Abstract
Objectives: This study assessed the effects of blue and Er:YAG lasers, fluoride varnish, and their combination on microhardness, and calcium and phosphorus content of demineralized enamel.
Materials and Methods: The primary Vickers microhardness of 28 third molars was measured and their enamel calcium and phosphorous content was quantified by energy-dispersive X-ray spectroscopy. They were then randomly assigned to five groups of 5% sodium fluoride (NaF) varnish, 445nm blue laser, Er:YAG laser, 5% NaF + 445nm blue laser, and 5% NaF + Er:YAG laser. The teeth then underwent pH-cycling to induce caries-like lesions. The surface microhardness of the teeth and the calcium and phosphorous content of demineralized enamel were measured again. Data were analyzed by one-way ANOVA and Tukey’s test (alpha=0.05).
Results: NaF, NaF-diode laser, and NaF-Er:YAG laser groups experienced a significant increase in microhardness of demineralized enamel close to the baseline value (P<0.05). The efficacy of NaF-blue laser and NaF-Er:YAG laser was higher than NaF . In blue and Er:YAG laser groups, the mean final microhardness was significantly lower than the baseline microhardness. The percentage of phosphorus in all groups was similar to that of sound enamel. The percentage of calcium in NaF group was significantly lower than that of sound enamel and all other groups. The calcium content in other groups was similar to that of sound enamel.
Conclusion: Fluoride varnish had a synergistic effect with Er:YAG and blue lasers to increase the demineralized enamel microhardness; blue and Er:YAG lasers alone were less effective.
Key Words: Dental Enamel, Tooth Demineralization, Lasers, Sodium Fluoride, Hardness, Dental Caries
Introduction
Enamel can be lost due to attrition, erosion, abrasion, or dental caries [1]. Despite the reduction in prevalence of dental caries, it is still a common oral health problem worldwide [2]. Dental caries is characterized by progressive demineralization of enamel as the result of activity of cariogenic microorganisms in dental plaque that produce acid by metabolizing the carbohydrates. Acid production leads to release of calcium and phosphorous ions from the hydroxyapatite structure [3]. It often occurs as the result of impaired balance between the remineralization and demineralization cycles [4]. White spot lesions are the first clinical symptom of dental caries, and indicate the first phase of enamel demineralization. If not stopped, the demineralization process continues and results in development of a cavitated lesion, requiring restoration. Thus, enhancement of remineralization of incipient carious lesions is an efficient non-invasive strategy to preserve the teeth [5].
Several preventive measures are currently employed to enhance the remineralization of incipient carious lesions and stop the progression of demineralization. Fluoride can prevent demineralization of the inner enamel surface, and enhance remineralization of the outer enamel surface [6]. It can increase the mineral content of enamel [7], and result in re-hardening (remineralization) of the soft (demineralized) enamel surface [8]. Fluoride provides a reservoir of calcium fluoride on the tooth surface that releases fluoride ions in case of a pH drop, conferring resistance to the enamel surface against caries [3]. Also, fluoride can lead to conversion of hydroxyapatite to fluorapatite, and increase enamel resistance to acid attacks as such. Moreover, fluoride decreases the ability of microorganisms to produce acids [9]. Evidence shows that use of sodium fluoride (NaF) can completely prevent enamel demineralization by decreasing acid production or changing the demineralization-remineralization equilibrium towards remineralization [10]. A previous study showed that NaF significantly decreased the depth of carious lesions by enhancement of remineralization [11].
Different laser types such as erbium-doped yttrium aluminum garnet (Er:YAG) laser have been used for caries prevention [12]. Er:YAG laser uses solid crystals as the conductor and erbium ion (Er3+) as the active ion [13]. Er:YAG laser in low-energy mode increases the uptake of fluoride by the enamel surface, and enhances the enamel resistance to acid attacks [14].
Al-Maliki et al. [15] demonstrated the optimal efficacy of blue (445nm) laser for caries prevention. Blue laser at 445nm wavelength is effective for soft tissue ablation and root canal disinfection. Lasers with high absorption coefficient (>1/1000cm) in hydroxyapatite such as Er:YAG laser are only absorbed by the first few micrometers of the enamel surface, and raise the temperature to 1000°C. Lasers in the visible wavelength spectrum have a low absorption coefficient (<1/1cm) in hydroxyapatite. Their effects on the enamel mainly depend on light scattering rather than absorption; thus, the surface temperature rise is expected to be lower [15].
Evidence shows that a combination of laser therapy and topical fluoride application has a synergistic effect on increasing the fluoride uptake and inhibiting enamel demineralization in comparison with laser irradiation or fluoride application alone [14]. Laser irradiation combined with fluoride therapy can result in deposition of high amounts of spherical or globular deposits on the enamel surface that morphologically resemble calcium fluoride [16]. Fluoride deposits can serve as a reservoir of fluoride for the purpose of enamel remineralization [17]. Photothermal effects of lasers can affect the tooth enamel. For example, at 420°C, reduced enamel carbonate reduces enamel permeability. The laser also affects the organic matrix of the enamel and can increase enamel acid resistance [18]. Many studies have shown the effects of lasers alone or with the help of fluoride on reducing the dissolution of tooth enamel by acids [19, 20]. Lasers can increase the deposition of fluoride on the surface of tooth enamel and also induce the formation of fluorapatite to create an enamel structure more resistant to acid attacks [21]. Blue laser was recently introduced to dentistry due to its cariostatic properties. However, no information is available regarding its effect on the microhardness of demineralized enamel or its acid resistance.
The efficacy of combined use of Er:YAG laser and fluoride varnish for improvement of enamel microhardness is a matter of question. Considering the gap of information on this topic, this study aimed to assess the effect of blue laser and Er:YAG laser, fluoride varnish, and their combination on microhardness, and calcium and phosphorus content of demineralized enamel. The obtained results can help in finding a treatment strategy with high efficacy for enhancement of enamel microhardness and resistance to acid attacks in presence of incipient enamel lesions.
MATERIALS AND METHODS
This study was approved by the ethics committee of Tehran University of Medical Sciences (IR.TUMS.DENTISTRY.REC.1398.184). This in vitro, experimental study was conducted on 28 erupted or impacted human third molars extracted within the past 3 months. The teeth were stored in saline (0.85% NaCl) at 4°C. After removing the calculus and soft tissue residues from the tooth surface, the teeth were immersed in 0.5% chloramine T solution for 1 week and were then transferred to saline. Teeth with cracks, caries, hypomineralization, or hypoplasia were excluded. Each tooth was split into buccal and lingual halves mesiodistally under water coolant. Each specimen was then mounted in dental stone. The surface of each tooth block was then polished with 400, 800, 1200, and 3000-grit abrasive papers, and the entire surface, except for a window measuring 3 x 3mm, was coated with nail varnish. This window was created for the conduction of interventions and tests. The specimens were then stored in saline until the experiments.
The baseline surface microhardness of each specimen was subsequently measured by a Vickers microhardness tester (KB HardWin XL; KB Pruftechnik GmbH, Germany) by applying 200g load for 10 seconds. The measurements were repeated in triplicate, and the mean of the three measurements was calculated and reported. Microhardness was calculated using the formula below:
Where VH is the Vickers hardness number, F is the load applied in kilograms, and d is the diameter of the indentation in millimeters. VH was measured in kilograms per square-millimeters.
Each specimen was then immersed in 10mL of demineralizing solution composed of 2.9g NaCl, 0.12g CaCl2, 0.13g NaH2PO4, 5mL NaN4, and 1.5mL acetic acid with a pH of 4 at 37°C for 1 week for artificial induction of demineralization and development of white spot lesions [11]. This solution was refreshed every 4 hours during the day but not overnight (a total of 5 times a day). Next, the specimens were rinsed with distilled water and stored in 0.9% saline for 24 hours.
The specimens were then randomly assigned to five groups as follows:
NaF group: 5% NaF varnish (ADS, USA) was applied on the surface of specimens according to the manufacturer’s instructions and dried. Accordingly, the tooth surface was brushed and dried. A microbrush dipped in varnish was rubbed on the entire tooth surface; the varnish was allowed 4 minutes to dry.
Blue laser group: Blue (diode) laser (Sirona, USA) with 445nm wavelength, 0.3W power, 60 seconds time, 320μm fiber diameter, spot size of 0.196cm2, and 91.8J/cm2 energy density in continuous-wave mode was irradiated with 1mm distance from the tooth surface [15].
Er:YAG laser group: Er:YAG laser (Light Walker, Fotona, Ljubljana, Slovenia) with 2940nm wavelength, 100mJ pulse energy, 10Hz frequency, and 300µs pulse duration was irradiated with sweeping motion for 20 seconds under distilled water coolant with 1W average power at 1mm distance from the tooth surface. The total energy was 20J. The external diameter of the tip was 1mm [22].
NaF + blue laser group: 5% NaF varnish + blue laser were used in this group. The varnish was applied according to the manufacturer’s instructions, and then blue laser was irradiated as explained for the blue laser group.
NaF + Er:YAG laser group: 5% NaF varnish + Er:YAG laser were used in this group. The varnish was applied according to the manufacturer’s instructions, and then Er:YAG laser was irradiated as explained for Er:YAG laser group.
After the interventions, the teeth were stored in artificial saliva (Fusayama-Meyer) at room temperature for 1 week. The saliva was refreshed daily. The composition of artificial saliva used in this study was as follows:
KCl (0.4g/L), NaCl (0.4g/L), CaCl2.2H2O (0.906g/L), NaH2PO4.2H2O (0.690g/L), Na2S.9H2O (0.005g/L), and urea (1g/L) with a pH of 7.03. After 1 week, the specimens were brushed with a fluoridated toothpaste for 7 minutes. After a final rinse with distilled water, they were stored in 0.9% saline for 24 hours.
The surface microhardness of the specimens was measured again as explained earlier, and the percentage of surface microhardness recovery (final microhardness – primary microhardness/primary microhardness) was calculated for each specimen.
The amount of calcium (Ca) and phosphorus (P) ions in the enamel was also quantified by energy dispersive X-ray spectroscopy. All measurements were made by an examiner blinded to the group allocation of the specimens.
The enamel microhardness and the calcium and phosphorus content were quantitative variables that had a normal distribution in this study and were reported as mean and standard deviation values. To compare the five groups regarding these variables, one-way ANOVA was applied followed by pairwise comparisons by the Tukey’s test. P<0.05 was considered statistically significant.
Results
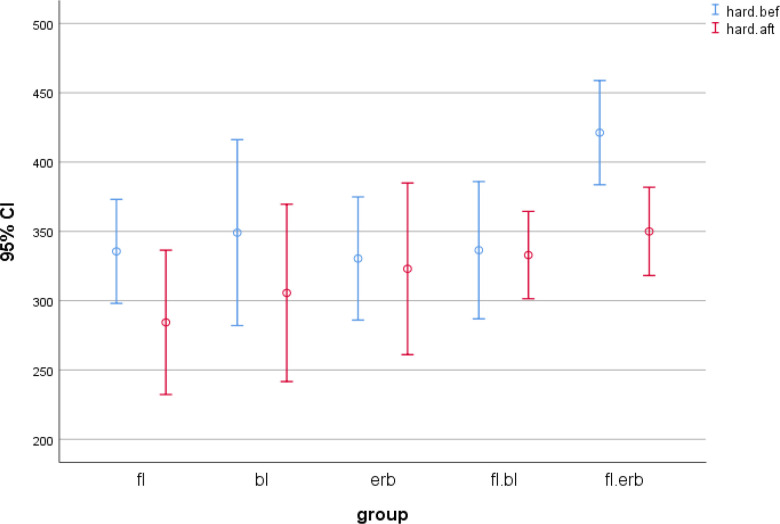
Comparison of the mean baseline and final microhardness values within NaF, NaF-blue laser, and NaF-Er:YAG laser groups revealed no significant difference (Fig. 1); in other words, these interventions successfully recovered the decreased microhardness (due to immersion in demineralizing solution) to a level close to that of the baseline microhardness; the NaF-blue laser and NaF-Er:YAG laser were more successful than NaF alone for this purpose. The difference between NaF-blue laser and NaF-Er:YAG laser was not significant (P=0.166). In blue laser (P=0.002) and Er:YAG laser (P=0.011) groups, the mean final microhardness was significantly lower than the baseline microhardness, and these interventions were not successful in recovering the baseline microhardness after immersion in demineralizing solution.
Fig 1.
Distribution of the mean microhardness values with 95% confidence interval (P<0.05, fl: 5% sodium fluoride (NaF) varnish, bl: 445nm diode laser, erb: Er:YAG laser, fl.bl: 5% NaF + 445nm diode laser, and fl.erb: 5% NaF + Er:YAG laser).
As shown in Table 1, the percentage of phosphorus ions was not significantly different in the experimental groups compared with sound enamel; in other words, the phosphorus content of the enamel in all groups remained the same as that in sound enamel after all interventions. Regarding the calcium content, only NaF group showed a significantly lower percentage of calcium compared with the sound enamel and all other groups. The calcium content of the enamel in blue laser, Er:YAG laser, NaF-blue laser, and NAF-Er:YAG laser groups was similar to the calcium content of sound enamel.
Table 1.
Calcium and phosphorus contents (percentage) of the enamel in the five groups
| Element | Groups | Mean Difference |
Std. Error | Sig. | 95% confidence interval | |
| Lower bound | Upper bound | |||||
| Ca | Fluoride | 16.41 | 3.42 | 0.001 | 5.98 | 26.84 |
| Blue Laser | 2.32 | 3.42 | 0.983 | -8.10 | 12.74 | |
| Er:YAG laser | 4.50 | 3.42 | 0.775 | -5.92 | 14.93 | |
| Fluoride + Blue laser | -0.32 | 3.42 | 1.000 | -10.75 | 10.89 | |
| Fluoride + Er:YAG laser | 3.84 | 3.42 | 0.869 | -6.58 | 14.27 | |
| P | Fluoride | 3.07 | 2.56 | 0.834 | -4.73 | 10.89 |
| Blue Laser | -2.00 | 2.56 | 0.969 | -0.81 | 5.80 | |
| Er:YAG laser | -2.05 | 2.56 | 0.965 | -9.86 | 5.75 | |
| Fluoride + Blue laser | -1.66 | 2.56 | 0.986 | -9.47 | 6.14 | |
| Fluoride + Er:YAG laser | 1.21 | 2.56 | 0.997 | -6.60 | 9.02 | |
Discussion
Fluoride is the most effective cariostatic agent that can prevent the development of carious lesions. Clinical studies have shown that topical fluoride has 20% to 40% efficacy for caries prevention. No significant difference has been found in the efficacy of different forms of topical fluoride such as varnish, gel, or solution. Thus, selection of the type of fluoride product depends on the cost, availability, patient acceptance, and safety [23].
Pandit et al. [24] confirmed the optimal efficacy of fluoride for caries prevention, and discussed that it can prevent demineralization, enhance remineralization, and decrease the metabolic activity of bacteria [24].
Evidence shows that following topical application of fluoride, CaF2 forms on the enamel surface and subsurface of carious enamel. When the oral environment is acidified, the fluoride ions are released from CaF2 and deposit again in the form of fluorapatite in the enamel structure [25].
Lasers were first used for caries prevention in 1965, and then clinicians started to use them with different protocols such as laser irradiation alone or in combination with fluoride [15]. One application of laser in dentistry is laser irradiation of dental hard tissue to increase its resistance to caries [26]. Fekrazad et al. [23] found that in addition to prevention of enamel demineralization, laser therapy can decrease the pH threshold at which dissolution occurs.
The mechanisms of the effects of laser on dental hard tissue can be divided into three main categories of physical changes, chemical changes, and kinetic changes and alterations of the polarity of crystals, due to the conversion of laser energy to heat [27].
Yamamoto and Sato [28] concluded that use of laser for caries prevention would better not be in the form of enamel melting or ablation. Thus, sub-ablative laser parameters were adopted in the present study.
Maung et al. [29] demonstrated that laser therapy prevented enamel demineralization and decreased enamel permeability. On the other hand, Chinelatti et al. [30] indicated that Er:YAG laser irradiation had no significant effect on enamel microhardness. Delbem et al. [31] showed that low-level Er:YAG laser irradiation only increased the internal enamel pressure due to temperature rise and water evaporation without melting or re-crystallization. Er:YAG laser is commonly used for dental hard tissue ablation. However, limited studies have shown that low-level Er:YAG laser has cariostatic properties as well. Moreover, it has been reported that sub-ablative Er:YAG laser irradiation combined with fluoride therapy can cause immediate conversion of hydroxyapatite to fluorapatite [32]. In line with the present results, the synergistic effect of laser therapy and fluoride therapy for enhancement of enamel resistance to acid attacks has been previously reported. Daily use of fluoride mouthwash can be tiresome for many patients. However, laser irradiation is only performed in dental office setting, and not on a daily basis. Thus, a combination of fluoride therapy and laser can decrease the need for frequent use of fluoride products, and is simpler for patients [23]. Laser irradiation can strengthen the bond between the fluoride-containing compounds on the surface and enamel, and decrease enamel solubility [23].
Previous studies have evaluated the efficacy of different laser types in combination with topical fluoride, indicating that fluoride concentration increases following laser irradiation. Liu et al. [14] evaluated the effect of combined use of laser therapy and fluoride therapy on enamel demineralization and demonstrated that the efficacy of laser therapy was comparable to that of fluoride therapy for reduction of demineralization. According to Valizadeh et al. [33] Er:YAG laser irradiation alone had no significant effect on enamel resistance to cariogenic solution. Nonetheless, they showed its synergistic effect when used in combination with NaF varnish. They concluded that application of fluoride varnish prior to laser irradiation conferred greater resistance to tooth structure and enhanced its hardness. Mathew et al. [34] reported that enhancement of enamel resistance following Er:YAG laser irradiation and fluoride application was greater than that caused by monotherapy with fluoride or laser. Liu et al. [14] in another study demonstrated that fluoride therapy along with Er:YAG laser irradiation had a synergistic effect on inhibition of enamel demineralization and increasing the enamel resistance to acid attacks. Delbem et al. [31] showed that Er:YAG laser therapy plus fluoride application resulted in minimum microhardness reduction following a demineralization cycle. However, Chinelatti et al. [30] observed that Er:YAG laser irradiation along with fluoride therapy could not prevent enamel erosion in primary or permanent teeth.
Several mechanisms have been suggested to explain the cariostatic effects of blue laser such as the photothermal interaction of laser and hydroxyapatite, interaction of laser with enamel organic matrix, and enhanced fluoride uptake by the enamel as the result of laser irradiation [29, 35]. According to Nammour et al. [36] blue laser irradiation of enamel results in fluoride retention by 42.3% after 7 days, compared with 12.3% in non-laser irradiated enamel.
The photon energy of blue laser (445nm) is 2.8eV, which is higher than that of other laser types, and suggests the possibility of a photochemical interaction in addition to photothermal effects. These interactions increase the formation of fluorapatite by replacement of carbonate or hydroxyl groups with fluoride. This replacement is favorable since it would result in a more stable molecular arrangement [37]. Hsu et al. [38] demonstrated that combined use of blue laser and fluoride conferred greater resistance to enamel against acid attacks compared with monotherapy with laser or fluoride. According to Ceballos-Jiménez et al. [39] combined use of Er:YAG laser and NaF, and also monotherapy with NaF caused a smaller reduction in calcium ions, and resulted in higher enamel resistance to demineralization; however, this increase was not statistically significant. Da Cunha et al. [40] indicated the optimal efficacy of NaF for inhibition of enamel erosion following an acidic challenge with 1% citric acid based on the magnitude of phosphorus content reduction. However, based on the magnitude of calcium content reduction, they concluded that NaF did not have a significant effect on inhibition of enamel erosion following acidic challenge with 1% and 10% citric acid.
Although the present results revealed that fluoride therapy and laser therapy can decrease enamel demineralization, further in situ studies are required to acquire a better understanding of the behavior of laser-irradiated enamel in an acidic challenge. However, mineral loss is expected to be lower under in situ and in vivo conditions due to the presence of saliva and its buffering capacity and remineralizing potential that maintains the balance of minerals.
CONCLUSION
Considering the current results and the limitations of this study, it appears that use of fluoride, and also Er:YAG and blue lasers along with fluoride (due to their synergistic effect) can increase the microhardness of demineralized enamel close to the baseline level.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Mohammad Javad Kharazifard for statistical analysis. The authors wish to thank the Dental Research Center, Dentistry Research Institute, Tehran University of Medical Sciences for the financial support with grant number 99-2-133-48323.
Notes:
Cite this article as: Hashemikamangar SS, Merati H, Valizadeh S, Saberi S. Effects of Lasers and Fluoride Varnish on Microhardness and Calcium and Phosphorus Content of Demineralized Enamel. Front Dent. 2024:21:27.
CONFLICT OF INTEREST STATEMENT
None declared.
References
- 1.West NX, Joiner A. Enamel mineral loss. J Dent. 2014 Jun;42:S2–11. doi: 10.1016/S0300-5712(14)50002-4. [DOI] [PubMed] [Google Scholar]
- 2.Kermanshah H, Hashemikamangar SS, Arami S, Mirsalehian A, Kamalinegad M, Karimi M, Jabalameli F. Comparison of antibacterial effect of hydroalcoholic extract of four plants against cariogenic microorganisms by two in vitro Methods. J Babol Univ Med Sci. 13(6):21–9. [Google Scholar]
- 3.Llena C, Leyda AM, Forner L. CPP-ACP and CPP-ACFP versus fluoride varnish in remineralisation of early caries lesions. A prospective study. Eur J Paediatr Dent. 2015 Sep;16(3):181–6. [PubMed] [Google Scholar]
- 4.Petersson GH. Assessing caries risk--using the Cariogram model. Swed Dent J. 2003 Jan;(158):1–65. [PubMed] [Google Scholar]
- 5.Oliveira PR, Fonseca AB, Silva ED, Coutinho TC, Tostes MA. Remineralizing potential of CPP‐ACP creams with and without fluoride in artificial enamel lesions. Aust Dent J. 2016 Mar;61(1):45–52. doi: 10.1111/adj.12305. [DOI] [PubMed] [Google Scholar]
- 6.Tatevossian A. Fluoride in dental plaque and its effects. J Dent Res. 1990 Feb;69(2_suppl):645–52. doi: 10.1177/00220345900690S126. [DOI] [PubMed] [Google Scholar]
- 7.Savas S, Kavrìk F, Kucukyilmaz E. Evaluation of the remineralization capacity of CPP-ACP containing fluoride varnish by different quantitative methods. J Appl Oral Sci. 2016 May;24:198–203. doi: 10.1590/1678-775720150583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poosti M, Ahrari F, Moosavi H, Najjaran H. The effect of fractional CO 2 laser irradiation on remineralization of enamel white spot lesions. Lasers Med Sci. 2014 Jul;29:1349–55. doi: 10.1007/s10103-013-1290-9. [DOI] [PubMed] [Google Scholar]
- 9.Jones S, Burt BA, Petersen PE, Lennon MA. The effective use of fluorides in public health. Bull World Health Organ. 2005;83:670–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Thurnheer T, Belibasakis GN. Effect of sodium fluoride on oral biofilm microbiota and enamel demineralization. Arch Oral Biol. 2018 May;89:77–83. doi: 10.1016/j.archoralbio.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Ekambaram M, Itthagarun A, King NM. Comparison of the remineralizing potential of child formula dentifrices. Int J Paediatr Dent. 2011 Mar;21(2):132–40. doi: 10.1111/j.1365-263X.2010.01101.x. [DOI] [PubMed] [Google Scholar]
- 12.Gerard DE, Fried D, Featherstone JD, Nancollas GH. Influence of laser irradiation on the constant composition kinetics of enamel dissolution. Caries Res. 2005 Aug;39(5):387–92. doi: 10.1159/000086845. [DOI] [PubMed] [Google Scholar]
- 13.Verma SK, Maheshwari S, Singh RK, Chaudhari PK. Laser in dentistry: An innovative tool in modern dental practice. Natl J Maxillofac Surg. 2012 Jul;3(2):124–32. doi: 10.4103/0975-5950.111342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Hsu CY, Teo CM, Teoh SH. Potential mechanism for the laser-fluoride effect on enamel demineralization. J Dent Res. 2013 Jan;92(1):71–5. doi: 10.1177/0022034512466412. [DOI] [PubMed] [Google Scholar]
- 15.Al-Maliky MA, Frentzen M, Meister J. Artificial caries resistance in enamel after topical fluoride treatment and 445 nm laser irradiation. BioMed Res Int. 2019;2019(1):9101642. doi: 10.1155/2019/9101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chin-Ying SH, Xiaoli G, Jisheng P, Wefel JS. Effects of CO2 laser on fluoride uptake in enamel. J Dent. 2004 Feb;32(2):161–7. doi: 10.1016/j.jdent.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Zancopé BR, Rodrigues LP, Parisotto TM, Steiner-Oliveira C, Rodrigues LK, Nobre-dos-Santos M. CO 2 laser irradiation enhances CaF 2 formation and inhibits lesion progression on demineralized dental enamel—In vitro study. Lasers Med Sci. 2016 Apr;31:539–47. doi: 10.1007/s10103-016-1900-4. [DOI] [PubMed] [Google Scholar]
- 18.Ana PA, Bachmann L, Zezell DM. Lasers effects on enamel for caries prevention. Laser Phys. 2006 May;16:865–75. [Google Scholar]
- 19.Korytnicki D, Mayer MP, Daronch M, Singer JD, Grande RH. Effects of Nd: YAG laser on enamel microhardness and dental plaque composition: an in situ study. Photomed Laser Surg. 2006 Feb;24(1):59–63. doi: 10.1089/pho.2006.24.59. [DOI] [PubMed] [Google Scholar]
- 20.Can AM, Darling CL, Ho C, Fried D. Non‐destructive assessment of inhibition of demineralization in dental enamel irradiated by a λ= 9.3‐µm CO2 laser at ablative irradiation intensities with PS‐OCT. Lasers Surg Med. 2008 Jul;40(5):342–9. doi: 10.1002/lsm.20633. [DOI] [PubMed] [Google Scholar]
- 21.Zancopé BR, Cesar MM, Rodrigues LK, Nobre-dos-Santos M. Evaluation of the effect of a CO2 laser and fluoride on the reduction of carious lesions progression in primary teeth: an in vitro study. Lasers Dent. 2014 Feb;8929:147–55. [Google Scholar]
- 22.Yassaei S, Motallaei MN. The effect of the Er: YAG laser and MI paste plus on the treatment of white spot lesions. J Lasers Med Sci. 2020;11(1):50. doi: 10.15171/jlms.2020.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fekrazad R, Ebrahimpour L. Evaluation of acquired acid resistance of enamel surrounding orthodontic brackets irradiated by laser and fluoride application. Lasers Med Sci. 2014 Nov;29:1793–8. doi: 10.1007/s10103-013-1328-z. [DOI] [PubMed] [Google Scholar]
- 24.Pandit S, Kim JE, Jung KH, Chang KW, Jeon JG. Effect of sodium fluoride on the virulence factors and composition of Streptococcus mutans biofilms. Arch Oral Biol. 2011 Jul;56(7):643–9. doi: 10.1016/j.archoralbio.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Borggreven JM, Van Dijk JW, Driessens FC. Effect of laser irradiation on the permeability of bovine dental enamel. Arch Oral Biol. 1980 Jan;25(11-12):831–2. doi: 10.1016/0003-9969(80)90142-9. [DOI] [PubMed] [Google Scholar]
- 26.Davari A, Daneshkazemi A, Assarzadeh H, Shafiee F. Evaluation of Er: YAG laser and fluoride ion effects on the remineralization of enamel white spot lesions. J Mashhad Dent Sch. 2018 Sep;42(3):220–10. [Google Scholar]
- 27.Amaechi BT, Higham SM. In vitro remineralisation of eroded enamel lesions by saliva. J Dent. 2001 Jul;29(5):371–6. doi: 10.1016/s0300-5712(01)00026-4. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto H, Sato K. Prevention of dental caries by acousto-optically Q-switched Nd: YAG laser irradiation. J Dent Res. 1980 Feb;59(2):137. doi: 10.1177/00220345800590020801. [DOI] [PubMed] [Google Scholar]
- 29.Maung NL, Wohland T, Hsu CY. Enamel diffusion modulated by Er: YAG laser:(part 1)—FRAP. J Dent. 2007 Oct;35(10):787–93. doi: 10.1016/j.jdent.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Chinelatti MA, Rocha CT, Colucci V, Serra MC, Rodrigues-Júnior AL, Corona SA. Effect of Er: Yag laser on dentin demineralization around restorations. Lasers Med Sci. 2017 Feb;32:413–8. doi: 10.1007/s10103-016-2136-z. [DOI] [PubMed] [Google Scholar]
- 31.Delbem AC, Cury JA, Nakassima CK, Gouveia VG, Theodoro LH. Effect of Er: YAG laser on CaF2 formation and its anti-cariogenic action on human enamel: an in vitro study. J Clin Laser Med Surg, 2003;21(4):197–201. doi: 10.1089/104454703768247765. [DOI] [PubMed] [Google Scholar]
- 32.Demito CF, Vivaldi‐Rodrigues G, Ramos AL, Bowman SJ. The efficacy of a fluoride varnish in reducing enamel demineralization adjacent to orthodontic brackets: an in vitro study. Orthod Craniofac Res. 2004 Nov;7(4):205–10. doi: 10.1111/j.1601-6343.2004.00305.x. [DOI] [PubMed] [Google Scholar]
- 33.Valizadeh S, Khub MR, Chiniforush N, Kharazifard MJ, Hashemikamangar SS. Effect of laser irradiance and fluoride varnish on demineralization around dental composite restorations. J Lasers Med Sci. 2020;11(4):450–5. doi: 10.34172/jlms.2020.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathew A, Reddy NV, Sugumaran DK, Peter J, Shameer M, Dauravu LM. Acquired acid resistance of human enamel treated with laser (Er: YAG laser and Co: 2: laser) and acidulated phosphate fluoride treatment: An: in vitro: atomic emission spectrometry analysis. Contemp Clin Dent. 2013 Apr;4(2):170–5. doi: 10.4103/0976-237X.114864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holcomb DW, Young RA. Thermal decomposition of human tooth enamel. Calcif Tissue Int. 1980 Dec;31:189–201. doi: 10.1007/BF02407181. [DOI] [PubMed] [Google Scholar]
- 36.Nammour S, Demortier G, Florio P, Delhaye Y, Pireaux JJ, Morciaux Y, Powell L. Increase of enamel fluoride retention by low fluence argon laser in vivo. Lasers Surg Med. 2003 Oct;33(4):260–3. doi: 10.1002/lsm.10219. [DOI] [PubMed] [Google Scholar]
- 37.Robinson C, Shore RC, Brookes SJ, Strafford S, Wood SR, Kirkham J. The chemistry of enamel caries. Crit Rev Oral Biol Med. 2000 Oct;11(4):481–95. doi: 10.1177/10454411000110040601. [DOI] [PubMed] [Google Scholar]
- 38.Hsu CY, Jordan TH, Dederich DN, Wefel JS. Laser-matrix-fluoride effects on enamel demineralization. J Dent Res. 2001 Sep;80(9):1797–801. doi: 10.1177/00220345010800090501. [DOI] [PubMed] [Google Scholar]
- 39.Ceballos-Jiménez AY, Rodríguez-Vilchis LE, Contreras-Bulnes R, Alatorre JÁ, Velazquez-Enriquez U, García-Fabila MM. Acid resistance of dental enamel treated with remineralizing agents, Er: YAG laser and combined treatments. Dent Med Probl. 2018;55(3):255–9. doi: 10.17219/dmp/93960. [DOI] [PubMed] [Google Scholar]
- 40.Da Cunha WA, Palma LF, Shitsuka C, Corrêa FN, Duarte DA, Corrêa MS. Efficacy of silver diamine fluoride and sodium fluoride in inhibiting enamel erosion: an ex vivo study with primary teeth. Eur Arch of Paediatr Dent. 2021 Jun;22:387–92. doi: 10.1007/s40368-020-00559-1. [DOI] [PubMed] [Google Scholar]