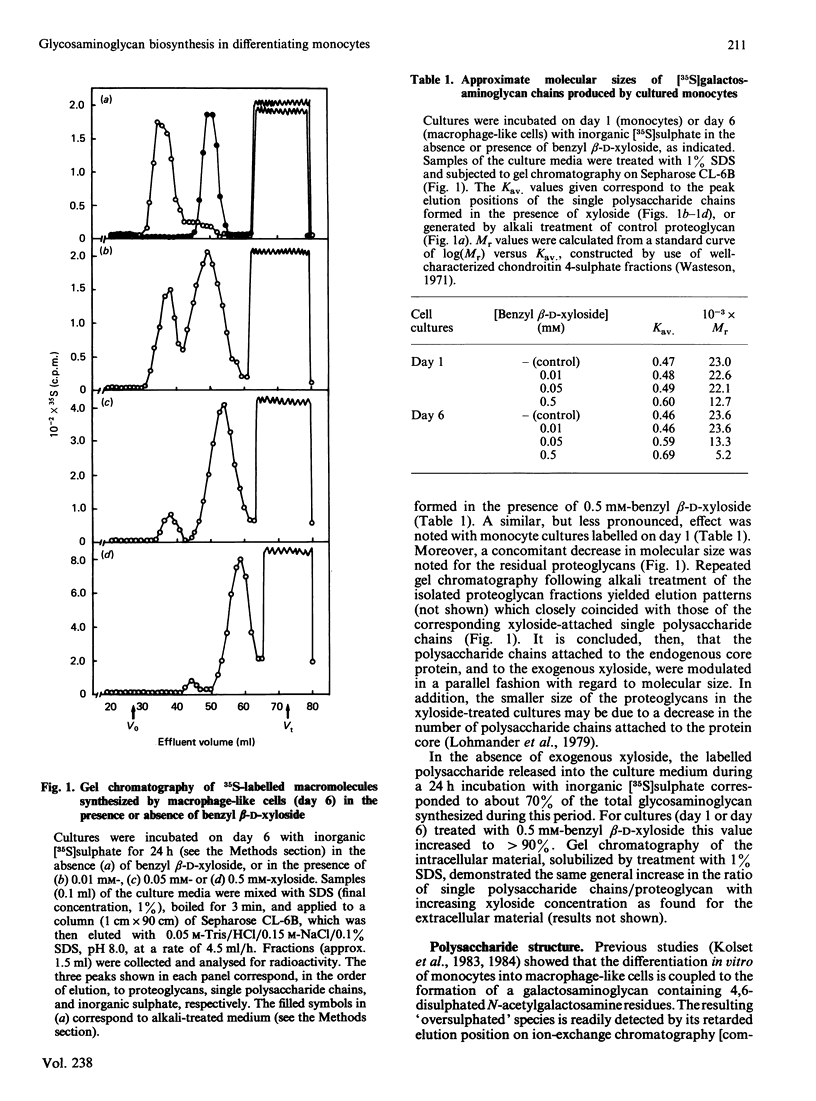
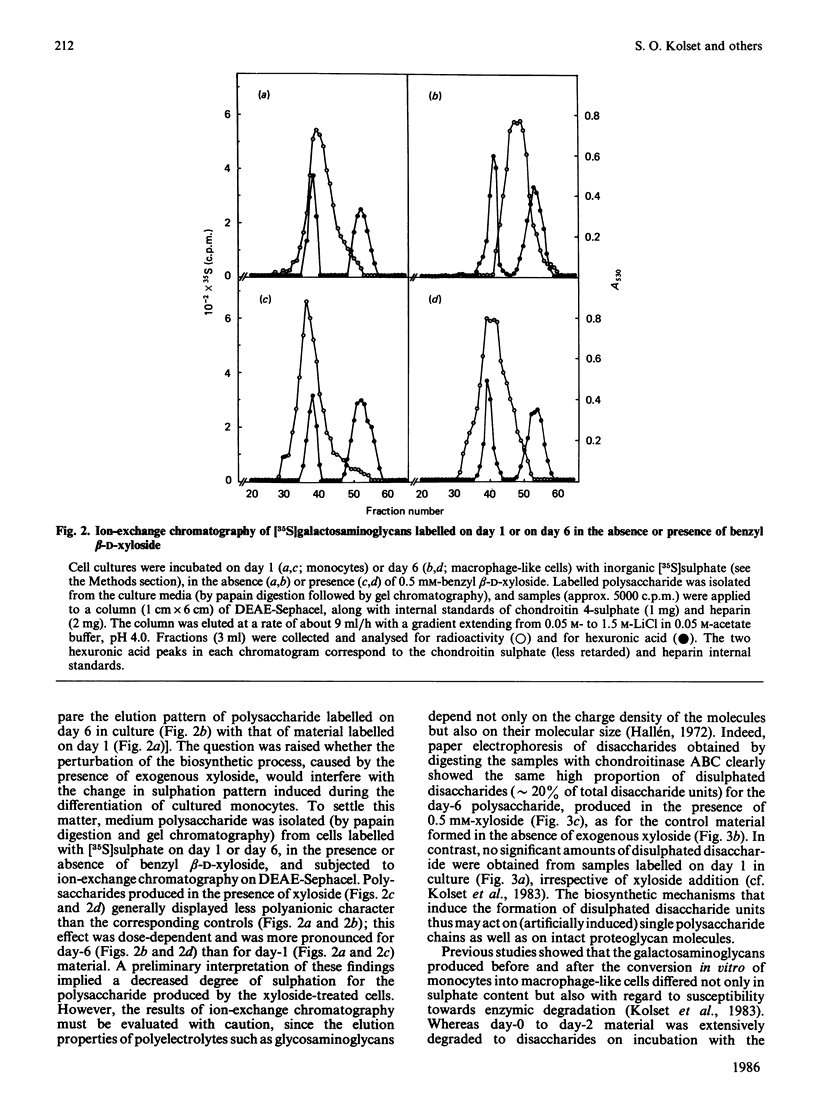
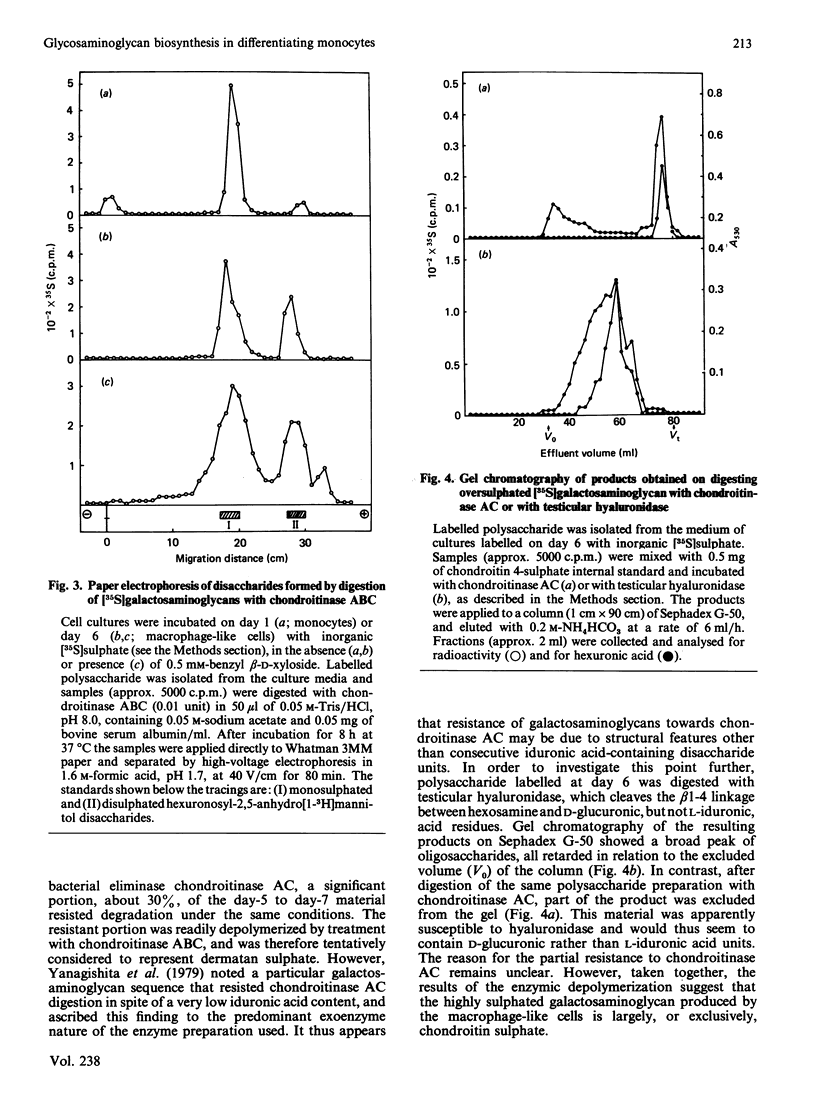
Abstract
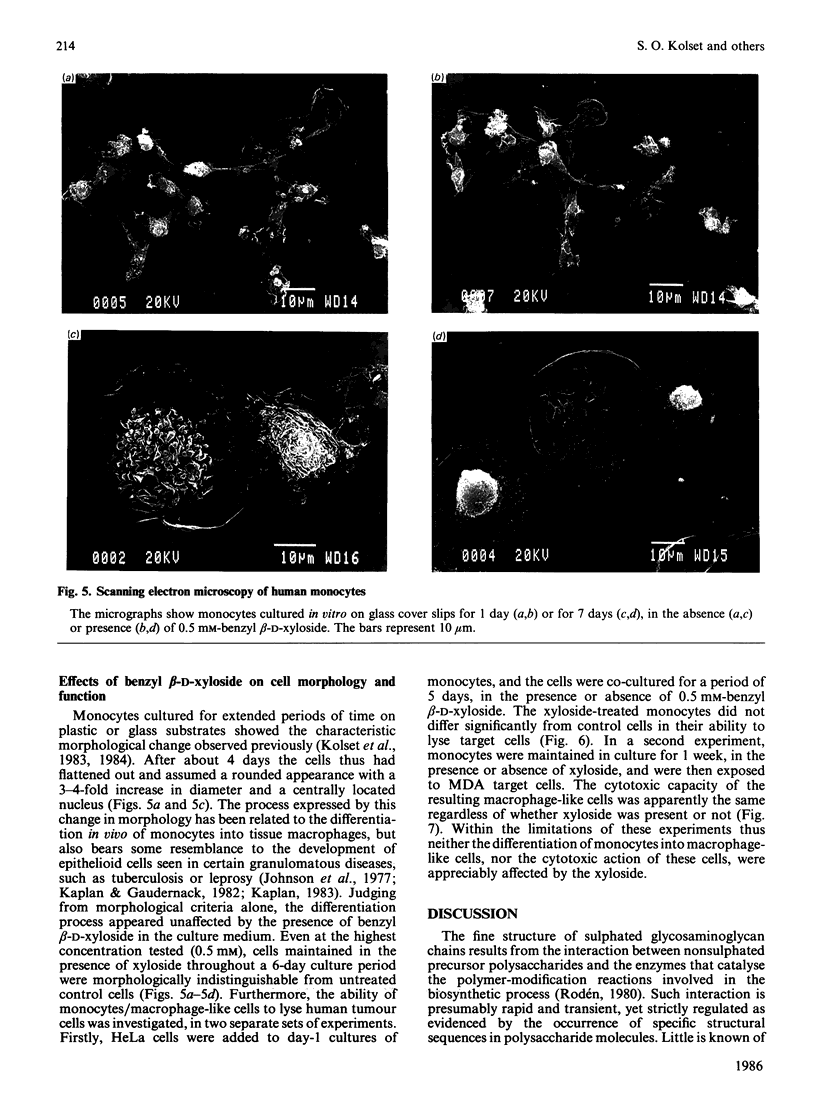
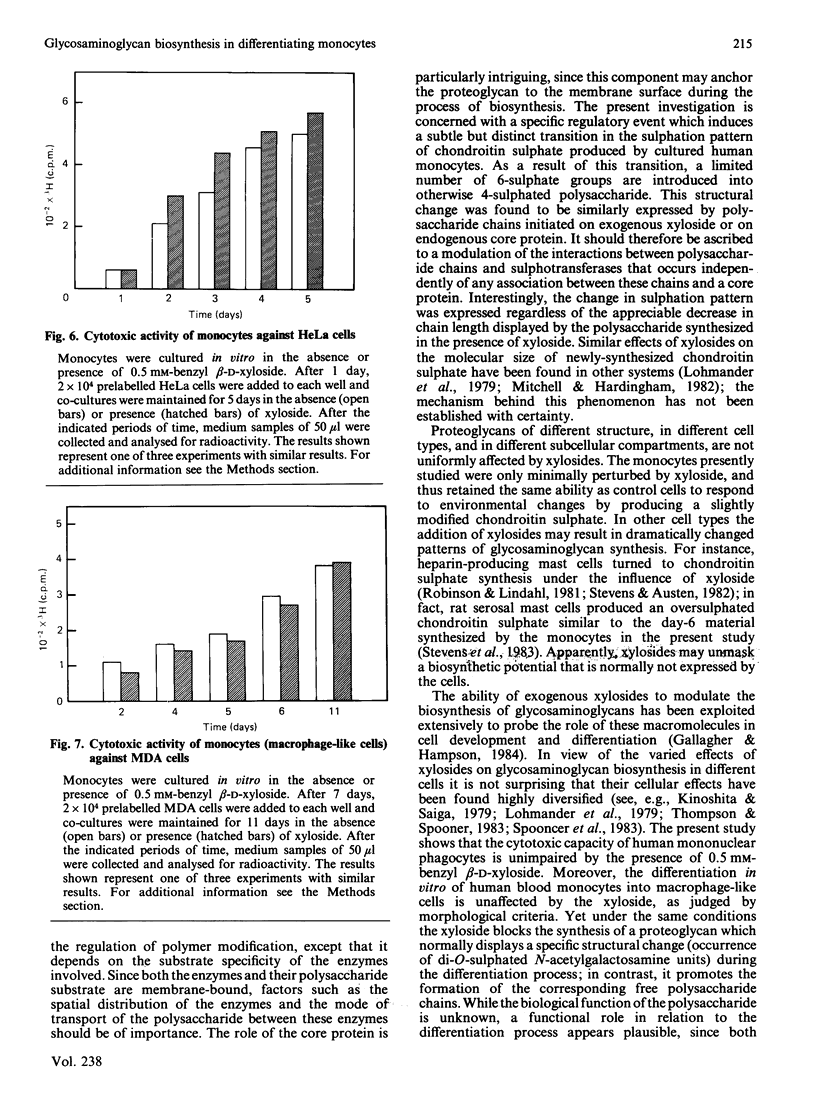
Monocytes isolated from human blood differentiate into macrophage-like cells when maintained in vitro for 3-5 days on plastic or glass culture dishes. In the process the cells display characteristic morphological changes, and in addition, a transition in glycosaminoglycan biosynthesis, from the production of chondroitin 4-sulphate to the formation of a polysaccharide containing 20% 4,6-disulphated disaccharide units [Kolset, Kjellén, Seljelid & Lindahl (1983) Biochem. J. 210, 661-667]. Cells were incubated with inorganic [35S]sulphate on day 1 or day 6 in culture, in the presence or absence of benzyl beta-D-xyloside, and labelled polysaccharide was isolated from the culture medium. In the presence of xyloside, the secretion of proteoglycans (90% galactosaminoglycan) was inhibited in a dose-dependent fashion and replaced by release of single polysaccharide chains, the size of which decreased with increasing dose of xyloside. The single polysaccharide chains produced on day 6 in the presence of 0.5 mM-xyloside showed the same proportion of disulphated disaccharide units as did the corresponding control material. Day-1 polysaccharide contained negligible amounts of this component, irrespective of the presence or absence of xyloside. It is concluded that the regulatory mechanism that induces 'oversulphation' during the differentiation process operates independently of any association between the polysaccharide chains and the core protein. Moreover, cells maintained in the presence of 0.5 mM-xyloside throughout a 6-day culture period showed the same morphological change, indicative of differentiation into macrophage-like cells, as did untreated control cells. The xyloside did not significantly affect the cytotoxicity of the monocytes, or of the differentiated macrophage-like cells, toward tumour cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Hampson I. N. Proteoglycans in cellular differentiation and neoplasia. Biochem Soc Trans. 1984 Jun;12(3):541–543. doi: 10.1042/bst0120541. [DOI] [PubMed] [Google Scholar]
- Hallén A. Chromatography of acidic glycosaminoglycans on DEAE-cellulose. J Chromatogr. 1972 Aug 23;71(1):83–91. doi: 10.1016/s0021-9673(01)85691-0. [DOI] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Gaudernack G. In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med. 1982 Oct 1;156(4):1101–1114. doi: 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G. In vitro differentiation of human monocytes. Monocytes cultured on glass are cytotoxic to tumor cells but monocytes cultured on collagen are not. J Exp Med. 1983 Jun 1;157(6):2061–2072. doi: 10.1084/jem.157.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S., Saiga H. The role of proteoglycan in the development of sea urchins. I. Abnormal development of sea urchin embryos caused by the disturbance of proteoglycan synthesis. Exp Cell Res. 1979 Oct 15;123(2):229–236. doi: 10.1016/0014-4827(79)90463-4. [DOI] [PubMed] [Google Scholar]
- Kolset S. O., Kjellén L., Seljelid R., Lindahl U. Changes in glycosaminoglycan biosynthesis during differentiation in vitro of human monocytes. Biochem J. 1983 Mar 15;210(3):661–667. doi: 10.1042/bj2100661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset S. O., Seljelid R., Lindahl U. Modulation of the morphology and glycosaminoglycan biosynthesis of human monocytes, induced by culture substrates. Biochem J. 1984 May 1;219(3):793–799. doi: 10.1042/bj2190793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Thunberg L. The antithrombin-binding sequence in heparin. Identification of an essential 6-O-sulfate group. J Biol Chem. 1983 Aug 25;258(16):9826–9830. [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Hascall V. C., Caplan A. I. Effects of 4-methyl umbelliferyl-beta-D-xylopyranoside on chondrogenesis and proteoglycan synthesis in chick limb bud mesenchymal cell cultures. J Biol Chem. 1979 Oct 25;254(20):10551–10561. [PubMed] [Google Scholar]
- Mitchell D., Hardingham T. The control of chondroitin sulphate biosynthesis and its influence on the structure of cartilage proteoglycans. Biochem J. 1982 Feb 15;202(2):387–395. doi: 10.1042/bj2020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama M., Kimata K., Suzuki S. The influence of p-nitrophenyl beta-d-xyloside on the synthesis of proteochondroitin sulfate by slices of embryonic chick cartilage. J Biochem. 1973 Nov;74(5):1069–1073. [PubMed] [Google Scholar]
- Robinson H. C., Brett M. J., Tralaggan P. J., Lowther D. A., Okayama M. The effect of D-xylose, beta-D-xylosides and beta-D-galactosides on chondroitin sulphate biosynthesis in embryonic chicken cartilage. Biochem J. 1975 Apr;148(1):25–34. doi: 10.1042/bj1480025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. C., Lindahl U. Effect of cycloheximide, beta-D-xylosides and beta-D-galactosides on heparin biosynthesis in mouse mastocytoma. Biochem J. 1981 Feb 15;194(2):575–586. doi: 10.1042/bj1940575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N. B., Galligani L., Ho P. L., Dorfman A. Stimulation of synthesis of free chondroitin sulfate chains by beta-D-xylosides in cultured cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4047–4051. doi: 10.1073/pnas.71.10.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooncer E., Gallagher J. T., Krizsa F., Dexter T. M. Regulation of haemopoiesis in long-term bone marrow cultures. IV. Glycosaminoglycan synthesis and the stimulation of haemopoiesis by beta-D-xylosides. J Cell Biol. 1983 Feb;96(2):510–514. doi: 10.1083/jcb.96.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. L., Austen K. F. Effect of p-nitrophenyl-beta-D-xyloside on proteoglycan and glycosaminoglycan biosynthesis in rat serosal mast cell cultures. J Biol Chem. 1982 Jan 10;257(1):253–259. [PubMed] [Google Scholar]
- Stevens R. L., Razin E., Austen K. F., Hein A., Caulfield J. P., Seno N., Schmid K., Akiyama F. Synthesis of chondroitin sulfate E glycosaminoglycan onto p-nitrophenyl-beta-D-xyloside and its localization to the secretory granules of rat serosal mast cells and mouse bone marrow-derived mast cells. J Biol Chem. 1983 May 10;258(9):5977–5984. [PubMed] [Google Scholar]
- Thompson H. A., Spooner B. S. Proteoglycan and glycosaminoglycan synthesis in embryonic mouse salivary glands: effects of beta-D-xyloside, an inhibitor of branching morphogenesis. J Cell Biol. 1983 May;96(5):1443–1450. doi: 10.1083/jcb.96.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Yanagishita M., Rodbard D., Hascall V. C. Isolation and characterization of proteoglycans from porcine ovarian follicular fluid. J Biol Chem. 1979 Feb 10;254(3):911–920. [PubMed] [Google Scholar]