Abstract
Objectives
Intradiscal steroid injection (ISI) use has been proven as a low-risk and rapid treatment for disc degeneration disease (DDD). However, the histological effects of steroids on human discs remain poorly understood. The purpose of this study is to investigate whether ISI induces histologic degeneration of the disc.
Methods
In this study, a histological analysis was carried out on the nucleus pulposus obtained from 150 patients who underwent posterior lumbar interbody fusion. Among these individuals, 59 received ISI before the surgery, while 91 did not. After staining with hematoxylin and eosin, the histological classification was performed based on chondrocyte proliferation (C1, C2, and C3) and granular matrix change (M1 and M2). Logistic regression analysis was used to identify the main factors influencing chondrocyte proliferation and granular matrix change. Additionally, histological differences between the ISI group and the non-ISI group were analyzed.
Results
Chondrocyte proliferation and granular matrix changes were not significantly different between the ISI and non-ISI groups. The logistic regression analysis indicated that age is the most significant risk factor for both chondrocyte proliferation (P = 0.02) and granular matrix changes (P < 0.01).
Conclusions
The most crucial factor in disc degeneration is age. ISI does not accelerate the histological degeneration of chondrocyte proliferation and granular matrix. Therefore, the ISI could be considered as a histologically safe alternative in patients with DDD.
Keywords: Intervertebral disc degeneration, Intradiscal injection, Steroids, Histology
1. Introduction
With disc degeneration disease (DDD), degeneration is accelerated compared to that occurring during the normal aging process [1]. Many modalities have been introduced for the treatment of DDD, including medications, molecular therapy, and replacement or rigid fusion surgery [2]. However, from now on, the replacement or arthrodesis of degenerated disc are the main stream of the treatment for late stage of DDD. And the treatment of regenerating the degenerated discs such as gene therapy or mesenchymal stem cell therapy at the early stage of the disease is still investigated but not applied clinically [3]. Recent studies have reported the effectiveness of intradiscal steroid injection (ISI) for discogenic pain in DDD [4,5]. Buttermann [5] concluded that ISI is a low-risk and rapid treatment option for DDD. Pain relief following steroid injection is attributed to the suppression of inflammatory mediators in the disc; steroid seeps out through the annular tear and spreads to the neural elements on the periphery of the disc and the posterior longitudinal ligament [5,6]. Therefore, ISI was one of the main treatments which relieve the discogenic pain in patient with DDD [4,5,7].
Steroid injection is a common practice that relieves pain experienced with musculoskeletal conditions; furthermore, the use of steroid injections is supported by their anti-inflammatory properties [8,9]. However, several side effects, including tendon rupture, systemic effects, and steroid-induced arthropathy, can occur with steroid injection [8]. Furthermore, studies on the histological effects of ISI on human discs remain scarce. Studies using animal models have shown that the histological effects of steroids on discs differed based on the type of animal [10,11]. Disc degeneration is histologically represented by increased chondrocyte proliferation, granular matrix changes, mucous degeneration, neovascularity, cleft, and others [12,13]. These changes occur similarly during the aging process as well as during the pathological process. If ISI causes further histologic degeneration of discs in humans, then the clinical usefulness of ISI should be reconsidered in patients undergoing ISI for pain relief purpose. This study investigated whether ISI induces histologic degeneration of the disc.
2. Methods
2.1. Study design and participants
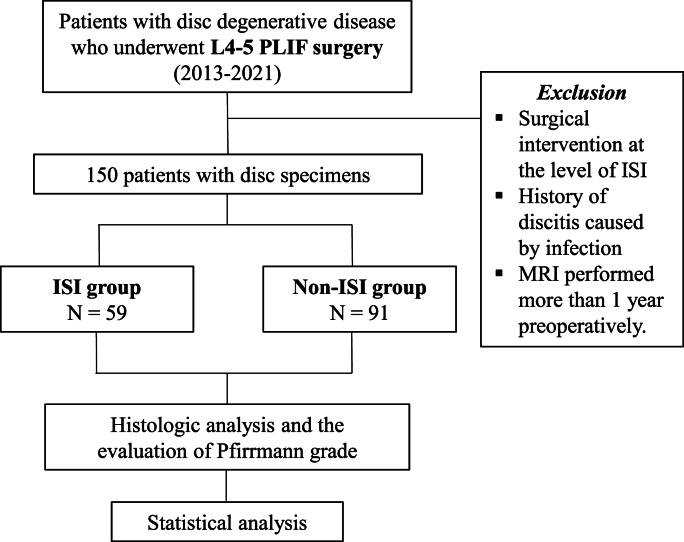
We retrospectively reviewed 150 patients who had undergone L4-5 posterior lumbar interbody fusion (PLIF) at out institution between January 2013 and April 2021. The inclusion criteria were defined as follows: patients diagnosed with DDD through clinical examinations and magnetic resonance imaging (MRI); confirmation of disc degeneration with a Pfirrmann (PM) grade ≥ 2 as indicated by preoperative MRI; and patients undergoing PLIF surgery at the L4-5 level.
The exclusion criteria were as follows: history of surgical intervention at the level of ISI; history of discitis caused by infection; and MRI performed more than 1 year preoperatively (Fig. 1).
Fig. 1.
Patient selection process.
L, lumbar; PLIF, posterior lumbar interbody fusion; ISI, intradiscal steroid injection; MRI, magnetic resonance imaging.
This retrospective study was approved by Ilsan Paik's Hospital Institutional Review Board (IRB number: 2020-10-028). Informed consent was waived because of the retrospective nature of the study.
2.2. Intradiscal steroid injection
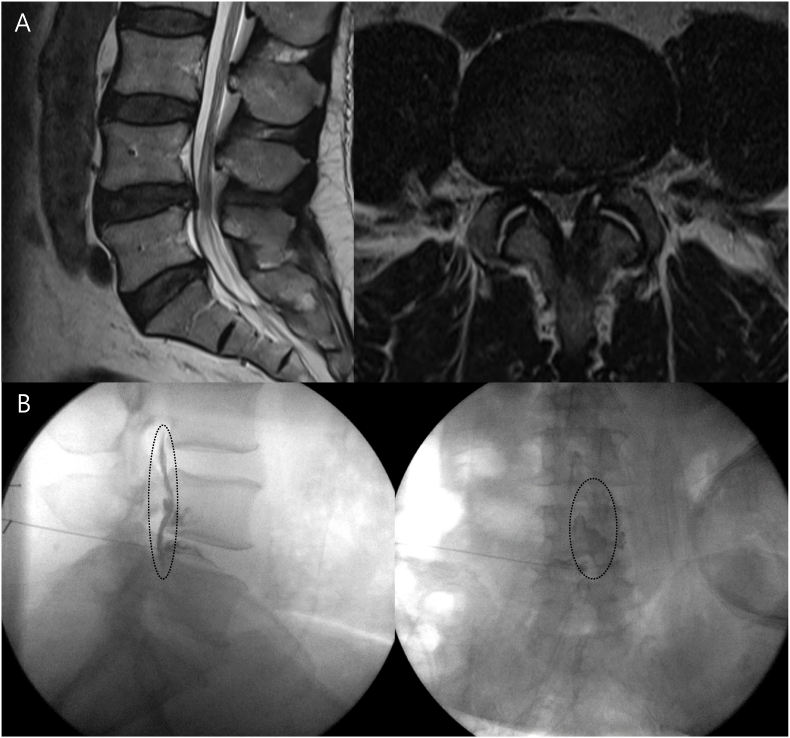
In this study, ISI was performed in DDD patients for the purpose of pain relief effect from steroids. The spinal needle (22-gauge and 18-cm) was advanced to the center of the disc space under C-arm. After the position of the needle tip was confirmed by the C-arm, 1 mL (40 mg) triamcinolone was injected in the disc (Fig. 2). In cases where patients continued to experience persistent discogenic back pain and exhibited spinal instability about three months after ISI, PLIF surgery was performed.
Fig. 2.
A representative case of a patient treated with intradiscal steroid injection.
A: Preoperative magnetic resonance imaging of a patient with L4-5 disc degeneration. B: The C-arm image after dye injection showed that the dye leaked out from the annulus fibrosus and spread to the anterior aspect of the dura (dotted).
2.3. Histology evaluation
During discectomy procedure in PLIF surgery, nucleus pulposus specimens were collected for histologic analysis. After annulotomy with surgical grade 11 scalpels, the surgeon performed discectomy of the nucleus pulposus using a pituitary forcep. The extracted specimens were sent to the pathology department for histological analyses. All disc specimens were immediately fixed in 10% neutral buffered formaldehyde for 24 h, decalcified using the HCl protocol for approximately 8 h, and routinely processed for embedding in paraffin. The paraffin-embedded specimens were cut to 4 μm, and the sections were stained with hematoxylin and eosin (HE). According to a previous study by Boo et al. [13], chondrocyte proliferation, granular change of matrix, mucous degeneration, edge neovascularity, tears, scar formation, and tissue defects were used as criteria for histologic evaluation of degenerated disc. However, in this study, disc specimens were gathered by pituitary forceps during discectomy procedure not by enbloc excision. Therefore, tears or defects were not used as a histological classification criterion. In addition, mucous degeneration and edge neovascularity were rarely seen in pilot study. As a result, only chondrocyte proliferation and granular change of matrix, which are usually carefully addressed in DDD, were taken as criteria for degeneration of disc in this study.
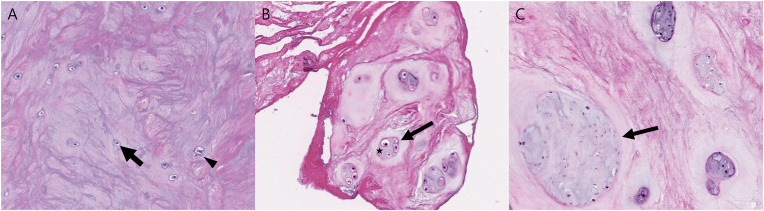
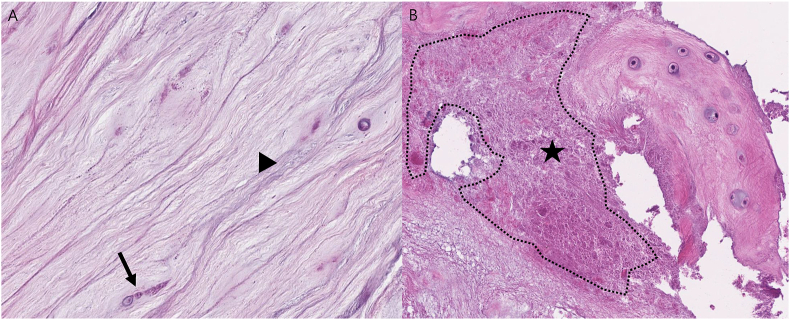
Chondrocyte proliferation and granular matrix changes were assessed through HE staining, with reference to existing literature [14]. Chondrocyte proliferation was defined as multiple chondrocytes growing in small, rounded groups or clusters sharply demarcated by a rim of the territorial matrix and graded as 1 (C1, single or two connected chondrocytes), 2 (C2, 3 to 7 chondrocytes grouped together), or 3 (C3, eight or more chondrocytes grouped together) (Fig. 3A–C). Chondrocyte proliferation grades were determined by counting chondrocytes in groups or clusters in the three regions showing the highest proliferation at a magnification of 200× and calculating the average. Granular matrix changes were defined as eosinophilic-stained amorphous granules within the fibrocartilage and graded as 1 (M1, < 5%) or 2 (M2, ≥ 5%) (Fig. 4A and B). In the analysis of pathology results, a single pathologist evaluated the slides twice at one-month intervals. When discrepancies arose, the slides were reviewed again to determine the most appropriate grading.
Fig. 3.
Histologic classification for the chondrocyte proliferation. (Hematoxylin and eosin, ×200, original magnification).
A: Grade 1. Single round chondrocyte (arrow) and two connected chondrocytes (arrowhead). B: Grade 2. Clusterization of four chondrocytes (arrow), surrounded by a pericellular matrix (star). C: Grade 3. Numerous chondrocytes grouped together (arrow).
Fig. 4.
Histologic classification for the matrix granular change. (Hematoxylin and eosin, ×200, original magnification).
A: Grade 1. Structurally well-organized fibrocartilage in uniform arrangement (arrowhead) and single chondrocyte (arrow). The matrix granular change is under 5% of matrix. B: Grade 2. Eosinophilic-staining amorphous granules in fibrocartilage (star).
2.4. Evaluation of the PM grade based on preoperative MRI
The PM grading system assesses degenerated intervertebral discs using the MRI signal intensity and height of the intervertebral disc [[15], [16], [17]]. It has been found that PM grade is related to the degree of actual disc degeneration [15]. Therefore, the author further analyzed whether the histological classification is related to the preoperative PM grade. The PM grade was confirmed retrospectively by two orthopaedic surgeons using MRI performed within 1 year preoperatively. If their evaluations differed, they discussed and confirmed the final grade together. The correlation between the PM grade and disc histology of patients who did not undergo ISI was analyzed.
2.5. Statistical analysis
A logistic regression analysis was performed to determine risk factors for chondrocyte proliferation and granular matrix changes. To reduce the confounding factor as much as possible, patients were divided into PM grade 3 (early degeneration) and PM grade 4 and 5 (advanced degeneration) based on preoperative taken MRI in order to compare the groups with similar degrees of degeneration [18,19]. In addition, patients were further subdivided based on the approximate median value of 65 years old, considering that age varies clearly according to chondrocyte proliferation and granular matrix change. Therefore, patients were categorized into four groups according to age (older than 65 years and 65 years or younger) and PM grade (grade 3, early degeneration, and grades 4 and 5, advanced degeneration). And a comparison of histology was performed according to whether ISI was performed.
If the variables were normally distributed, then parametric statistical analyses were performed; otherwise, nonparametric statistical analyses were performed. Comparisons of the continuous variables of each group were performed using the independent sample t-test. For nominal variables, Fisher's exact test, the chi-square test, and the Cochran-Armitage trend test were used. The intraobserver reliability for chondrocyte proliferation and granular matrix changes showed kappa coefficients of 0.95 and 0.92, respectively. For the PM grade, the kappa coefficient was 0.88. All statistical analyses were performed using SPSS software version 20.0. (IBM Corp., Armonk, NY, USA). Statistical significance was set at P < 0.05.
3. Results
A total of 150 patients (73 men and 77 women) underwent L4-5 PLIF surgery. Fifty-nine patients received ISI (ISI group) while 91 patients did not (non-ISI group). On average, the ISI group received PLIF surgery 7.64 months (SD, 5.50) after undergoing ISI. Thirty-eight patients and 112 patients had grade 1 and grade 2 granular matrix changes, respectively. Seventeen, 41, and 92 patients had grade 1, grade 2, and grade 3 chondrocyte proliferation, respectively. Twenty-five, 111, and 14 patients had PM grades 3, 4, and 5, respectively (Table 1).
Table 1.
Characteristics of patients.
| ISI (N = 59) | Non-ISI (N = 91) | P-value | |
|---|---|---|---|
| Age, yrs | 66.05 ± 9.65 | 67.77 ± 10.50 | 0.16 |
| Sex | |||
| Men | 34 (57.6%) | 39 (42.9%) | 0.08 |
| Women | 25 (42.4%) | 52 (57.1%) | |
| Histologic grade | |||
| Chondrocyte proliferation | |||
| C1 | 7 (11.9%) | 10 (11.0%) | 0.92 |
| C2 | 17 (28.8%) | 24 (26.4%) | |
| C3 | 35 (59.3%) | 57 (62.6%) | |
| Granular matrix change | |||
| M1 | 11 (18.6%) | 27 (29.7%) | 0.13 |
| M2 | 48 (81.4%) | 64 (70.3%) | |
| Pfirrmann grade | |||
| P3 | 7 (11.9%) | 18 (19.8%) | 0.40 |
| P4 | 47 (79.7%) | 64 (70.3%) | |
| P5 | 5 (8.5%) | 9 (9.9%) | |
Values are presented as number (%), or mean ± standard deviation unless otherwise indicated.
ISI, intradiscal steroid injection.
Categorical variables were analyzed using the chi-square test.
Continuous variables were analyzed using Student's t-test.
The logistic regression analysis indicated that age was the most significant risk factor for both chondrocyte proliferation (P = 0.02) and granular matrix changes (P < 0.01).
When age was compared among different grades of chondrocyte proliferation in patients in the non-ISI group, the mean age of patients with C3 (69.9 years; standard deviation [SD], 8.9) was higher than that of patients with C2 (65.5 years; SD, 11.9) and C1 (60.8 years; SD, 12.5) (P = 0.02). Additionally, age was significantly different between those with M1 (62.6 years; SD, 12.4) and M2 (69.9 years; SD, 8.9) grades of granular matrix changes in the non-ISI group (P < 0.01).
There was an age difference based on the PM grade (P < 0.01). Patients with PM grades 4 and 5 (severe disc generation; 68.4 years; SD, 9.7) were significantly older than those with PM grade 3 (60.4 years; SD, 10.3). Furthermore, the PM grade was related to histological degeneration (chondrocyte proliferation, P = 0.03; granular matrix changes, P = 0.04) for patients in the non-ISI group (Table 2).
Table 2.
Histological differences according to PM grade in non-ISI group.
| PM grade 3 (N = 18) | PM grade 4, 5 (N = 73) | P-value | |
|---|---|---|---|
| Age, yrs | 51.83 ± 6.95 | 71.7 ± 6.88 | < 0.01a |
| Sex | |||
| Men | 7 (38.9%) | 32 (43.8%) | 0.70 |
| Women | 11 (61.1%) | 41 (56.2%) | |
| Chondrocyte proliferation | |||
| C1 | 5 (27.8%) | 5 (6.8%) | 0.03a |
| C2 | 5 (27.8%) | 19 (26.0%) | |
| C3 | 8 (44.4%) | 49 (67.1%) | |
| Granular matrix change | |||
| M1 | 9 (50.0%) | 18 (24.7%) | 0.04a |
| M2 | 9 (50.0%) | 55 (75.3%) | |
Values are presented as number (%), or mean ± standard deviation unless otherwise indicated.
PM, pfirrmann; ISI, intradiscal steroid injection.
Continuous variables were analyzed using student's t-test.
Categorical variables were analyzed using the chi-square test.
P-value < 0.05.
From the results of analysis of patients classified by age and PM grade, chondrocyte proliferation and granular matrix changes were not significantly different in patients with and without ISI in all four groups (Table 3, Table 4).
Table 3.
Comparison between the ISI and non-ISI group in patients ≤ 65 years old.
| ISI (N = 26) | Non-ISI (N = 35) | P-value | |
|---|---|---|---|
| Age, yrs | 57.35 ± 6.65 | 57.06 ± 7.48 | 0.44 |
| Sex | |||
| Men | 11 (42.3%) | 15 (42.9%) | 0.97 |
| Women | 15 (57.7%) | 20 (57.1%) | |
| PM grade 3 | |||
| Chondrocyte proliferation | |||
| C1 | 1 (25.0%) | 5 (41.7%) | 0.51 |
| C2 | 1 (25.0%) | 3 (25.0%) | |
| C3 | 2 (50.0%) | 4 (33.3%) | |
| Granular matrix change | |||
| M1 | 2 (50.0%) | 7 (58.3%) | 1.00 |
| M2 | 2 (50.0%) | 5 (41.7%) | |
| PM grade 4, 5 | |||
| Chondrocyte proliferation | |||
| C1 | 3 (13.6%) | 2 (8.7%) | 0.74 |
| C2 | 6 (27.3%) | 7 (30.4%) | |
| C3 | 13 (59.1%) | 14 (60.9%) | |
| Granular matrix change | |||
| M1 | 7 (31.8%) | 10 (43.5%) | 0.42 |
| M2 | 15 (68.2%) | 13 (56.5%) | |
Values are presented as number (%), or mean ± standard deviation unless otherwise indicated.
ISI, intradiscal steroid injection; PM, pfirrmann.
Continuous variables were analyzed using student's t-test.
Categorical variables were analyzed using the Cochran-Armitage trend test, Fisher's exact test, and chi-square test.
Table 4.
Comparison between the ISI and non-ISI group in patients > 65 years old.
| ISI (N = 33) | Non-ISI (N = 56) | P-value | |
|---|---|---|---|
| Age, yrs | 72.91 ± 4.69 | 74.46 ± 5.22 | 0.08 |
| Sex | |||
| Men | 23 (69.7%) | 24 (42.9%) | 0.01a |
| Women | 10 (30.3%) | 32 (57.1%) | |
| PM grade 3 | |||
| Chondrocyte proliferation | |||
| C1 | 0 (0.0%) | 0 (0.0%) | 0.34 |
| C2 | 2 (66.7%) | 2 (33.3%) | |
| C3 | 1 (33.3%) | 4 (66.7%) | |
| Granular matrix change | |||
| M1 | 0 (0.0%) | 2 (33.3%) | 0.50 |
| M2 | 3 (100.0%) | 4 (66.7%) | |
| PM grade 4, 5 | |||
| Chondrocyte proliferation | |||
| C1 | 3 (10.0%) | 3 (6.0%) | 0.46 |
| C2 | 8 (26.7%) | 12 (24.0%) | |
| C3 | 19 (63.3%) | 35 (70.0%) | |
| Granular matrix change | |||
| M1 | 2 (6.7%) | 8 (16.0%) | 0.31 |
| M2 | 28 (93.3%) | 42 (84.0%) | |
Values are presented as number (%), or mean ± standard deviation unless otherwise indicated.
ISI, intradiscal steroid injection; PM, pfirrmann.
Continuous variables were analyzed using student's t-test.
Categorical variables were analyzed using the Cochran-Armitage trend test and Fisher's exact test.
P-value < 0.05.
4. Discussion
ISI did not cause further degenerative changes in human discs. The effects of steroids on disc histology have been studied mainly using animal models, and varying results were found based on the animal type and steroid type [10,11,20]. Aoki et al. [10] evaluated degeneration of intervertebral discs of rabbits after ISI and found that rabbits receiving methylprednisolone acetate injection had matrix vehicles one day post ISI, which is indicative of primary tissue calcification and degenerative changes of the nucleus pulposus and annulus fibrosus. And they concluded that polyethylene glycol in methylprednisolone acetate cause degeneration and primary calcification of disc because of its toxicity and irritative effect. However, when a different type of steroid was injected in dog discs, different results were obtained [11]. Imke et al. [11] evaluated the safety of the intradiscal release of triamcinolone acetonide (TAA) in a canine degenerated intervertebral disc model with 8.4 μg and 0.84 mg TAA. Immediately before TAA injection and 12 weeks after TAA injection, histological and radiological analyses of intervertebral discs were performed. Regardless of the TAA dosage, local injection of TAA did not affect the disc height index, PM grading, collagen content, or glycosaminoglycan content. It was concluded that ISI appeared safe, suggesting their potential applicability for pain relief. Both Imke's study and our study showed that triamcinolone injection in degenerated discs did not lead to further degenerative changes. Given the similarity between the intervertebral disc degeneration process in dogs and humans, both studies provide evidence that steroids do not induce disc degeneration in humans [21].
Intervertebral disc degeneration includes a catabolic cell response, changed extracellular matrix (ECM), and altered biomechanics [22]. According to Baptista et al. [1], ECM remodelers and cytokines such as interleukin-1 and tumor necrosis factor-α are involved in the catabolic process of disc degeneration. Matrix metalloproteinases are the enzymes that collectively degrade every type of ECM protein, causing degenerative disc changes [23,24]. Despite stab injuries, which are known to induce degenerative disc changes, occurring during this study [25,26], disc degeneration did not progress histologically after ISI. This is probably because of the suppression of the proinflammatory mediators (matrix metalloproteinases, interleukin-1, and tumor necrosis factor-α) and catabolic action caused by steroids during the disc degeneration process. When steroids are injected in discs, they diffuse to the nucleus pulposus and bind to the glucocorticoid receptor, causing suppression or stimulation of genes related to inflammation; this is called transrepression or transactivation. As a result, these factors inhibit the synthesis of inflammatory mediators [27]. In addition to the triamcinolone used in this study, there are dextamethasone, hydrocortisone, betamethasone, etc., which have slightly different chemical structures, but are basically combined with the glucocorticoid response element and linked to inflammation related pathway; transactivation for anti-inflammatory response and transrepression for pro-inflammatory response. However, for each steroid type, there is a difference in the duration of action due to the difference in affinity with the glucocorticoid receptor. Triamcinolone used in this study also combined with glucocorticoid response element as a composite of glucocorticoid, resulting in anti-inflammatory action and not causing further degeneration of the disc.
During this study, the degree of disc degeneration was histologically analyzed by referring to Boo's classification method [13]. However, only chondrocyte proliferation and granular matrix changes were analyzed using several classification criteria. Discs were not totally intact when collected because they were acquired by pituitary forceps during discectomy. When degeneration occurred in discs, the production of collagen type 2 and aggrecan decreased in chondrocytes, causing a reduced matrix around the cell and resulting in a lack of nutrients around the chondrocyte and clusterization of chondrocytes to compensate [12,13]. Granular matrix changes are also the result of disc degeneration caused by ECM degradation. As a result, the degraded products of collagen and other molecules composing the matrix were eosinophilic stained [13,28]. As these two criteria are associated with age and PM grade, they were considered suitable for determining the extent of disc tissue degeneration.
This study had some limitations. First, this study did not analyze discs based on the molecular level. Although there was no further histologic damage of the chondrocytes and matrix, steroids could directly affect the molecular aspects of collagen, as shown by a previous study on rabbits [29]. Therefore, conducting molecular-based studies in the future is essential. Second, the group comprising patients with stab injury who did not receive ISI and group comprising patients with stab injury who received ISI were not compared. However, stab injuries are known to cause disc degeneration, and the fact that disc degeneration did not progress histologically in the ISI group supports the idea that steroids do not adversely affect the disc histology. Third, further prospective studies are needed to serially examine the histological changes over time following steroid injection into the disc. Finally, although this study analyzed a total of 150 human disc samples, it has the limitation of a relatively small sample size. However, considering that clinical analyses of human disc tissue through fusion surgery are rare, this research holds significant value.
5. Conclusions
Chondrocyte proliferation and granular matrix changes were not significantly different between the ISI and non-ISI groups. The histological degeneration of the human disc is not expedited by ISI. Consequently, ISI may be regarded as a histologically secure alternative for individuals with DDD.
CRediT author statement
Jin Hwan Kim: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Writing-Original draft, Writing-Review & Editing. Sunhee Chang: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing-Review & Editing. Byung Ho Kim: Investigation, Methodology. Gyu Heon Lee: Investigation, Methodology. Sung Tan Cho: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing-Original draft, Writing-Review & Editing.
Conflicts of interest
The authors declare no competing interests.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank Ji Yeon Lee in the Medical record office for helping to collect patient data. This work was supported in part by the Korea Health Industry Development Institute (HI21C1137). ORCID Jin Hwan Kim: 0000-0001-8695-3547. Sunhee Chang: 0000-0002-7775-4711. Byung Ho Kim: 0000-0001-9234-2937. Gyu Heon Lee: 0000-0002-2012-5068. Sung Tan Cho: 0000-0002-2559-4066.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Baptista J, Fontes R, Liberti E. Aging and degeneration of the intervertebral disc: review of basic science. Coluna/Columna. 2015;14:144–148. [Google Scholar]
- 2.Wu P H, Kim H S, Jang IT. Intervertebral disc diseases PART 2: a review of the current diagnostic and treatment strategies for intervertebral disc disease. Int J Mol Sci. 2020;21:2135. doi: 10.3390/ijms21062135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serhan H. Advancements in the treatment of degenerative disc disease. Hamdan Med J. 2018;11:175. [Google Scholar]
- 4.Muzin S, Isaac Z, Walker J., 3rd. The role of intradiscal steroids in the treatment of discogenic low back pain. Curr Rev Musculoskelet Med. 2008;1:103–107. doi: 10.1007/s12178-007-9015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buttermann GR. The effect of spinal steroid injections for degenerative disc disease. Spine J. 2004;4:495–505. doi: 10.1016/j.spinee.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Singjie LC, Kusuma SA, Saleh I, Kholinne E. The potency of platelet-rich plasma for chronic low back pain: a systematic review and metaanalysis of randomized controlled trial. Asian Spine J. 2023;17:782–789. doi: 10.31616/asj.2022.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khot A, Bowditch M, Powell J, Sharp D. The use of intradiscal steroid therapy for lumbar spinal discogenic pain: a randomized controlled trial. Spine. 2004;29:833–836. doi: 10.1097/00007632-200404150-00002. ; discussion 7. [DOI] [PubMed] [Google Scholar]
- 8.Shah A, Mak D, Davies AM, James SL, Botchu R. Musculoskeletal corticosteroid administration: current concepts. Can Assoc Radiol J. 2019;70:29–36. doi: 10.1016/j.carj.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 9.MacMahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology. 2009;252:647–661. doi: 10.1148/radiol.2523081929. [DOI] [PubMed] [Google Scholar]
- 10.Aoki M, Kato F, Mimatsu K, Iwata H. Histologic changes in the intervertebral disc after intradiscal injections of methylprednisolone acetate in rabbits. Spine. 1997;22:127–131. doi: 10.1097/00007632-199701150-00001. ; discussion 32. [DOI] [PubMed] [Google Scholar]
- 11.Rudnik-Jansen I, Tellegen A, Beukers M, Öner F, Woike N, Mihov G., et al. Safety of intradiscal delivery of triamcinolone acetonide by a poly(esteramide) microsphere platform in a large animal model of intervertebral disc degeneration. Spine J. 2019;19:905–919. doi: 10.1016/j.spinee.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88:10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 13.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Weiler C, Lopez-Ramos M, Mayer H M, Korge A, Siepe C J, Wuertz K., et al. Histological analysis of surgical lumbar intervertebral disc tissue provides evidence for an association between disc degeneration and increased body mass index. BMC Res Notes. 2011;4:497. doi: 10.1186/1756-0500-4-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu LP, Qian WW, Yin GY, Ren YX, Hu ZY. MRI assessment of lumbar intervertebral disc degeneration with lumbar degenerative disease using the pfirrmann grading systems. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfirrmann C W A, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 17.Kim G U, Chang M C, Kim T U, Lee GW. Diagnostic modality in spine disease: a review. Asian Spine J. 2020;14:910–920. doi: 10.31616/asj.2020.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stich S, Jagielski M, Fleischmann A, Meier C, Bussmann P, Kohl B., et al. Degeneration of lumbar intervertebral discs: characterization of anulus fibrosus tissue and cells of different degeneration grades. Int J Mol Sci. 2020;21:2165. doi: 10.3390/ijms21062165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teichtahl A J, Urquhart D M, Wang Y, Wluka A E, O’Sullivan R, Jones G., et al. Lumbar disc degeneration is associated with modic change and high paraspinal fat content – a 3.0T magnetic resonance imaging study. BMC Muscoskel Disord. 2016;17:439. doi: 10.1186/s12891-016-1297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito S, Usui H, Maruyama K, Muro T. Roentgenographic evaluation of ossification and calcification of the lumbar spinal canal after intradiscal betamethasone injection. J Spinal Disord. 2001;14:434–438. doi: 10.1097/00002517-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Bergknut N, Rutges JP, Kranenburg HJ., Smolders LA, Hagman R, Smidt HJ., et al. The dog as an animal model for intervertebral disc degeneration? Spine. 2012;37:351–358. doi: 10.1097/BRS.0b013e31821e5665. [DOI] [PubMed] [Google Scholar]
- 22.Vergroesen PPA, Kingma I, Emanuel KS, Hoogendoorn RJW, Welting TJ, van Royen BJ., et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23:1057–1070. doi: 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35:652–655. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- 24.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology. 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Wang L, Park J B, Park P, Yang V C, Hollister SJ., et al. Intradiscal injection of simvastatin retards progression of intervertebral disc degeneration induced by stab injury. Arthritis Res Ther. 2009;11 doi: 10.1186/ar2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Yang S, Wang L, Park P, La Marca F, Hollister SJ., et al. Time course investigation of intervertebral disc degeneration produced by needle-stab injury of the rat caudal spine: laboratory investigation. J Neurosurg Spine. 2011;15:404–413. doi: 10.3171/2011.5.SPINE10811. [DOI] [PubMed] [Google Scholar]
- 27.Williams DM. Clinical pharmacology of corticosteroids. Respir Care. 2018;63:655–670. doi: 10.4187/respcare.06314. [DOI] [PubMed] [Google Scholar]
- 28.Rutges JP, Duit RA, Kummer JA, Bekkers JE, Oner FC, Castelein RM., et al. A validated new histological classification for intervertebral disc degeneration. Osteoarthritis Cartilage. 2013;21:2039–2047. doi: 10.1016/j.joca.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Balasubramaniam P. In: Trends in research and treatment of joint diseases. Hirohata K, Mizuno K, Matsubara T., editors. Springer; Tokyo: 1992. Mechanism of collagen breakdown by local infiltration of steroids; pp. 51–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.