Abstract
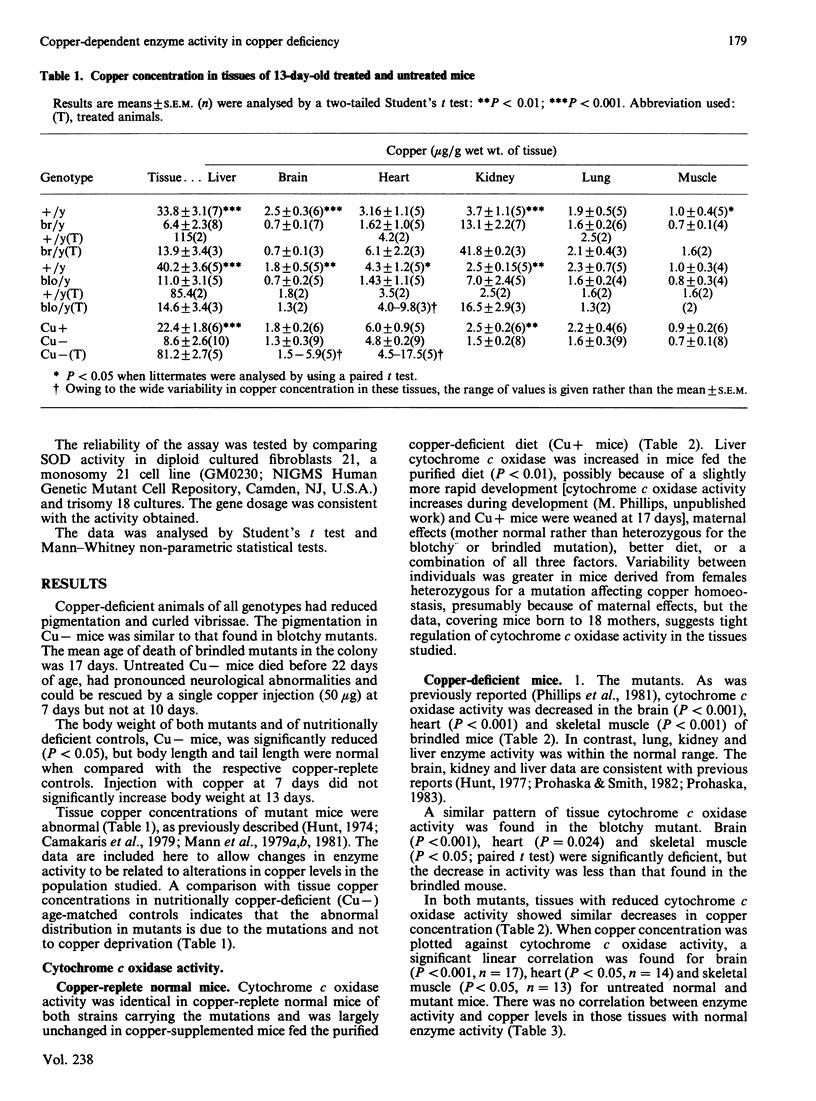
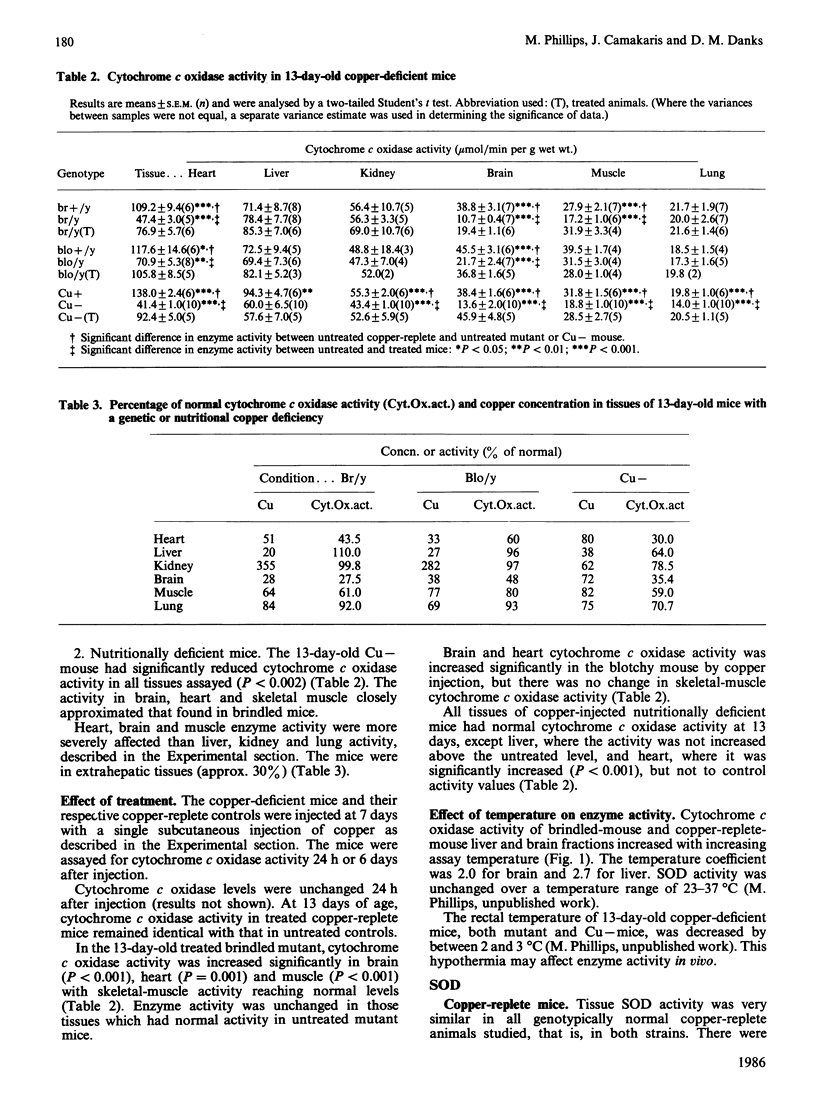
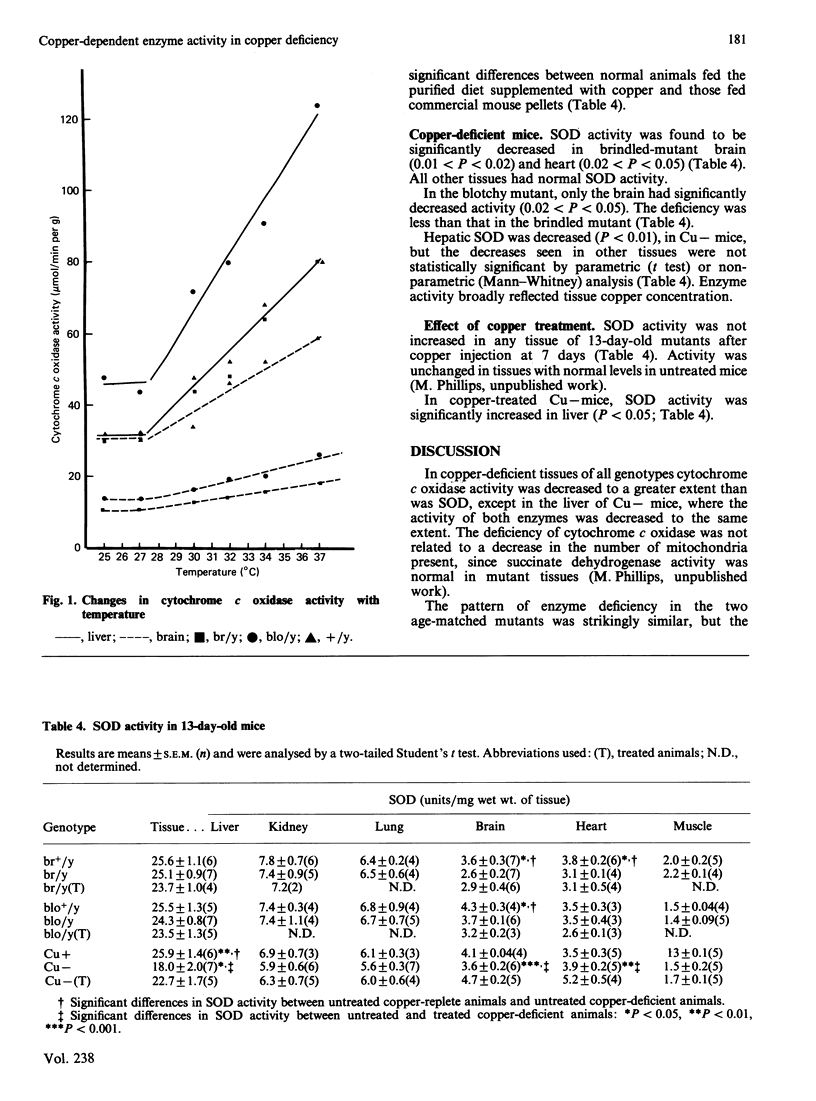
The activity of two copper-dependent enzymes, cytochrome c oxidase and copper, zinc-superoxide dismutase, was determined in six tissues of age-matched (13-day-old) copper-deficient mutant and normal mice. In the two mutants 'brindled' and 'blotchy', brain, heart and skeletal muscle had significant enzyme deficiencies. Cytochrome c oxidase was more severely affected than was superoxide dismutase. In these three tissues the degree of deficiency could be correlated with decreased copper concentration; however, enzyme activity was normal in liver, kidney and lung, despite abnormal copper concentrations in these tissues. In nutritionally copper-deficient mice, all six tissues showed decreased enzyme activity, which was most marked in brain, heart and skeletal muscle, the tissues which showed enzyme deficiencies in the mutants. Analysis in vitro of cytochrome c oxidase (temperature coefficient = 2) at a single temperature was found to underestimate the deficiency of this enzyme in hypothermic copper-deficient animals. Cytochrome c oxidase deficiency may therefore be sufficiently severe in vivo to account for the clinical manifestations of copper deficiency. An injection of copper (50 micrograms of Cu+) at 7 days increased cytochrome c oxidase activity by 13 days in all deficient tissues of brindled mice, and in brain and heart from blotchy mice. However, skeletal-muscle cytochrome c oxidase in blotchy mutants did not respond to copper injection. Cytochrome c oxidase activity increased to normal in all tissues of nutritionally copper-deficient mice after copper injection, except in the liver. Hepatic enzyme activity remained severely deficient despite a liver copper concentration three times that found in copper-replete controls. Superoxide dismutase activity did not increase with treatment in either mutant, but its activity was higher than control levels in nutritionally deficient mice after injection. This difference is probably due to sequestration of copper in mutant tissue such as kidney, but a defect in the copper transport pathway to superoxide dismutase cannot be excluded.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Montecucco C., Richter C. The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophys Res Commun. 1975 Jul 22;65(2):597–603. doi: 10.1016/s0006-291x(75)80188-4. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Camakaris J., Mann J. R., Danks D. M. Copper metabolism in mottled mouse mutants: copper concentrations in tissues during development. Biochem J. 1979 Jun 15;180(3):597–604. doi: 10.1042/bj1800597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GALLAGHER C. H., JUDAH J. D., REES K. R. The biochemistry of copper deficiency. II. Synthetic processes. Proc R Soc Lond B Biol Sci. 1956 May 29;145(919):195–205. doi: 10.1098/rspb.1956.0027. [DOI] [PubMed] [Google Scholar]
- Hunt D. M. A study of copper treatment and tissue copper levels in the murine congenital copper deficiency, mottled. Life Sci. 1976 Dec 15;19(12):1913–1919. doi: 10.1016/0024-3205(76)90124-7. [DOI] [PubMed] [Google Scholar]
- Hunt D. M. Catecholamine biosynthesis and the activity of a number of copper-dependent enzymes in the copper deficient mottled mouse mutants. Comp Biochem Physiol C. 1977;57(1):79–83. doi: 10.1016/0306-4492(77)90082-x. [DOI] [PubMed] [Google Scholar]
- Hunt D. M. Primary defect in copper transport underlies mottled mutants in the mouse. Nature. 1974 Jun 28;249(460):852–854. doi: 10.1038/249852a0. [DOI] [PubMed] [Google Scholar]
- Mann J. R., Camakaris J., Danks D. M. Copper metabolism in mottled mouse mutants: distribution of 64Cu in brindled (Mobr) mice. Biochem J. 1979 Jun 15;180(3):613–619. doi: 10.1042/bj1800613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J. R., Camakaris J., Danks D. M., Walliczek E. G. Copper metabolism in mottled mouse mutants: copper therapy of brindled (Mobr) mice. Biochem J. 1979 Jun 15;180(3):605–612. doi: 10.1042/bj1800605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J. R., Camakaris J., Francis N., Danks D. M. Copper metabolism in mottled mouse (Mus musculus) mutants. Studies of blotchy (Moblo) mice and a comparison with brindled (Mobr) mice. Biochem J. 1981 Apr 15;196(1):81–88. doi: 10.1042/bj1960081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Prohaska J. R., Smith T. L. Effect of dietary or genetic copper deficiency on brain catecholamines, trace metals and enzymes in mice and rats. J Nutr. 1982 Sep;112(9):1706–1717. doi: 10.1093/jn/112.9.1706. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., McGoodwin E. B., Martin G. R., Grahn D. Decreased lysyl oxidase activity in the aneurysm-prone, mottled mouse. J Biol Chem. 1977 Feb 10;252(3):939–942. [PubMed] [Google Scholar]
- Rowe D. W., McGoodwin E. B., Martin G. R., Sussman M. D., Grahn D., Faris B., Franzblau C. A sex-linked defect in the cross-linking of collagen and elastin associated with the mottled locus in mice. J Exp Med. 1974 Jan 1;139(1):180–192. doi: 10.1084/jem.139.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce P. M., Camakaris J., Mann J. R., Danks D. M. Copper metabolism in mottled mouse mutants. The effect of copper therapy on lysyl oxidase activity in brindled (Mobr) mice. Biochem J. 1982 Feb 15;202(2):369–371. doi: 10.1042/bj2020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. M., Osborne-White W. S., O'Dell B. L. Cytochromes in brain mitochondria from lambs with enzootic ataxia. J Neurochem. 1976 Jun;26(6):1145–1148. doi: 10.1111/j.1471-4159.1976.tb06998.x. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]
- Wenk G., Suzuki K. Congenital copper deficiency: copper therapy and dopamine-beta-hydroxylase activity in the mottled (brindled) mouse. J Neurochem. 1983 Dec;41(6):1648–1652. doi: 10.1111/j.1471-4159.1983.tb00876.x. [DOI] [PubMed] [Google Scholar]
- Wenk G., Suzuki K. The effect of copper supplementation on the concentration of copper in the brain of the brindled mouse. Biochem J. 1982 Sep 1;205(3):485–487. doi: 10.1042/bj2050485. [DOI] [PMC free article] [PubMed] [Google Scholar]


