Abstract
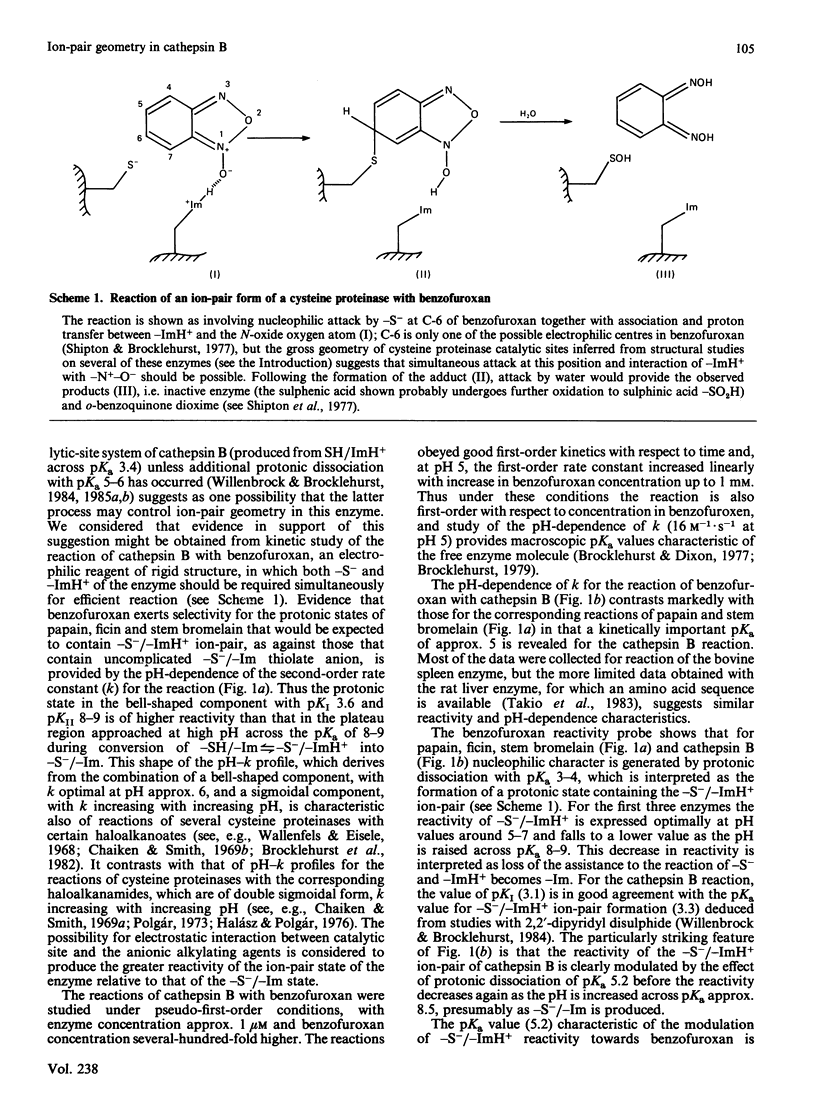
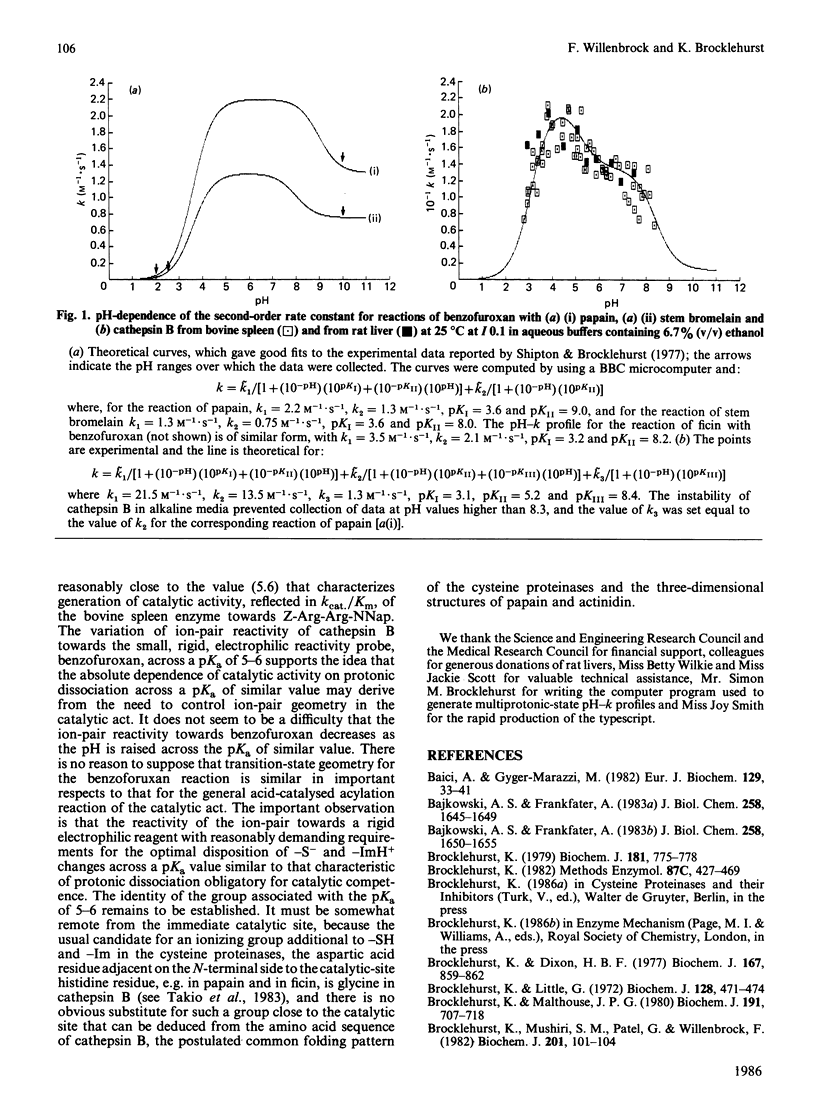
Benzofuroxan reacts with the catalytic-site thiol group of cathepsin B (EC 3.4.22.1) to produce stoichiometric amount of the chromophoric reduction product, o-benzoquinone dioxime. In a study of the pH-dependence of the kinetics of this reaction, most data were collected for the bovine spleen enzyme, but the more limited data collected for the rat liver enzyme were closely similar both in the magnitude of the values of the second-order rate constants (k) and in the shape of the pH-k profile. In acidic and weakly alkaline media, the reaction is faster than the reactions of benzofuroxan with some other cysteine proteinases. For example, in the pH region around 5-6, the reaction of cathepsin B is about 10 times faster than that of papain, 15 times faster than that of stem bromelain and 6 times faster than that of ficin. The pH-dependence of k for the reaction of cathepsin B with benzofuroxan was determined in the pH range 2.7-8.3. In marked contrast with the analogous reactions of papain, ficin and stem bromelain [reported by Shipton & Brocklehurst (1977) Biochem. J. 167, 799-810], the pH-k profile for the cathepsin B reaction contains a sigmoidal component with pKa 5.2 in which k increases with decrease in pH. This modulation of the reactivity of the catalytic-site -S-/-ImH+ ion-pair state of cathepsin B (produced by protonic dissociation from -SH/-ImH+ with pKa approx. 3) towards a small, rigid, electrophilic reagent, in a reaction that appears to involve both components of the ion-pair for efficient reaction, suggests that the state of ionization of a group associated with a molecular pKa of approx. 5 may control ion-pair geometry. This might account for the remarkable finding [reported by Willenbrock & Brocklehurst (1984) Biochem. J. 222, 805-814] that, although the ion-pair appears to be generated in cathepsin B as the pH is increased across pKa 3.4, catalytic competence is not generated until the pH is increased across pKa 5-6.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baici A., Gyger-Marazzi M. The slow, tight-binding inhibition of cathepsin B by leupeptin. A hysteretic effect. Eur J Biochem. 1982 Dec;129(1):33–41. doi: 10.1111/j.1432-1033.1982.tb07017.x. [DOI] [PubMed] [Google Scholar]
- Bajkowski A. S., Frankfater A. Steady state kinetic evidence for an acyl-enzyme intermediate in reactions catalyzed by bovine spleen cathepsin B. J Biol Chem. 1983 Feb 10;258(3):1645–1649. [PubMed] [Google Scholar]
- Bajkowski A. S., Frankfater A. The pH dependency of bovine spleen cathepsin B-catalyzed transfer of N alpha-benzyloxycarbonyl-L-lysine from p-nitrophenol to water and dipeptide nucleophiles. Comparisons with papain. J Biol Chem. 1983 Feb 10;258(3):1650–1655. [PubMed] [Google Scholar]
- Brocklehurst K., Dixon H. B. The pH-dependence of second-order rate constants of enzyme modification may provide free-reactant pKa values. Biochem J. 1977 Dec 1;167(3):859–862. doi: 10.1042/bj1670859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactivities of the various protonic states in the reactions of papain and of L-cysteine with 2,2'- and with 4,4'- dipyridyl disulphide: evidence for nucleophilic reactivity in the un-ionized thiol group of the cysteine-25 residue of papain occasioned by its interaction with the histidine-159-asparagine-175 hydrogen-bonded system. Biochem J. 1972 Jun;128(2):471–474. doi: 10.1042/bj1280471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P. Evidence for a two-state transition in papain that may have no close analogue in ficin. Differences in the disposition of cationic sites and hydrophobic binding areas in the active centres of papain and ficin. Biochem J. 1980 Dec 1;191(3):707–718. doi: 10.1042/bj1910707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Mushiri S. M., Patel G., Willenbrock F. Evidence for a close similarity in the catalytic sites of papain and ficin in near-neutral media despite differences in acidic and alkaline media. Kinetics of the reactions of papain and ficin with chloroacetate. Biochem J. 1982 Jan 1;201(1):101–104. doi: 10.1042/bj2010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. The equilibrium assumption is valid for the kinetic treatment of most time-dependent protein-modification reactions. Biochem J. 1979 Sep 1;181(3):775–778. doi: 10.1042/bj1810775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. Two-protonic-state electrophiles as probes of enzyme mechanisms. Methods Enzymol. 1982;87:427–469. doi: 10.1016/s0076-6879(82)87026-2. [DOI] [PubMed] [Google Scholar]
- Chaiken I. M., Smith E. L. Reaction of chloroacetamide with the sulfhydryl group of papain. J Biol Chem. 1969 Oct 10;244(19):5087–5094. [PubMed] [Google Scholar]
- Chaiken I. M., Smith E. L. Reaction of the sulfhydryl group of papain with chloroacetic acid. J Biol Chem. 1969 Oct 10;244(19):5095–5099. [PubMed] [Google Scholar]
- Halász P., Polgár L. Effect of the immediate environment on the reactivity of the essential -SH group of papain. Eur J Biochem. 1976 Dec 11;71(2):571–575. doi: 10.1111/j.1432-1033.1976.tb11147.x. [DOI] [PubMed] [Google Scholar]
- Kamphuis I. G., Drenth J., Baker E. N. Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain. J Mol Biol. 1985 Mar 20;182(2):317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Lewis S. D., Johnson F. A., Ohno A. K., Shafer J. A. Dependence of the catalytic activity of papain on the ionization of two acidic groups. J Biol Chem. 1978 Jul 25;253(14):5080–5086. [PubMed] [Google Scholar]
- Polgár L. On the mode of activation of the catalytically essential sulfhydryl group of papain. Eur J Biochem. 1973 Feb 15;33(1):104–109. doi: 10.1111/j.1432-1033.1973.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Salih E., Brocklehurst K. Investigation of the catalytic site of actinidin by using benzofuroxan as a reactivity probe with selectivity for the thiolate-imidazolium ion-pair systems of cysteine proteinases. Evidence that the reaction of the ion-pair of actinidin (pKI 3.0, pKII 9.6) is modulated by the state of ionization of a group associated with a molecular pKa of 5.5. Biochem J. 1983 Sep 1;213(3):713–718. doi: 10.1042/bj2130713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Brochlehurst K. Characterization of the papain active centre by using two-protonic-state electrophiles as reactivity probes. Evidence for nucleophilic reactivity in the un-interrupted cysteine-25-histidine-159 interactive system. Biochem J. 1978 May 1;171(2):385–401. doi: 10.1042/bj1710385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Brocklehurst K. Benzofuroxan as a thiol-specific reactivity probe. Kinetics of its reactions with papain, ficin, bromelain and low-molecular-weight thiols. Biochem J. 1977 Dec 1;167(3):799–810. doi: 10.1042/bj1670799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Stuchbury T., Brocklehurst K. Evaluation of benzofuroxan as a chromophoric oxidizing agent for thiol groups by using its reactions with papain, ficin, bromelain and low-molecular-weight thiols. Biochem J. 1977 Mar 1;161(3):627–637. doi: 10.1042/bj1610627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyterman L. A., Wijdenes J. Benzoylamidoacetonitrile as an inhibitor of papain. Biochim Biophys Acta. 1973 Mar 15;302(1):95–101. doi: 10.1016/0005-2744(73)90012-0. [DOI] [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Teller D. C., Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenfels K., Eisele B. Stereospecific alkylation with asymmetric reagents. Eur J Biochem. 1968 Jan;3(3):267–275. doi: 10.1111/j.1432-1033.1968.tb19526.x. [DOI] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. A general framework of cysteine-proteinase mechanism deduced from studies on enzymes with structurally different analogous catalytic-site residues Asp-158 and -161 (papain and actinidin), Gly-196 (cathepsin B) and Asn-165 (cathepsin H). Kinetic studies up to pH 8 of the hydrolysis of N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide catalysed by cathepsin B and of L-arginine 2-naphthylamide catalysed by cathepsin H. Biochem J. 1985 Apr 15;227(2):521–528. doi: 10.1042/bj2270521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Natural structural variation in enzymes as a tool in the study of mechanism exemplified by a comparison of the catalytic-site structure and characteristics of cathepsin B and papain. pH-dependent kinetics of the reactions of cathepsin B from bovine spleen and from rat liver with a thiol-specific two-protonic-state probe (2,2'-dipyridyl disulphide) and with a specific synthetic substrate (N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide). Biochem J. 1984 Sep 15;222(3):805–814. doi: 10.1042/bj2220805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Preparation of cathepsins B and H by covalent chromatography and characterization of their catalytic sites by reaction with a thiol-specific two-protonic-state reactivity probe. Kinetic study of cathepsins B and H extending into alkaline media and a rapid spectroscopic titration of cathepsin H at pH 3-4. Biochem J. 1985 Apr 15;227(2):511–519. doi: 10.1042/bj2270511. [DOI] [PMC free article] [PubMed] [Google Scholar]


