Abstract
Highly biocompatible microcarriers are culture materials designed to enhance the efficiency of cell spheroid culture. Typically, collagen or specially processed plastic materials serve as these microcarriers. In the context of cultured-cell-based food production, however, both collagen and plastic materials present challenges regarding their cost-effectiveness and edibility. A notable issue with collagen, especially when derived from fish scales, is its low denaturation temperature, making it unsuitable for use with mammalian cells unless cross-linked. To address this issue, our research pivoted towards utilizing dried fish, a rich source of proteins including collagen. For this study, Medaka fish were selected. The fish were dried, ground into fine particles, and then impregnated with ethanol to create dried fish powder (DFP). Its efficacy was then evaluated as a microcarrier in spheroid cultures. The results revealed that DFP supports the adhesion and proliferation of various cell types, including human epidermal cells, human malignant melanoma cells, mouse fibroblasts, mouse endothelial cells and fish fibroblasts. Furthermore, Western blot analysis was used to verify the expression of mitogen-activated protein kinase-related proteins in both human epidermal cells and mouse fibroblasts cultured with DFP. This fish-derived powdered microcarrier offers a cost-effective production method requiring only a few steps. Its affordability and high performance as a carrier position it as a potentially revolutionary material for use in biological research and food production science.
Keywords: Medaka, Dried fish, Microcarrier, Spheroid
Highlights
-
•
Specialized microcarriers were developed from dried fish for use in spheroid cultures.
-
•
Dried fish powder exhibits high biocompatibility with both fish and mammalian cells.
-
•
Protein analysis in spheroid cultures that utilized dried fish powder was feasible.
1. Introduction
Cell spheroid culture represents one of the methodologies in cell culture systems [1]. Several methods are available for forming cell spheroids, including the hanging drop technique, the use of U-shaped or V-shaped well plates, and methods employing a non-adhesive culture dish [2]. Specifically, microcarriers are employed as culture substrates for facilitating large-scale cultivation of cell spheroid culture [3]. Collagen is recognized as an optimal microcarrier for cell culture due to its favorable properties [4,5]. However, its relatively high cost in general renders it unsuitable for economical culture applications [6]. Collagen sourced from fish scales is comparatively more affordable than collagen derived from bovine and porcine sources. However, with the exception of certain large fish species such as Tilapia, Labeo rohita and Catla catia, fish scale-derived collagen typically exhibits a lower denaturation temperature [6,7]. This characteristic poses challenges for its utilization as a culture carrier for mammalian cells.
In this research, we focused on dried fish, categorizing it as a biologically derived dried product. Dried fish, a product manufactured globally, is commonly consumed after undergoing a heating process [8]. Upon heating, dried fish largely conserves its structural integrity, with minimal melting observed. This observation served as an inspiration for our concept of utilizing fish powder as a microcarrier in cell spheroid culture applications. The Medaka (Oryzias latipes), a small eurythermal fish species, exhibits widespread prevalence across diverse aquatic habitats including streams, ponds and creeks in Asian countries [9]. Due to their compact body size and the relative ease of cultivation, Medaka fish are utilized not only as ornamental species but also as valuable models in experimental research [10]. In consideration of potential industrial applications, we emphasize the compact size and the simplicity of cultivating Medaka fish, and consequently selecting this species as the primary source for fish powder production.
In the present study, the feasibility of using Medaka fish-derived DFP as a microcarrier in spheroid cultures of mammalian cells was investigated.
2. Materials & methods
2.1. Medaka fish-derived DFP manufacturing process
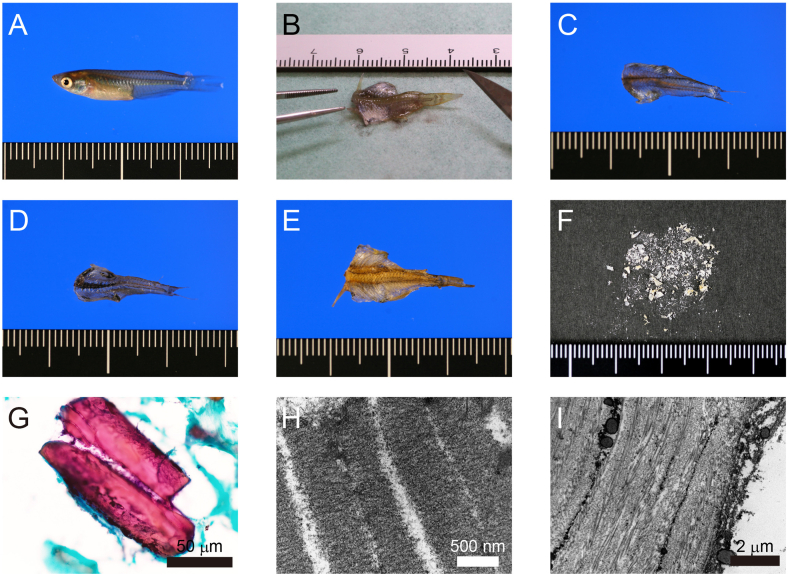
Adult conventional Medaka fish was purchased from a fish breeder (Yutoku, Saga, Japan) (Fig. 1A) and subjected to hypothermic immersion in ice water for euthanasia, followed by immediate processing to excise the head and internal organs (Fig. 1B–D). After 24-h in a freeze dryer (FDU-2100, EYELA, Tokyo, Japan), the weights of 10 fish were reduced from 0.66-0.86 g to 0.15–0.32 g (Fig. 1E). The dried product was pulverized into powder form using a crusher (Force Mill, OSAKA CHEMICAL, Osaka, Japan) (Fig. 1F). The DFP was fixed by immersion in 80 % ethanol for 48 h. Ethanol was eliminated using an evaporator (miVac Duo, ATS, Warminster, PA, USA), after which the mixture was reprocessed into a powdered form.
Fig. 1.
Preparation of dried fish powder (DFP) using Medaka (A) Macroscopic image of Medaka. (B) Processing treatment of Medaka bodies. (C) Outer surface after processing. (D) Inner surface after processing. (E) Dried body. (F) Macroscopic image of DFP. (G) Papanicolaou staining of DFP. (H, I) Transmission electron microscope image of DFP.
2.2. Ingredient analysis of medaka fish-derived DFP
To clarify the chemical composition, a chemical analysis was performed using 100 g of material, equivalent to 5000 medaka fish after the formation and drying process. The analysis of the basic components of medaka fish-derived DFP was outsourced to the Japan Functional Food Analysis and Research Center (Fukuoka, Japan). Amino acid analysis was outsourced to the Industrial Technology Center of Saga (Saga, Japan). The samples were hydrolyzed in hydrochloric acid containing 0.2 % phenol and 0.2 % sodium azide. The hydrolysate was then derivatized using the AccQ-Tag Ultra Derivatization Kit (Waters, MA, USA), and the derivatives were analyzed via pre-column derivatization HPLC. During hydrolysis, asparagine and glutamine are converted to aspartic acid and glutamic acid, respectively. Therefore, the measured amounts of aspartic acid include asparagine, and the measured amounts of glutamic acid include glutamine.
2.3. Cells
Human keratinocyte HaCaT cell lines were obtained from CLI (Eppelheim, Germany). Human melanoma HMY-1 and mouse fibroblast NIH/3T3 cell lines were obtained from the Japanese Cancer Research Bank (Osaka, Japan). MS-1 cell line was obtained from ATCC (Manassas, VA, USA). Pinna fibroblast CAF cells were sourced from the RIKEN Cell Bank (Ibaraki, Japan). Mammalian cells were cultured in RPMI-1640 medium (Fujifilm, Tokyo, Japan) containing 10 % fetal bovine serum (NICHIREI BIOSCIENCES, Tokyo, Japan), 100 μg/mL streptomycin and 100 μg/mL penicillin. Fish cells were cultured in Medium 199 (Gibco, Tokyo, Japan) containing 15 % fetal bovine serum, 100 μg/mL streptomycin and 100 μg/mL penicillin. The culture medium was replaced every alternate day.
Mammalian cells were incubated in a 5 % CO2 and 20 % O2 mixture at 37 °C in a CO2 incubator. Fish cells were incubated in a 5 % CO2 and 15 % O2 environment at 27 °C using an AnaeroPack-CO2 (MITSUBISHI GAS CHEMICAL, Tokyo, Japan) in a Gas-tight Container 2.5L (MITSUBISHI GAS CHEMICAL) that generated the specified gas mixture.
2.4. Cell culture using DFP
An average of 7 mg (range 4–8 mg) of DFP was mixed with 1 × 106 cells of each type in 10 mL of culture medium and they were left to stand for 5 h. After confirming the adhesion of the cells to the DFP, the mixture was transferred to a suspension cell culture dish (1020-100, AGC Techno Glass, Tokyo, Japan) and cultured in a rotating medium within an incubator. For the rotational culture of mammalian cells, a cell shaker (CS-LR, TAITEC, Tokyo Japan) was employed set to a rotation speed of 50 rpm. Conversely, an NR-3 (TAITEC) was used for fish cells at a rotation speed of 20 rpm. For the culture without DFP, the cell culture was conducted in the same manner, excluding the addition of DFP.
2.5. Histology and immunohistochemistry
After completion of the culture process, cell blocks were prepared from the samples using standard methods [11]. Histological analyses were conducted on sections stained with standard hematoxylin-eosin (HE) and Papanicolaou staining methods. Papanicolaou staining, which allows for accurate evaluation of the morphology of small cell clusters, was employed to clarify the shape of DFPs and to measure their size. For observation under a transmission electron microscope, DFPs were fixed with 2.5 % glutaraldehyde and 1 % osmium tetroxide, dehydrated with alcohol, and embedded in epoxy resin. Thin sections were then examined using an electron microscope (JEM-2100, JEOL Ltd., Tokyo). For the identification of epithelial cells and myofibroblasts, the mouse monoclonal anti-cytokeratin antibody (CK AE1/AE3, Dako, Glostrup, Denmark) and anti-α smooth muscle actin (αSMA, M0851, Dako) were employed, respectively. A rabbit polyclonal antibody against vimentin (#10366-1-AP, Proteintech, IL, USA) was used to identify mesenchymal cells. Cell proliferation in HaCaT cells forming spheroids (n = 5) was evaluated using a mouse monoclonal anti-Ki-67 antibody (Dako) and phospho-Histone H3 antibody (#9701; Cell Signaling Technology [CST], Danvers, MA, USA), and in NIH/3T3 cells forming spheroids (n = 4) a mouse monoclonal anti-proliferating cell nuclear antigen (PCNA) antibody (M0879; Dako). In the analysis of the labeling indices for Ki67 and PCNA, over 500 cells from each experimental group were counted under high magnification, and their rates of positivity analyzed. For immunofluorescence analyses, Alexa Fluor 488-conjugated goat anti-rabbit IgG (Thermo Fisher Scientific, Waltham, MA, USA) was used as the secondary antibody.
2.6. Western blot analysis
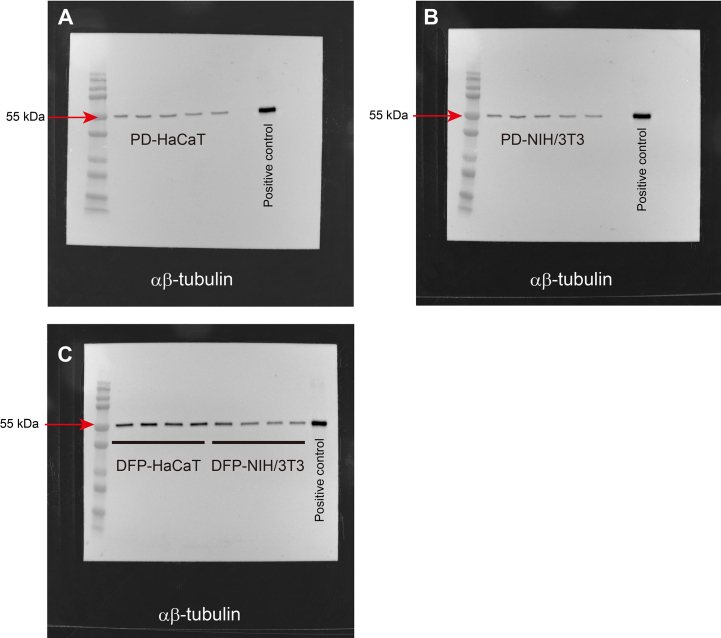
After 48 h of cell spheroid and 2-dimensional standard culture, samples were lysed using 300 μL of M-PER Reagent (Thermo Fisher Scientific), supplemented with the Protease/Phosphatase Inhibitor Cocktail (#5872; CST). Cell lysates, each standardized to contain an equal amount of protein, were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12 % Bis-Tris gels and then transferred to polyvinylidene difluoride membranes. The membranes were incubated overnight at 4 °C with antibodies targeting extracellular signal-regulated kinase (ERK) 1/2 (#9102; CST), phosphorylated ERK (#4370; CST), p38 (#8690; CST) and phosphorylated p38 (#4511; CST). α/β-Tubulin (#2148; CST) served as the internal control. The antibody-bound antigens on the membranes were visualized using a chemiluminescent immunodetection system (Western Breeze; Thermo Fisher Scientific). The band densities were captured using a FUSION system (Vilber-Lourmat, Eberhardzell, Germany) and analyzed with ImageJ software (National Institutes of Health, Bethesda, MD, USA). Data are presented as ratios relative to established control values.
2.7. Statistical analysis
Data obtained from 4 to 5 independent experiments were analyzed using Student's t-test or a Wilcoxon test, depending on the results of equality of variance. Values are presented as the mean ± SD, together with the number of experiments conducted. The mean value of replicates in experiments was used to determine statistical significance; P-values <0.05 were deemed to be statistically significant findings. All statistical analyses were performed using JMP 17 for Windows (SAS, Cary, N.C., USA).
3. Results
3.1. Characteristics of DFP derived from medaka fish
The size of DFP from medaka was measured using Papanicolaou staining (Fig. 1G). The size was 207 ± 81.1 μm, although a few hard tissues, such as scales and bone tissue, measured up to 1 mm. Observation under a transmission electron microscope confirmed a striated structure in the muscle tissue (Fig. 1H) and orderly arranged collagen fibers in the dermal tissue (Fig. 1I). As shown in Table 1, the content per 100 g of DFP was: 317 kcal of energy, 70.1 g of protein, 4.1 g of lipid, 0.0 g of carbohydrate, 1.4 g of salt equivalent, 540 mg of sodium, 12.0 g of water, and 13.8 g of ash. Amino acid analysis revealed that the content per 100 g of DFP was: 4224 mg of Ala, 4452 mg of Arg, 5878 mg of Asp/Asn, 748 mg of Cys, 9159 mg of Glu/Gln, 5361 mg of Gly, 1583 mg of His, 2699 mg of Ile, 5059 mg of Leu, 5147 mg of Lys, 1310 mg of Met, 2973 mg of Phe, 3254 mg of Pro, 2989 mg of Ser, 2904 mg of Thr, 1255 mg of Tyr, and 2993 mg of Val (Table 2).
Table 1.
Component analysis results of DFP.
| Item | Value | Note |
|---|---|---|
| Energy | 317 kcal/100 g | Energy conversion factors were calculated as 4 kcal/g for protein, 9 kcal/g for lipids, and 4 kcal/g for carbohydrates. |
| Protein | 70.1 g/100 g | Combustion method |
| Lipid | 4.1 g/100 g | Ether extraction method |
| Carbohydrate | 0.0 g/100 g | The carbohydrate content was calculated using the formula: 100 - (water + protein + lipid + ash). |
| Salt equivalent | 1.4 g/100 g | Sodium equivalent value |
| Sodium | 540 mg/100 g | Atomic absorption spectrometry |
| Water content | 12.0 g/100 g | Ambient temperature heating drying method |
| Ash content | 13.8 g/100 g | Direct incineration method |
Table 2.
Amino acid analysis results of DFP.
| Amino acid | Value |
|---|---|
| Ala | 4224 mg/100 g |
| Arg | 4452 mg/100 g |
| Asp/Asn | 5878 mg/100 g |
| Cys | 748 mg/100 g |
| Glu/Gln | 9159 mg/100 g |
| Gly | 5, 361 mg/100 g |
| His | 1583 mg/100 g |
| Ile | 2699 mg/100 g |
| Leu | 5059 mg/100 g |
| Lys | 5147 mg/100 g |
| Met | 1310 mg/100 g |
| Phe | 2973 mg/100 g |
| Pro | 3254 mg/100 g |
| Ser | 2989 mg/100 g |
| Thr | 2904 mg/100 g |
| Tyr | 1255 mg/100 g |
| Val | 2993 mg/100 g |
3.2. Cell spheroid formation using DFP
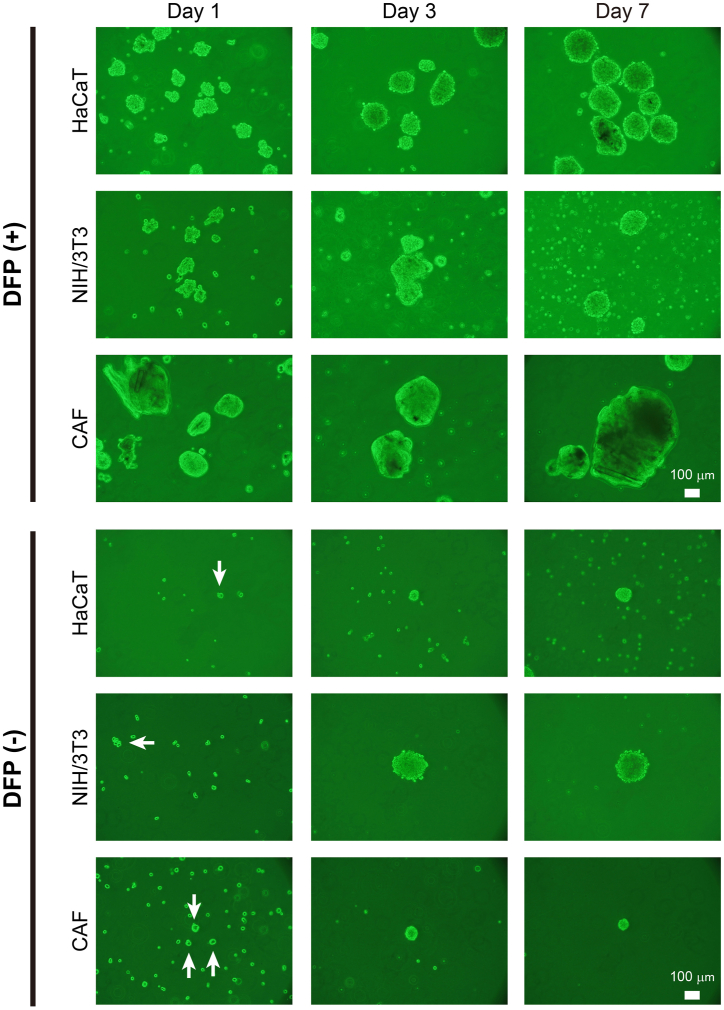
In this study, we combined DFP, which was generated as previously described (vide supra), with various cell lines to determine if cell spheroids could be formed. As shown in Fig. 2, the formation of cell spheroids was observed on the first day of culture for HaCaT, NIH/3T3 and CAF cells, after mixing them with DFP. Under all conditions, certain areas failed to completely envelop the DFP surface, resulting in noticeable unevenness on the surfaces of the spheroids. In all examined cell lines, there was a trend towards a slight increase in the size of cell spheroids on the third day of culture compared to the first day. By the seventh day of culture, while the size of spheroids in HaCaT and NIH/3T3 cells remained largely unchanged, those for CAF cells exhibited further enlargement.
Fig. 2.
Morphology of various cell spheroids with or without DFP Phase contrast microscopy revealed that HaCaT cells, NIH/3T3 cells and CAF cells initiate cell spheroid formation from Day 1. Notably, spheroids comprised of CAF cells exhibited a progressive increase in size over time. In the absence of DFP, the spheroids observed on Day 1 were small for HaCaT, NIH/3T3 and CAF cells. In the absence of DFP, the large spheroids observed with DFP were not present on Day 7. Arrows indicate small spheroids.
For comparison, suspensions of HaCaT, NIH/3T3, and CAF cells were cultured using a similar procedure but without DFP. On the first day of culture, all three types of cells formed spheroids composed of several adhering cells, which were limited in both number and size. No noticeable increase in the number of spheroids was observed for any of the cell types even after the culture period. Notably, spheroids by HaCaT cells and CAF cells without DFP were markedly smaller compared to those cultured with DFP. In the absence of DFP, the size of the spheroids formed by NIH/3T3 cells remained relatively unchanged from day 3 to day 7.
3.3. Histological analysis of cell spheroids using DFP
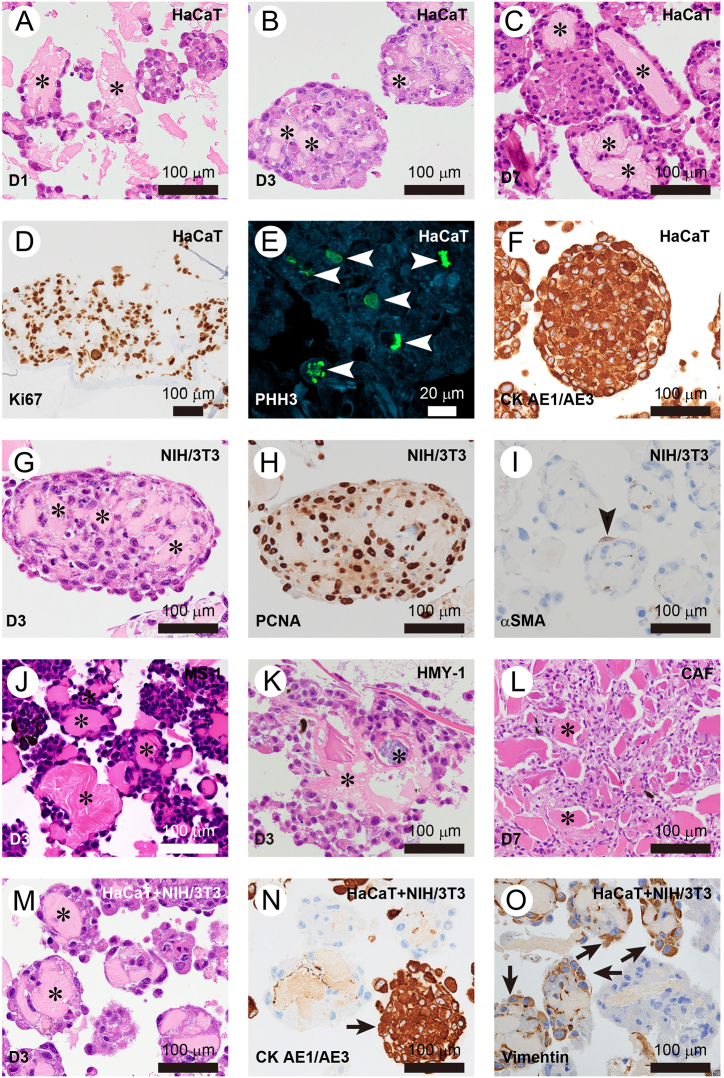
Histological analysis was conducted on cell spheroid types generated using DFP. On the initial day of culture, HaCaT cells effectively adhered to the DFP surface, maintaining viability (Fig. 3A). Regions of DFP not covered by cells were also noted. By day three of culture, a significant portion of the DFP surface within the spheroids was encompassed by cells (Fig. 3B). Even on the 7th day of culture, DFP remained present, with HaCaT cells adhering to and proliferating around it (Fig. 3C). The Ki67 labeling index, a marker for cell proliferation in HaCaT cells, was observed to be 86.3 ± 3.4 % on the third day (Fig. 3D). Phospho-Histone H3 is recognized as a marker of cell proliferation, and positive cells were detected in HaCaT cell spheroids on the third day of culture (Fig. 3E). Furthermore, immunostaining revealed that HaCaT cells, derived from epidermal tissue, tested positive for pan-cytokeratin (CK AE1/AE3) (Fig. 3F). On the third day of culture, NIH/3T3 cells had proliferated, covering the surface of the DFP (Fig. 3G). Immunostaining using PCNA, a marker for cell proliferation, revealed a labeling index of 93.0 ± 2.1 % (Fig. 3H). Furthermore, immunostaining with αSMA indicated that only a small fraction of cells differentiated into myofibroblasts, with the majority of NIH/3T3 cells testing negative for αSMA (Fig. 3I). MS-1 cells (Fig. 3J), an endothelial cell line, and HMY-1 cells (Fig. 3K), a malignant melanoma cell line, both adhered to DFP and survived on the third day of culture. On the seventh day of culture, CAF cells demonstrated proliferation on the DFP surface, and a unique structure characterized by the connection of numerous DFPs was observed (Fig. 3L). Cell spheroids also developed in co-cultures of HaCaT cells and NIH/3T3 cells utilizing DFP (Fig. 3M). Intriguingly, most spheroids were predominantly composed of a single cell type, yet a small proportion exhibit a mosaic composition of different cell types (Fig. 3N and O).
Fig. 3.
Histological analysis of various spheroids using DFP (A) Organization of cell spheroids by HaCaT cells on Day 1. Asterisks indicate DFP. (B) Organization of cell spheroids by HaCaT cells on Day 3. Asterisks indicate DFP. (C) Organization of cell spheroids by HaCaT cells on Day 7. Asterisks indicate DFP. (D) Image showing HaCaT cell spheroids stained for the cell proliferation marker Ki67. (E) Image showing HaCaT cell spheroids stained for the cell proliferation marker phospho-Histone H3 (PHH3). Arrows indicate PHH3-positive cells. (F) Image showing HaCaT cell spheroids stained for the cytokeratin marker CK AE1/AE3. (G) Organization of cell spheroids by NIH/3T3 cells on Day 3. Asterisks indicate DFP. (H) Image showing NIH/3T3 cell spheroids stained for the cell proliferation marker PCNA. (I) Image showing NIH/3T3 cell spheroids stained for the myofibroblast marker αSMA. The arrowhead indicates a myofibroblast. (J) Organization of cell spheroids by MS-1 cells on Day 1. Asterisks indicate DFP. (K) Organization of cell spheroids by HMY-1 cells on Day 3. Asterisks indicate DFP. (L) Organization of cell spheroids by CAF cells on Day 7. Asterisk indicates DFP. (M) Organization of cell spheroids by HaCaT cells and NIH/3T3 cells on Day 3. Asterisk indicates DFP. (N) Image showing spheroids of HaCaT cells and NIH/3T3 cells stained for the cytokeratin marker CK AE1/AE3. Arrows indicate CK AE1/AE3 positive HaCaT cells. (O) Image showing spheroids of HaCaT cells and NIH/3T3 cells stained for the intermediate filament marker vimentin. Arrows indicate vimentin positive NIH3T3 cells.
3.4. Protein expression of DFP-used cell spheroids
Protein expression in cells cultured using DFP was compared to the expression in cells cultured in standard plastic dishes (PD). In this study, the proteins assessed were ERK and p38, key components of the mitogen-activated protein kinase (MAPK) signaling pathway, which play crucial roles in regulating cell proliferation and differentiation [12].
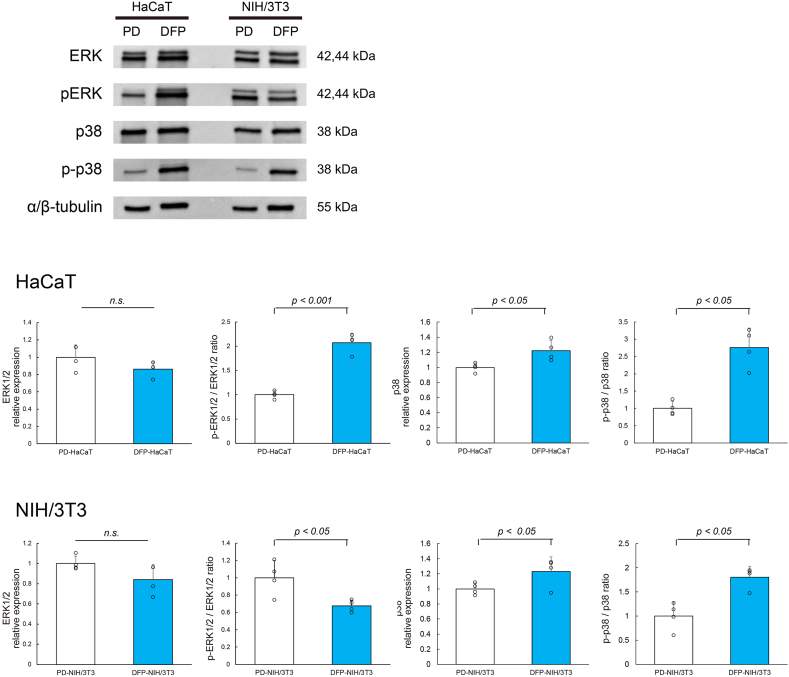
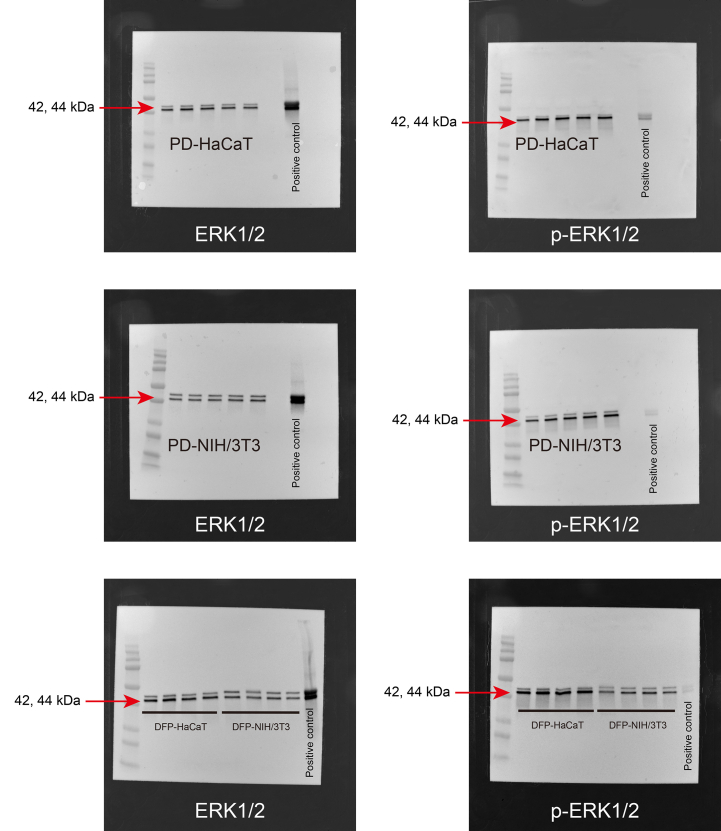
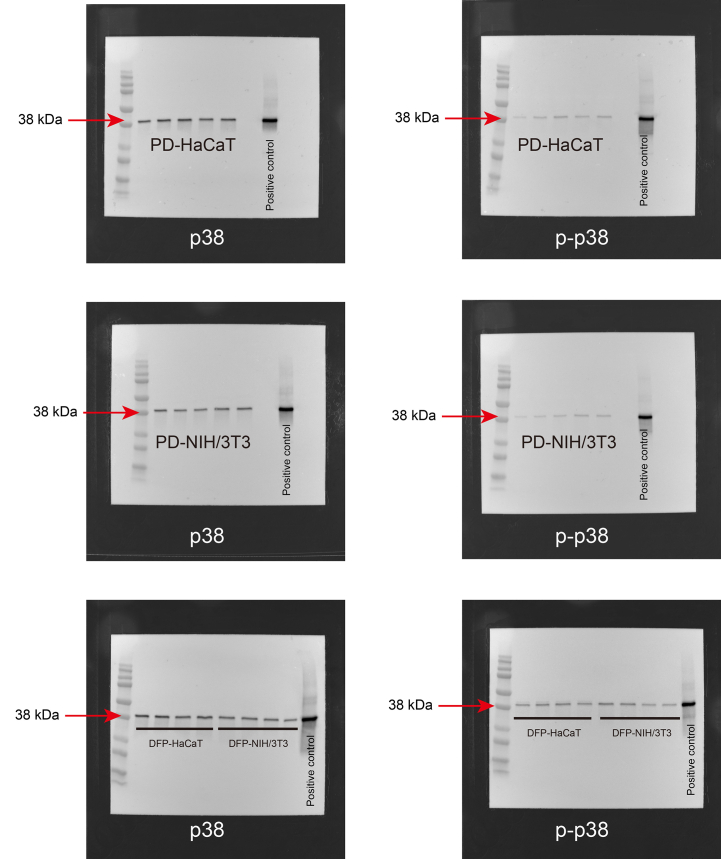
As shown in Fig. 4, no significant difference was observed in the expression levels of total ERK1/2 between HaCaT cells cultured with DFP (DFP-HaCaT group) and those cultured in standard plastic dishes (PD-HaCaT group). However, the phosphorylation rate of ERK1/2 was significantly higher in the DFP-HaCaT group compared to the PD-HaCaT group. The expression rate of total p38 was significantly higher in the DFP-HaCaT group compared to the PD-HaCaT group, and its phosphorylation rate was significantly higher in the DFP-HaCaT group. For NIH/3T3 cells, no significant difference was observed in the total ERK1/2 expression levels between those cultured in plastic dishes (PD-NIH/3T3 group) and those cultured using DFP (DFP-NIH/3T3 group). However, the phosphorylation rate of ERK1/2 was significantly lower in the DFP-NIH/3T3 group compared to the PD-NIH/3T3 group. There was no significant difference in the expression levels of total p38 between the DFP-NIH/3T3 and PD-NIH/3T3 groups, and the p38 phosphorylation rate was significantly higher in the DFP-NIH/3T3 group.
Fig. 4.
Western blotting analysis of cell spheroids MAPK family protein, ERK1/2, p-ERK1/2, p38 and p-p38 expression levels in spheroid by HaCaT cell (n = 4) or NIH/3T3 (n = 4) cells were evaluated by western blotting. Relative expression is depicted as the ratio of target protein expression to α/β-tubulin expression. PD, plastic dish; DFP, dried fish powder.
4. Discussion
Cell spheroid culture, compared to 2D culture, yields a higher number of cells per unit volume and better mimics physiological cell functions [13,14]. For adherent cells in spheroid culture, a carrier material is essential for survival and proliferation [15]. Typically collagen or modified plastic bases coated with an extracellular matrix or electrically charged are used as carriers [16]. While essential in medical and biological research for consistent quality, these materials face challenges in the food industry due to cost-effectiveness and edibility issues. Particularly, fish scale-derived collagen, despite being cheaper than mammalian collagen, is costly due to its required acid extraction process. Additionally, its low denaturation temperature makes it unsuitable for mammalian cell cultures in the food industry, as it lacks the necessary stability for effective cell culture [6,17]. This aspect highlights the distinct material requirements and challenges between research and food production applications.
In the present study, our focus was on the unique properties of dried fish. It was observed that when boiled, dried fish retained its structure, resisting complete melting. Through the drying process, the water content in fish was significantly reduced, resulting in an increase in tissue density and hardness [18,19]. This alteration in texture makes it feasible to grind the dried fish into a fine powder. We developed the concept of utilizing this fish powder as a cell carrier, recognizing its potential application in various biological and agricultural contexts. Medaka was chosen as the species for fish powder production due to its small size and ease of cultivation. Notably, Medaka has a history of use in space research, illustrating its adaptability and resilience in varied environments [20,21]. This background positions it as a versatile candidate for future applications in space exploration, aligned with the advancing trajectory of human space endeavors. In addition to Medaka, the utilization of fish powder derived from other small fish species, such as Zebrafish, also shows promise and warrants further investigation. This area of study will reveal additional effective applications of fish powder in various fields, marking it as a topic of significant interest for future research.
DFP derived from Medaka, employed as a spheroid culture carrier, exhibited high cell adhesion and proliferation for various cell types, including human epidermal cells, human malignant melanoma, mouse fibroblasts, mouse endothelium and fish fibroblasts. Comparing the presence and absence of DFP revealed that the spheroid size of HaCaT, NIH/3T3, and CAF cells tended to be larger on the first day of culture with the use of DFP. The use of DFP is considered particularly effective for obtaining large spheroids from the early stages of culture. Its effectiveness rivals or surpasses conventional plastic dishes. Moreover, DFP streamlines protein expression analysis in cell spheroids and enhances cell culture quality assessment. The production process of the Medaka-derived DFP, involving straightforward forming, drying, crushing and ethanol impregnation, is significantly more cost-effective than traditional collagen production methods. This cost-efficiency, coupled with its robust performance, makes it a promising tool for advancing both terrestrial and extraterrestrial industrial food production technologies in the future.
Biocompatibility and cytotoxicity are crucial considerations for cell culture carriers. Allergic reactions to fish occur in the same manner as allergic reactions to mammalian products [22]. DFP, derived from fish, contains numerous allergy-inducing substances, including histamine and various proteins [23]. If used in a living organism, there is a high risk of triggering a severe allergic reaction. Additionally, DFP may currently contain trace amounts of endotoxin, which is considered toxic to living organisms. The effectiveness of fish-derived collagen as a material for regenerative medicine has been confirmed by several studies [24,25]. Collagen is recognized as a biological material with low antigenicity, but its telopeptides are also antigenic [26]. Therefore, it is not possible to use DFP directly in the living organism. If DFP were to be used in vivo, various extraction and processing steps would be required. On the other hand, when using DFP for cultured cells, immune reactions are not a concern, and compatibility between the material and the cells is paramount. In this study, both epithelial and mesenchymal cells adhered to DFP and subsequently proliferated. The fact that the majority of cells in each cell line survived in the presence of DFP suggests that DFP has a low degree of cytotoxicity. Furthermore, the results of MAPK signal analysis suggested that there were no significant adverse effects on cell functions. However, because the number of cell lines used in this study was limited, it is difficult to conclude from these results alone that DFP does not have cytotoxicity. In the future, it will be necessary to investigate the cytotoxicity of DFP using various cell types.
We fully acknowledge the limitations of this study. Medaka was the only fish species used, and it is necessary to verify whether similar effects are observed in other small fish species to confirm the advantages of this microcarrier for cell culture. Additionally, the cell lines used in this study were limited in terms of both animal origin and cell type. To accurately assess the biological performance of DFP, it is essential to analyze cell dynamics using a broader range of cell types. Since the DFP we produced was fabricated in our laboratory, we were unable to ensure uniform quality. Consequently, its performance may not meet the standards required for industrial applications, making this a critical issue to address in the future.
In the present study, it was established that dried fish powder derived from Medaka fish is an effective culture carrier for spheroid cultures of both mammalian and fish cells. Looking ahead, the application of fish powder in cell spheroid culture holds significant promise for revolutionizing cell culture methodologies, offering a cost-effective alternative. This advancement is expected to make substantial contributions to the field of industrial food production.
Funding
This work was supported by research grants from the TSUNAGI project of Saga prefecture, Japan (to SA).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Takehisa Sakumoto: Writing – original draft, Investigation, Formal analysis. Takayuki Narita: Writing – original draft, Methodology, Investigation. Sayuri Morito: Investigation. Megumi Nishiyama: Writing – original draft, Investigation. Mariko Hashiguchi: Writing – original draft, Investigation. Yumeka Mine: Investigation. Shuhei Iwamoto: Investigation. Shuji Toda: Writing – review & editing, Supervision. Shigehisa Aoki: Writing – review & editing, Writing – original draft, Validation, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. A patent application related to the content of this research has been filed in Japan.
Acknowledgements
We thank M. Nishida and S. Nishimura for excellent technical assistance. This research was also supported by the Analytical Research Center for Experimental Sciences, Saga University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e38418.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
References
- 1.Fennema E., Rivron N., Rouwkema J., van Blitterswijk C., De Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013;31:108–115. doi: 10.1016/j.tibtech.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Froehlich K., Haeger J.-D., Heger J., Pastuschek J., Photini S.M., Yan Y., Lupp A., Pfarrer C., Mrowka R., Schleußner E. Generation of multicellular breast cancer tumor spheroids: comparison of different protocols. J. Mammary Gland Biol. Neoplasia. 2016;21:89–98. doi: 10.1007/s10911-016-9359-2. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson K. Microcarrier cell culture. Biotechnol. Genet. Eng. Rev. 1988;6:404–439. [PubMed] [Google Scholar]
- 4.Overstreet M., Sohrabi A., Polotsky A., Hungerford D.S., Frondoza C.G. Collagen microcarrier spinner culture promotes osteoblast proliferation and synthesis of matrix proteins. In Vitro Cell. Dev. Biol. Anim. 2003;39:228–234. doi: 10.1290/1543-706X(2003)039<0228:CMSCPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Frondoza C., Sohrabi A., Hungerford D. Human chondrocytes proliferate and produce matrix components in microcarrier suspension culture. Biomaterials. 1996;17:879–888. doi: 10.1016/0142-9612(96)83283-2. [DOI] [PubMed] [Google Scholar]
- 6.Pati F., Adhikari B., Dhara S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol. 2010;101:3737–3742. doi: 10.1016/j.biortech.2009.12.133. [DOI] [PubMed] [Google Scholar]
- 7.Gauza-Włodarczyk M., Kubisz L., Mielcarek S., Włodarczyk D. Comparison of thermal properties of fish collagen and bovine collagen in the temperature range 298–670 K. Mater. Sci. Eng. C. 2017;80:468–471. doi: 10.1016/j.msec.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Kawarazuka N., Béné C. The potential role of small fish species in improving micronutrient deficiencies in developing countries: building evidence. Publ. Health Nutr. 2011;14:1927–1938. doi: 10.1017/S1368980011000814. [DOI] [PubMed] [Google Scholar]
- 9.Shima A., Mitani H. Medaka as a research organism: past, present and future. Mech. Dev. 2004;121:599–604. doi: 10.1016/j.mod.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Kinoshita M., Murata K., Naruse K., Tanaka M. John Wiley & Sons; 2009. Medaka: Biology, Management, and Experimental Protocols. [Google Scholar]
- 11.Nathan N.A., Narayan E., Smith M.M., Horn M.J. Cell block cytology: improved preparation and its efficacy in diagnostic cytology. Am. J. Clin. Pathol. 2000;114:599–606. doi: 10.1309/G035-P2MM-D1TM-T5QE. [DOI] [PubMed] [Google Scholar]
- 12.Seger R., Krebs E.G. The MAPK signaling cascade. Faseb. J. 1995;9:726–735. [PubMed] [Google Scholar]
- 13.Nyberg S.L., Hardin J., Amiot B., Argikar U.A., Remmel R.P., Rinaldo P. Rapid, large‐scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transplant. 2005;11:901–910. doi: 10.1002/lt.20446. [DOI] [PubMed] [Google Scholar]
- 14.Santo V.E., Estrada M.F., Rebelo S.P., Abreu S., Silva I., Pinto C., Veloso S.C., Serra A.T., Boghaert E., Alves P.M. Adaptable stirred-tank culture strategies for large scale production of multicellular spheroid-based tumor cell models. J. Biotechnol. 2016;221:118–129. doi: 10.1016/j.jbiotec.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Shao C., Chi J., Zhang H., Fan Q., Zhao Y., Ye F. Development of cell spheroids by advanced technologies. Adv. Mater. Technol. 2020;5 [Google Scholar]
- 16.Pinto B., Henriques A.C., Silva P.M., Bousbaa H. Three-dimensional spheroids as in vitro preclinical models for cancer research. Pharmaceutics. 2020;12:1186. doi: 10.3390/pharmaceutics12121186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yunoki S., Suzuki T., Takai M. Stabilization of low denaturation temperature collagen from fish by physical cross-linking methods. J. Biosci. Bioeng. 2003;96:575–577. doi: 10.1016/S1389-1723(04)70152-8. [DOI] [PubMed] [Google Scholar]
- 18.Azizah N., Asfiyanti N.A., Hasibuan M., Jannah M., Gustari R., Hasibuan R.S. The effect of fish thickness on dryness level and time for drying fish. Int. J. Nat. Sci. Eng. 2021;5:114–119. [Google Scholar]
- 19.Fitri N., Chan S.X.Y., Che Lah N.H., Jam F.A., Misnan N.M., Kamal N., Sarian M.N., Mohd Lazaldin M.A., Low C.F., Hamezah H.S. A comprehensive review on the processing of dried fish and the associated chemical and nutritional changes. Foods. 2022;11:2938. doi: 10.3390/foods11192938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ijiri K. Life-cycle experiments of medaka fish aboard the international space station. Adv. Space Biol. Med. 2003;9:201–216. doi: 10.1016/s1569-2574(03)09008-7. [DOI] [PubMed] [Google Scholar]
- 21.Murata Y., Yasuda T., Watanabe-Asaka T., Oda S., Mantoku A., Takeyama K., Chatani M., Kudo A., Uchida S., Suzuki H. Histological and transcriptomic analysis of adult Japanese Medaka sampled onboard the international space station. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor S., Kabourek J., Hefle S. Fish allergy: fish and products thereof. J. Food Sci. 2004;69:R175–R180. [Google Scholar]
- 23.Sharp M.F., Lopata A.L. Fish allergy: in review. Clin. Rev. Allergy Immunol. 2014;46:258–271. doi: 10.1007/s12016-013-8363-1. [DOI] [PubMed] [Google Scholar]
- 24.Yamada S., Yamamoto K., Ikeda T., Yanagiguchi K., Hayashi Y. Potency of fish collagen as a scaffold for regenerative medicine. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/302932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S., Lau C.-S., Liang K., Wen F., Teoh S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2022;74:92–103. doi: 10.1016/j.copbio.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Lynn A., Yannas I., Bonfield W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2004;71:343–354. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.