Abstract
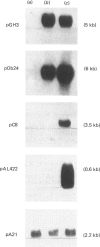
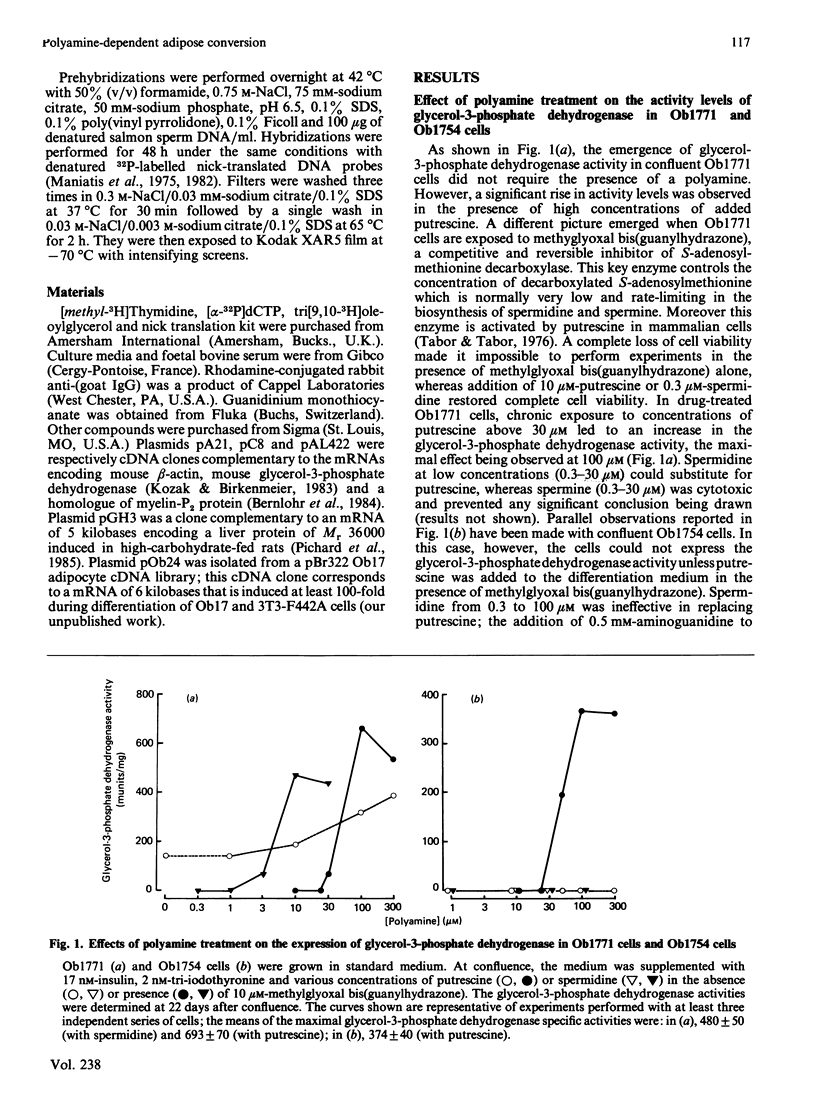
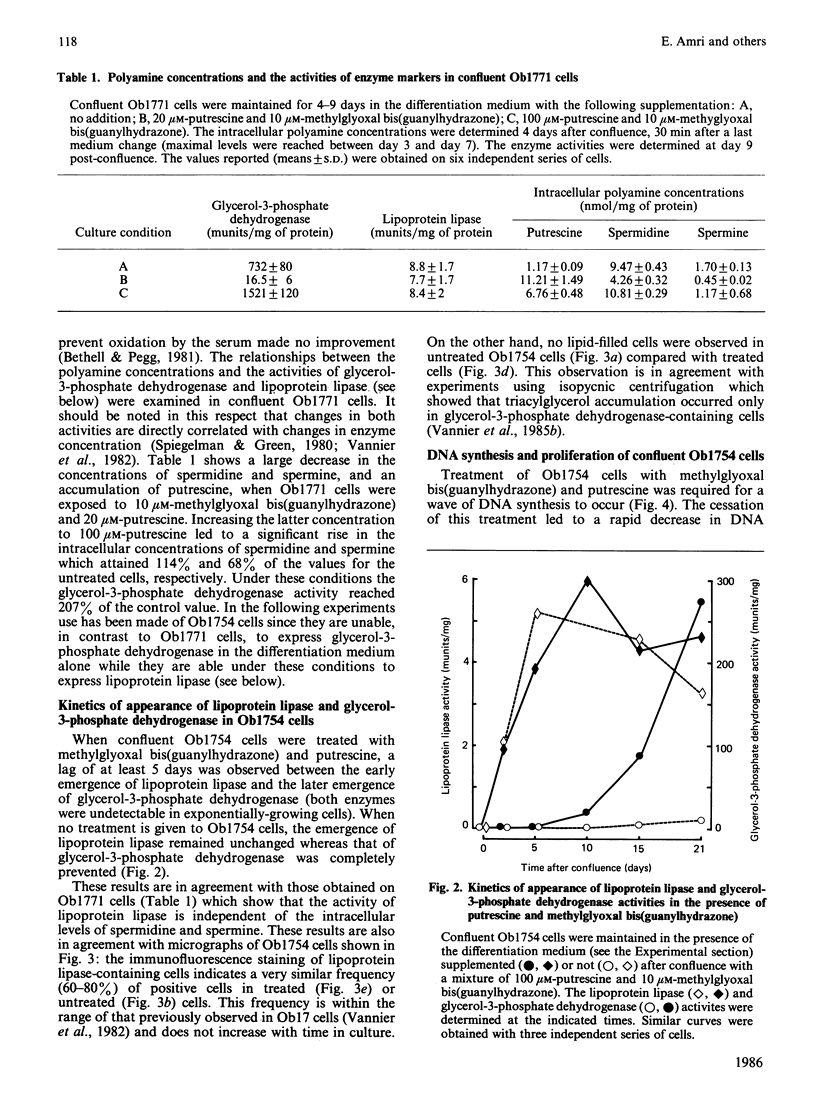
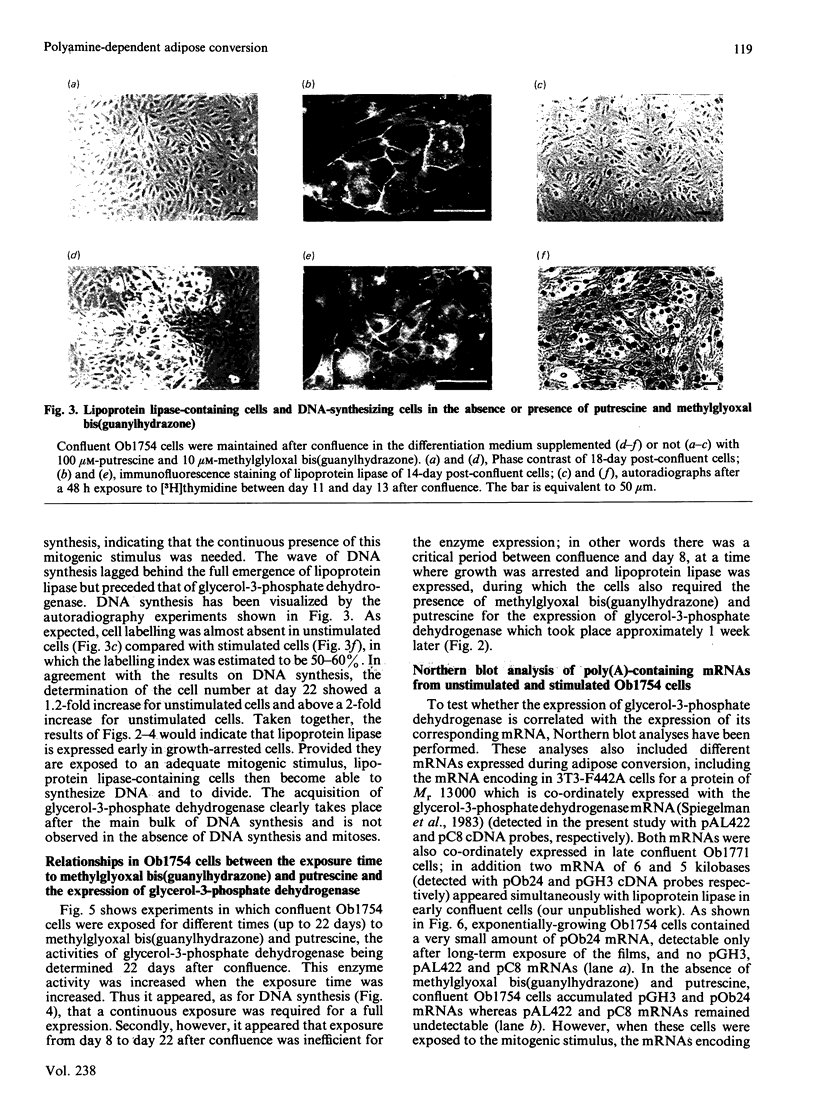
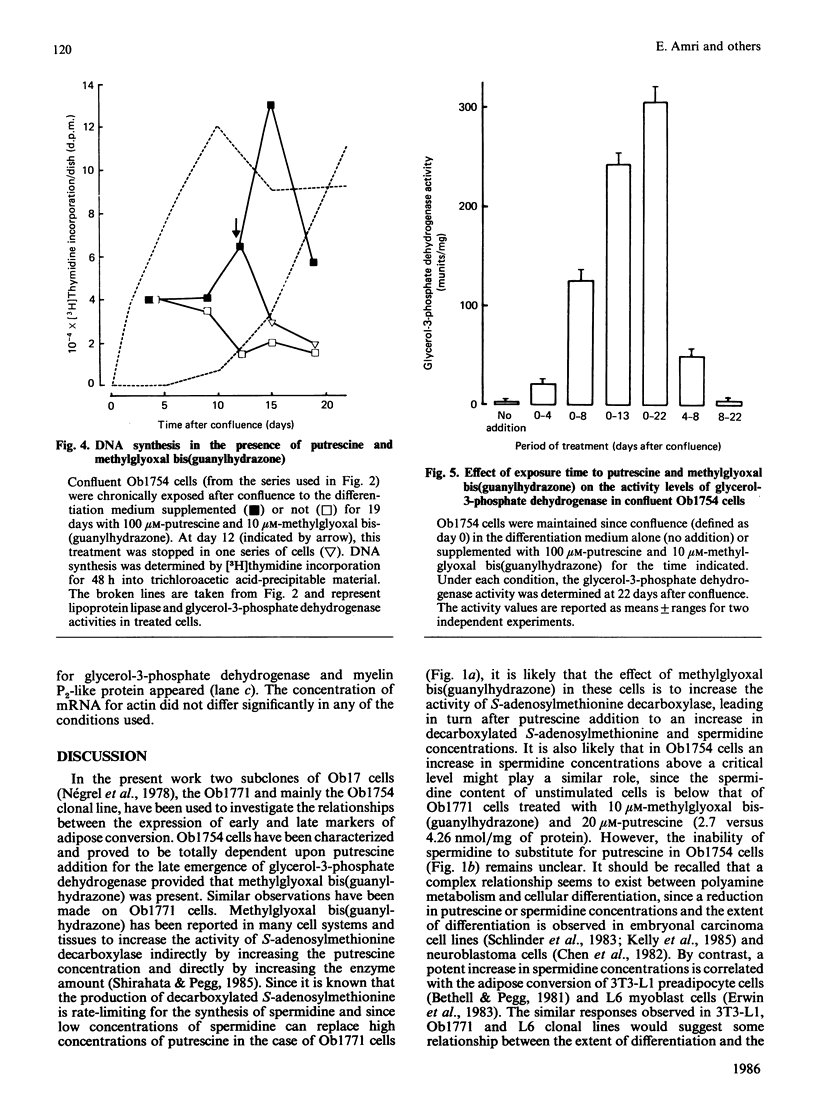
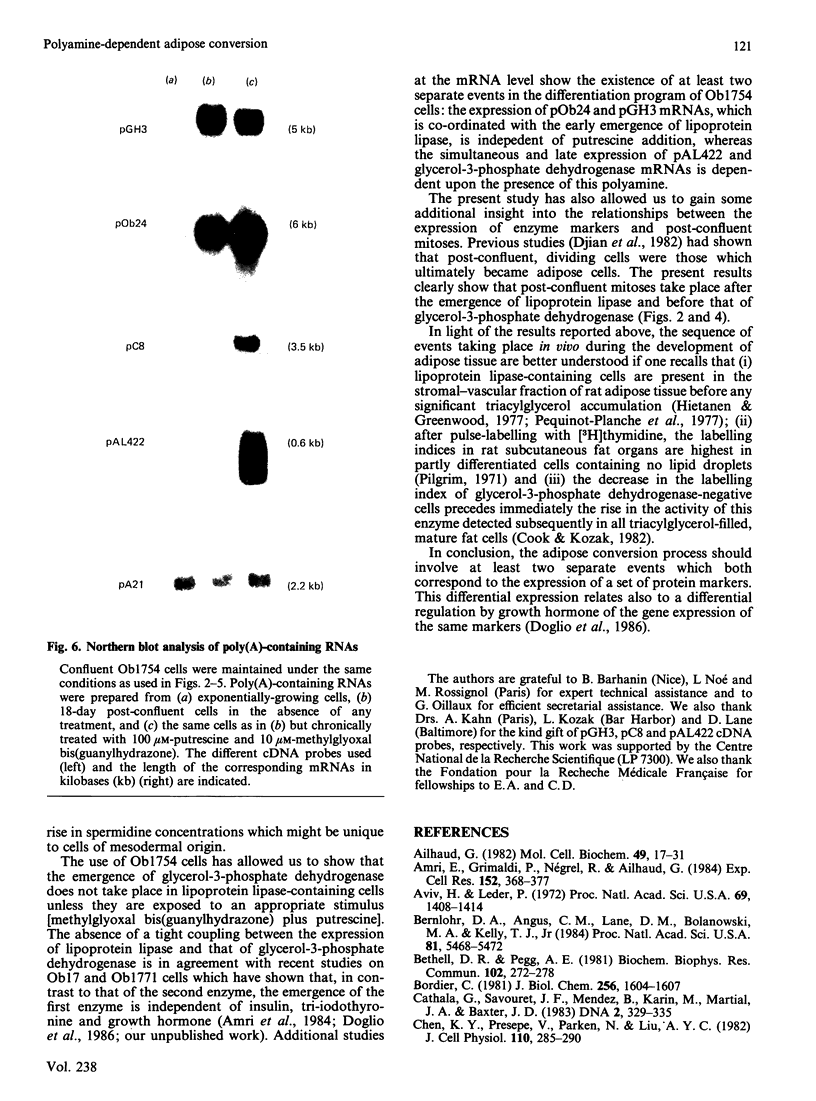
A subclone of preadipocyte Ob17 cells has been isolated (Ob1754 clonal line). Confluent Ob1754 cells treated with an inhibitor of spermidine and spermine synthesis, methylglyoxal bis(guanylhydrazone), were totally dependent upon putrescine addition for the expression of glycerol-3-phosphate dehydrogenase which behaved as a late marker of adipose conversion. Under these conditions, the early expression of lipoprotein lipase during growth arrest remained unchanged. Studies at the mRNA level showed that the expression of unidentified pOb24 and pGH3 mRNAs, which was parallel to that of lipoprotein lipase, is independent of polyamine addition whereas the late emergence of glycerol-3-phosphate dehydrogenase mRNA was putrescine-dependent and co-ordinated with the expression of pAL422 mRNA encoding for a myelin-P2 homologue [Bernlohr, Angus, Lane, Bolanowski & Kelly (1984) Proc. Natl. Acad. Sci. U.S.A. 81, 5468-5472]. The appearance of lipoprotein lipase preceded DNA synthesis and post-confluent mitoses which were both putrescine-dependent and which took place before the appearance of glycerol-3-phosphate dehydrogenase. Thus the adipose conversion of Ob1754 cells involves the expression of at least two separate sets of markers which are differently regulated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ailhaud G. Adipose cell differentiation in culture. Mol Cell Biochem. 1982 Nov 12;49(1):17–31. doi: 10.1007/BF00230992. [DOI] [PubMed] [Google Scholar]
- Amri E. Z., Grimaldi P., Négrel R., Ailhaud G. Adipose conversion of ob17 cells. Insulin acts solely as a modulator in the expression of the differentiation program. Exp Cell Res. 1984 Jun;152(2):368–377. doi: 10.1016/0014-4827(84)90638-4. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr D. A., Angus C. W., Lane M. D., Bolanowski M. A., Kelly T. J., Jr Expression of specific mRNAs during adipose differentiation: identification of an mRNA encoding a homologue of myelin P2 protein. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5468–5472. doi: 10.1073/pnas.81.17.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethell D. R., Pegg A. E. Polyamines are needed for the differentiation of 3T3-L1 fibroblasts into adipose cells. Biochem Biophys Res Commun. 1981 Sep 16;102(1):272–278. doi: 10.1016/0006-291x(81)91517-5. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chen K. Y., Presepe V., Parken N., Liu A. Y. Changes of ornithine decarboxylase activity and polyamine content upon differentiation of mouse NB-15 neuroblastoma cells. J Cell Physiol. 1982 Mar;110(3):285–290. doi: 10.1002/jcp.1041100311. [DOI] [PubMed] [Google Scholar]
- Cook J. R., Kozak L. P. Sn-glycerol-3-phosphate dehydrogenase gene expression during mouse adipocyte development in vivo. Dev Biol. 1982 Aug;92(2):440–448. doi: 10.1016/0012-1606(82)90189-0. [DOI] [PubMed] [Google Scholar]
- Cook K. S., Groves D. L., Min H. Y., Spiegelman B. M. A developmentally regulated mRNA from 3T3 adipocytes encodes a novel serine protease homologue. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6480–6484. doi: 10.1073/pnas.82.19.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djian P., Grimaldi P., Négrel R., Ailhaud G. Adipose conversion of OB17 preadipocytes. Relationships between cell division and fat cell cluster formation. Exp Cell Res. 1982 Dec;142(2):273–281. doi: 10.1016/0014-4827(82)90368-8. [DOI] [PubMed] [Google Scholar]
- Doglio A., Dani C., Grimaldi P., Ailhaud G. Growth hormone regulation of the expression of differentiation-dependent genes in preadipocyte Ob1771 cells. Biochem J. 1986 Aug 15;238(1):123–129. doi: 10.1042/bj2380123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin B. G., Ewton D. Z., Florini J. R., Pegg A. E. Polyamine depletion inhibits the differentiation of L6 myoblast cells. Biochem Biophys Res Commun. 1983 Aug 12;114(3):944–949. doi: 10.1016/0006-291x(83)90651-4. [DOI] [PubMed] [Google Scholar]
- Etienne J., Noé L., Rossignol M., Arnaud C., Vydelingum N., Kissebah A. H. Antibody against rat adipose tissue lipoprotein lipase. Biochim Biophys Acta. 1985 Mar 27;834(1):95–102. doi: 10.1016/0005-2760(85)90180-8. [DOI] [PubMed] [Google Scholar]
- Gaillard D., Ailhaud G., Négrel R. Fetuin modulates growth and differentiation of Ob17 preadipose cells in serum-free hormone-supplemented medium. Biochim Biophys Acta. 1985 Jul 30;846(1):185–191. doi: 10.1016/0167-4889(85)90125-9. [DOI] [PubMed] [Google Scholar]
- Hietanen E., Greenwood M. R. A comparison of lipoprotein lipase activity and adipocyte differentiation in growing male rats. J Lipid Res. 1977 Jul;18(4):480–490. [PubMed] [Google Scholar]
- Kelly M., McCann P. P., Schindler J. Alterations in polyamine metabolism during embryonal carcinoma cell differentiation in vitro. Dev Biol. 1985 Oct;111(2):510–514. doi: 10.1016/0012-1606(85)90502-0. [DOI] [PubMed] [Google Scholar]
- Kozak L. P., Birkenmeier E. H. Mouse sn-glycerol-3-phosphate dehydrogenase: molecular cloning and genetic mapping of a cDNA sequence. Proc Natl Acad Sci U S A. 1983 May;80(10):3020–3024. doi: 10.1073/pnas.80.10.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri-Harcuch W., Marsch-Moreno M. DNA synthesis and cell division related to adipose differentiation of 3T3 cells. J Cell Physiol. 1983 Jan;114(1):39–44. doi: 10.1002/jcp.1041140107. [DOI] [PubMed] [Google Scholar]
- Kuri-Harcuch W., Wise L. S., Green H. Interruption of the adipose conversion of 3T3 cells by biotin deficiency: differentiation without triglyceride accumulation. Cell. 1978 May;14(1):53–59. doi: 10.1016/0092-8674(78)90300-8. [DOI] [PubMed] [Google Scholar]
- Murphy M. G., Négrel R., Ailhaud G. Lipoprotein lipase and monoacylglycerol lipase activities during maturation of ob17 preadipocytes. Biochim Biophys Acta. 1981 May 22;664(2):240–248. doi: 10.1016/0005-2760(81)90046-1. [DOI] [PubMed] [Google Scholar]
- Négrel R., Grimaldi P., Ailhaud G. Establishment of preadipocyte clonal line from epididymal fat pad of ob/ob mouse that responds to insulin and to lipolytic hormones. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6054–6058. doi: 10.1073/pnas.75.12.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pairault J., Green H. A study of the adipose conversion of suspended 3T3 cells by using glycerophosphate dehydrogenase as differentiation marker. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5138–5142. doi: 10.1073/pnas.76.10.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichard A. L., Munnich A., Meienhofer M. C., Vaulont S., Simon M. P., Marie J., Dreyfus J. C., Kahn A. Characterization and metabolic regulation of a liver-specific 5.4-kilobase mRNA whose synthesis is transcriptionally induced by carbohydrates and repressed by glucagon and cyclic AMP. Biochem J. 1985 Mar 15;226(3):637–644. doi: 10.1042/bj2260637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim C. DNA synthesis and differentiation in developing white adipose tissue. Dev Biol. 1971 Sep;26(1):69–76. doi: 10.1016/0012-1606(71)90108-4. [DOI] [PubMed] [Google Scholar]
- Péquignot-Planche E., De Gasquet P., Boulangé A., Tonnu N. T. Lipoprotein lipase activity at onset of development of white adipose tissue in newborn rats. Biochem J. 1977 Feb 15;162(2):461–463. doi: 10.1042/bj1620461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell T. R., Files N., Ingram M. Mitosis of contact-inhibited 3T3 preadipocytes precedes chemically induced differentiation into adipocytes. Proc Soc Exp Biol Med. 1983 Sep;173(4):471–474. doi: 10.3181/00379727-173-41672. [DOI] [PubMed] [Google Scholar]
- Schindler J., Kelly M., McCann P. P. Inhibition of ornithine decarboxylase induces embryonal carcinoma cell differentiation. Biochem Biophys Res Commun. 1983 Jul 18;114(1):410–417. doi: 10.1016/0006-291x(83)91642-x. [DOI] [PubMed] [Google Scholar]
- Shirahata A., Pegg A. E. Regulation of S-adenosylmethionine decarboxylase activity in rat liver and prostate. J Biol Chem. 1985 Aug 15;260(17):9583–9588. [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980 Sep 25;255(18):8811–8818. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier C., Amri E. Z., Etienne J., Négrel R., Ailhaud G. Maturation and secretion of lipoprotein lipase in cultured adipose cells. I. Intracellular activation of the enzyme. J Biol Chem. 1985 Apr 10;260(7):4424–4431. [PubMed] [Google Scholar]
- Vannier C., Jansen H., Négrel R., Ailhaud G. Study of lipoprotein lipase content in Ob17 preadipocytes during adipose conversion. Immunofluorescent localization of the enzyme. J Biol Chem. 1982 Oct 25;257(20):12387–12393. [PubMed] [Google Scholar]