Abstract
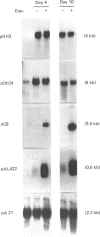
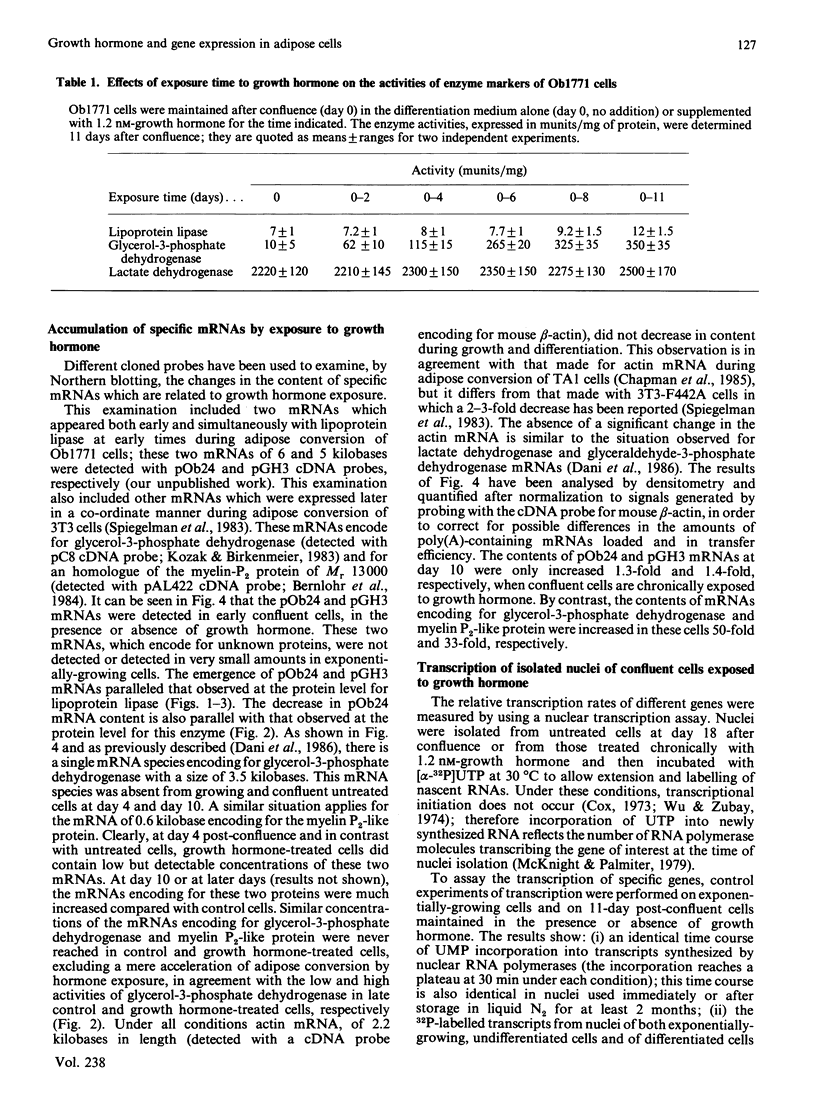
The adipose conversion of Ob1771 preadipocytes, during exposure to medium containing bovine serum and supplemented with growth hormone, is accompanied by the acquisition of phenotypic markers and the increased accumulation of specific mRNAs. The expression of lipoprotein lipase, and that of unidentified pOb24 and pGH3 mRNAs, are early events which are independent of growth hormone supplementation. By contrast, the late expression of mRNAs encoding for glycerol-3-phosphate dehydrogenase and p422 protein (a myelin-P2 homologue) and that of glycerol-3-phosphate dehydrogenase activity require the presence of growth hormone. The abundance of beta-actin mRNA does not change during differentiation. Runoff transcription by nuclei isolated from untreated or growth hormone-treated cells reveal little or no change in the rates of transcription of pOb24, pGH3 and beta-actin mRNAs. By contrast, the transcription rate of the p422 gene increases markedly (greater than 6-fold) in nuclei of growth hormone-treated cells. However, the p422 mRNA is more abundant than would be predicted by its nuclear transcription alone, suggesting, in Ob1771 cells exposed to growth hormone, that there is a post-transcriptional level of control. These results indicate that the permissive role of growth hormone during adipose cell differentiation is related to terminal events only and that its effects can be seen both at the protein and mRNA level. These results strongly suggest that an increased rate of specific transcription is primarily responsible for the accumulation of mRNAs during exposure to growth hormone.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ailhaud G., Amri E., Cermolacce C., Djian P., Forest C., Gaillard D., Grimaldi P., Khoo J., Négrel R., Serrero-Davé G. Hormonal requirements for growth and differentiation of ob17 preadipocyte cells in vitro. Diabete Metab. 1983 May-Jun;9(2):125–133. [PubMed] [Google Scholar]
- Amri E. Z., Dani C., Doglio A., Etienne J., Grimaldi P., Ailhaud G. Adipose cell differentiation: evidence for a two-step process in the polyamine-dependent Ob1754 clonal line. Biochem J. 1986 Aug 15;238(1):115–122. doi: 10.1042/bj2380115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri E. Z., Grimaldi P., Négrel R., Ailhaud G. Adipose conversion of ob17 cells. Insulin acts solely as a modulator in the expression of the differentiation program. Exp Cell Res. 1984 Jun;152(2):368–377. doi: 10.1016/0014-4827(84)90638-4. [DOI] [PubMed] [Google Scholar]
- Bernlohr D. A., Angus C. W., Lane M. D., Bolanowski M. A., Kelly T. J., Jr Expression of specific mRNAs during adipose differentiation: identification of an mRNA encoding a homologue of myelin P2 protein. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5468–5472. doi: 10.1073/pnas.81.17.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr D. A., Bolanowski M. A., Kelly T. J., Jr, Lane M. D. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1985 May 10;260(9):5563–5567. [PubMed] [Google Scholar]
- Bernlohr D. A., Doering T. L., Kelly T. J., Jr, Lane M. D. Tissue specific expression of p422 protein, a putative lipid carrier, in mouse adipocytes. Biochem Biophys Res Commun. 1985 Oct 30;132(2):850–855. doi: 10.1016/0006-291x(85)91209-4. [DOI] [PubMed] [Google Scholar]
- Chapman A. B., Knight D. M., Dieckmann B. S., Ringold G. M. Analysis of gene expression during differentiation of adipogenic cells in culture and hormonal control of the developmental program. J Biol Chem. 1984 Dec 25;259(24):15548–15555. [PubMed] [Google Scholar]
- Chapman A. B., Knight D. M., Ringold G. M. Glucocorticoid regulation of adipocyte differentiation: hormonal triggering of the developmental program and induction of a differentiation-dependent gene. J Cell Biol. 1985 Oct;101(4):1227–1235. doi: 10.1083/jcb.101.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. S., Hunt C. R., Spiegelman B. M. Developmentally regulated mRNAs in 3T3-adipocytes: analysis of transcriptional control. J Cell Biol. 1985 Feb;100(2):514–520. doi: 10.1083/jcb.100.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. F. Transcription of high-molecular-weight RNA from hen-oviduct chromatin by bacterial and endogenous form-B RNA polymerases. Eur J Biochem. 1973 Nov 1;39(1):49–61. doi: 10.1111/j.1432-1033.1973.tb03102.x. [DOI] [PubMed] [Google Scholar]
- Djian P., Grimaldi P., Négrel R., Ailhaud G. Adipose conversion of OB17 preadipocytes. Relationships between cell division and fat cell cluster formation. Exp Cell Res. 1982 Dec;142(2):273–281. doi: 10.1016/0014-4827(82)90368-8. [DOI] [PubMed] [Google Scholar]
- Djian P., Phillips M., Green H. The activation of specific gene transcription in the adipose conversion of 3T3 cells. J Cell Physiol. 1985 Sep;124(3):554–556. doi: 10.1002/jcp.1041240327. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard D., Poli P., Négrel R. Characterization of ouabain-resistant mutants of the preadipocyte Ob17 clonal line. Adipose conversion in vitro and in vivo. Exp Cell Res. 1985 Feb;156(2):513–527. doi: 10.1016/0014-4827(85)90558-0. [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Grumbach M. M., Kaplan S. L. The neuroendocrine regulation and function of growth hormone and prolactin in the mammalian fetus. Endocr Rev. 1981 Fall;2(4):363–395. doi: 10.1210/edrv-2-4-363. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol. 1979 Oct;101(1):169–171. doi: 10.1002/jcp.1041010119. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Grimaldi P., Czerucka D., Rassoulzadegan M., Cuzin F., Ailhaud G. ob17 cells transformed by the middle-T-only gene of polyoma virus differentiate in vitro and in vivo into adipose cells. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5440–5444. doi: 10.1073/pnas.81.17.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi P., Djian P., Negrel R., Ailhaud G. Differentiation of Ob17 preadipocytes to adipocytes: requirement of adipose conversion factor(s) for fat cell cluster formation. EMBO J. 1982;1(6):687–692. doi: 10.1002/j.1460-2075.1982.tb01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak L. P., Birkenmeier E. H. Mouse sn-glycerol-3-phosphate dehydrogenase: molecular cloning and genetic mapping of a cDNA sequence. Proc Natl Acad Sci U S A. 1983 May;80(10):3020–3024. doi: 10.1073/pnas.80.10.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler G., Feick P., Hauner H., Herberg L. An adipogenic serum factor in genetically obese rodents. FEBS Lett. 1983 Mar 7;153(1):179–182. doi: 10.1016/0014-5793(83)80143-4. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Morikawa M., Nixon T., Green H. Growth hormone and the adipose conversion of 3T3 cells. Cell. 1982 Jul;29(3):783–789. doi: 10.1016/0092-8674(82)90440-8. [DOI] [PubMed] [Google Scholar]
- Nixon T., Green H. Contribution of growth hormone to the adipogenic activity of serum. Endocrinology. 1984 Feb;114(2):527–532. doi: 10.1210/endo-114-2-527. [DOI] [PubMed] [Google Scholar]
- Nixon T., Green H. Properties of growth hormone receptors in relation to the adipose conversion of 3T3 cells. J Cell Physiol. 1983 Jun;115(3):291–296. doi: 10.1002/jcp.1041150312. [DOI] [PubMed] [Google Scholar]
- Négrel R., Grimaldi P., Ailhaud G. Establishment of preadipocyte clonal line from epididymal fat pad of ob/ob mouse that responds to insulin and to lipolytic hormones. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6054–6058. doi: 10.1073/pnas.75.12.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu R. S. Two-dimensional electrophoretic analyses of proteins synthesized during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1979 Nov 10;254(21):11111–11118. [PubMed] [Google Scholar]
- Spiegelman B. M., Farmer S. R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982 May;29(1):53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980 Sep 25;255(18):8811–8818. [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Cyclic AMP-mediated control of lipogenic enzyme synthesis during adipose differentiation of 3T3 cells. Cell. 1981 May;24(2):503–510. doi: 10.1016/0092-8674(81)90341-x. [DOI] [PubMed] [Google Scholar]
- Steinberg M. M., Brownstein B. L. A clonal analysis of the differentiation of 3T3-L1 preadipose cells: role of insulin. J Cell Physiol. 1982 Dec;113(3):359–364. doi: 10.1002/jcp.1041130303. [DOI] [PubMed] [Google Scholar]
- Weiss G. H., Rosen O. M., Rubin C. S. Regulation of fatty acid synthetase concentration and activity during adipocyte differentiation. Studies on 3T3-L1 cells. J Biol Chem. 1980 May 25;255(10):4751–4757. [PubMed] [Google Scholar]
- Wu G. J., Zubay G. Prolonged transcription in a cell-free system involving nuclei and cytoplasm. Proc Natl Acad Sci U S A. 1974 May;71(5):1803–1807. doi: 10.1073/pnas.71.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezulak K. M., Green H. Specificity of gene expression in adipocytes. Mol Cell Biol. 1985 Feb;5(2):419–421. doi: 10.1128/mcb.5.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]