Hadithi, S. A. & Goulder, R. Physiological state of epiphytic bacteria on submerged stems of the reed Phragmites australis compared with planktonic bacteria in gravel-pit ponds. J. Appl. Bacteriol. 66, 107–117. 10.1111/j.1365-2672.1989.tb02460.x (1989). [Google Scholar]
- 50.Borsodi, A. K., Farkas, I. & Kurdi, P. Numerical analysis of planktonic and reed biofilm bacterial communities of Lake Fertő (Neusiedlersee, Hungary/Austria). Water Res. 32, 1831–1840. 10.1016/S0043-1354(97)00423-5 (1998). [Google Scholar]
- 51.Parfenova, V. V., Gladkikh, A. S. & Belykh, O. I. Comparative analysis of biodiversity in the planktonic and biofilm bacterial communities in Lake Baikal. Microbiol. (N Y). 82, 91–101. 10.1134/S0026261713010128 (2013). [Google Scholar]
- 52.Yamamoto, M., Murai, H., Takeda, A., Okunishi, S. & Morisaki, H. Bacterial flora of the biofilm formed on the submerged surface of the reed Phragmites austmlis. Microbes Environ. 20, 14–24. 10.1264/jsme2.20.14 (2005). [Google Scholar]
- 53.Brooker, M. & Johnson, P. C. The behaviour of phosphate, nitrate, chloride and hardness in twelve Welsh rivers. Water Res. 18, 1155–1164. 10.1016/0043-1354(84)90232-X (1984). [Google Scholar]
- 54.Schoumans, O. F., Bouraoui, F., Kabbe, C., Oenema, O. & van Dijk, K. C. Phosphorus management in Europe in a changing world. Ambio. 44, 180–192. 10.1007/s13280-014-0613-9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barreto, S., Ács, É., Makk, J., Bugyi, G. & Böddi, B. Preliminary algological investigations in Soroksár-arm of River Danube. Limnologishe Berichte Donau. 32, 159–162 (1997). http://real.mtak.hu/id/eprint/4207 [Google Scholar]
- 56.Kiss, K. T., Pápista, É. K., Ács, É. & Makk, J. Comparison of phytoplankton of 80s and late 90s in a large side arm of the Danube River (Soroksár-Danube-Hungary). in International Assocation for danube Research of The International Association of Theoretical and Applied Limnology 103–110. (2000). http://real.mtak.hu/id/eprint/4211
- 57.Szabó, K., Ács, É. & Pápista, É. Periphhyton and phytoplankton in the Soroksár-Danube in Hungary. I. Periphytic algae on reed stems. Acta Bot. Hung. 43, 13–35. 10.1556/ABot.43.2001.1-2.2 (2001). [Google Scholar]
- 58.Palleroni, N. J. Pseudomonas. in Bergey’s Manual of Systematics of Archaea and Bacteria 1–1. (2015). 10.1002/9781118960608.gbm01210
- 59.Vargha, M., Takáts, Z. & Márialigeti, K. Degradation of atrazine in a laboratory scale model system with Danube River sediment. Water Res. 39, 1560–1568. 10.1016/j.watres.2004.10.013 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Xue, F. et al. Heterologous overexpression of a novel halohydrin dehalogenase from Pseudomonas pohangensis and modification of its enantioselectivity by semi-rational protein engineering. Int. J. Biol. Macromol. 146, 80–88. 10.1016/j.ijbiomac.2019.12.203 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Liébana, R. et al. Unravelling the interactions among microbial populations found in activated sludge during biofilm formation. FEMS Microbiol. Ecol. 92, fiw134. 10.1093/femsec/fiw134 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Yang, G., Gong, M., Mai, L., Zhuang, L. & Zeng, E. Y. Diversity and structure of microbial biofilms on microplastics in riverine waters of the Pearl River Delta, China. Chemosphere. 272, 129870. 10.1016/j.chemosphere.2021.129870 (2021). [DOI] [PubMed] [Google Scholar]
- 63.Idomeh, J. E. et al. Petroleum hydrocarbon impacted aquatic ecosystem reveals Methylotenera as the dominant genera in the Niger Delta Region of Nigeria. Geomicrobiol. J. 38, 879–894. 10.1080/01490451.2021.1973614 (2021). [Google Scholar]
- 64.Mohsin, H., Asif, A. & Rehman, Y. Anoxic growth optimization for metal respiration and photobiological hydrogen production by arsenic-resistant Rhodopseudomonas and Rhodobacter species. J. Basic. Microbiol. 59, 1208–1216. 10.1002/jobm.201900100 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Peng, W. et al. Bioremediation of Cadmium- and zinc-contaminated soil using Rhodobacter sphaeroides. Chemosphere. 197, 33–41. 10.1016/j.chemosphere.2018.01.017 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Wu, P. et al. The organophosphorus pesticides in soil was degradated by Rhodobacter sphaeroides after wastewater treatment. Biochem. Eng. J. 141, 247–251. 10.1016/j.bej.2018.07.019 (2019). [Google Scholar]
- 67.Capson-Tojo, G. et al. Purple phototrophic bacteria for resource recovery: challenges and opportunities. Biotechnol. Adv. 43, 107567. 10.1016/j.biotechadv.2020.107567 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Lv, P. et al. Diversity of culturable aerobic denitrifying bacteria in the sediment, water and biofilms in Liangshui River of Beijing, China. Sci. Rep. 7, 10032. 10.1038/s41598-017-09556-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rusznyák, A., Vladár, P., Szabó, G., Márialigeti, K. & Borsodi, A. K. Phylogenetic and metabolic bacterial diversity of Phragmites australis Periphyton communities in two Hungarian soda ponds. Extremophiles. 12, 763–773. 10.1007/s00792-008-0183-5 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Balzer, M., Witt, N., Flemming, H. C. & Wingender, J. Faecal indicator bacteria in river biofilms. Water Sci. Technol. 61, 1105–1111. 10.2166/wst.2010.022 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Yuan, L. et al. The complete genome sequence of Rahnella aquatilis ZF7 reveals potential beneficial properties and stress tolerance capabilities. Arch. Microbiol. 202, 483–499. 10.1007/s00203-019-01758-1 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Engloner, A. I. & Szegő, D. Genetic diversity of riverine reed stands indicating the water regime of the habitat. Ecol. Indic. 61, 846–849. 10.1016/j.ecolind.2015.10.037 (2016). [Google Scholar]
- 73.Vadadi-Fülöp, C. S., Mészáros, G., Jablonszky, G. & Hufnagel, L. Ecology of the Ráckeve-Soroksár Danube-a review. Appl. Ecol. Environ. Res. (2007). http://www.ecology.uni-corvinus.hu/
- 74.Engloner, A. I., Németh, K., Gere, D., Stefán, D. & Óvári, M. Effects of water depth and water level fluctuation on the total and bio-available element concentrations in riverine reed stands. Ecol. Indic. 114, 106328. 10.1016/j.ecolind.2020.106328 (2020). [Google Scholar]
- 75.Mátrai, I., Lakatos, G., Czudar, A. & Szlávik, L. Forecast of changes concerning the water budget in a wetland of Danube floodplain. J. Environ. Sci. Eng. 5, (2011).
- 76.Mátrai, I., Buzatzky, G. & Lakatos, G. Ecological status of waterfowl habitat on the Gemenc floodplain area in Hungary. J. Ecol. Nat. Environ. 1, 120–129 (2009). [Google Scholar]
- 77.Eaton, A. D., Clesceri, L. S., Rice, E. W. & Greenberg, A. E. Standard Methods for the Examination of Water and Wastewater 21st ed (APHA, AWWA, WEF, 2005).
- 78.Takahashi, S., Tomita, J., Nishioka, K., Hisada, T. & Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS One. 9, e105592. 10.1371/journal.pone.0105592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. 10.1038/s41587-019-0209-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bokulich, N. A. et al. Quality-filtering vastly improves diversity estimates from Illumina Amplicon sequencing. Nat. Methods. 10, 57–59. 10.1038/nmeth.2276 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahé, F. VSEARCH: a Versatile Open Source Tool for Metagenomics. PeerJ. 4, e2584. 10.7717/peerj.2584 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schloss, P. D. et al. Introducing Mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. 10.1128/AEM.01541-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.R Core Team. R: A language and environment for statistical computing. Preprint at. (2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences in fastq format are deposited in NCBI as BioProjects PRJNA 838445 and 1119742.