Abstract
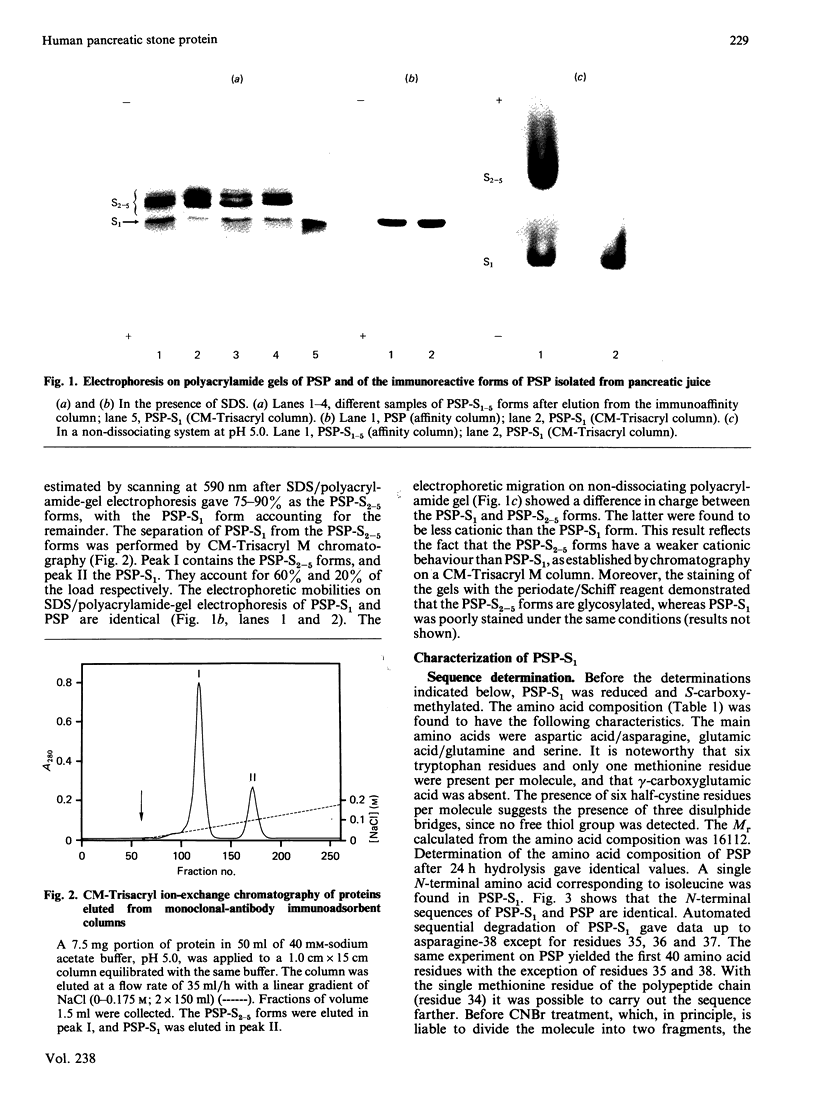
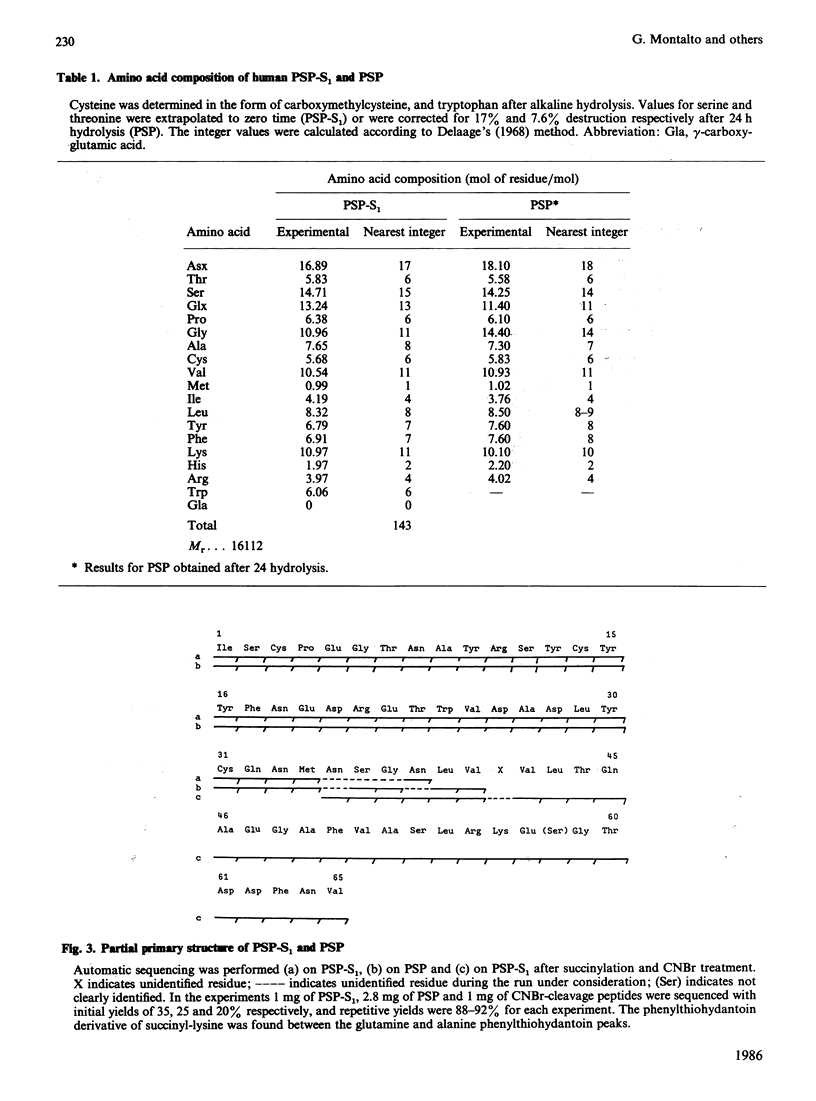
Pancreatic stone protein (PSP) is the major organic component of human pancreatic stones. With the use of monoclonal antibody immunoadsorbents, five immunoreactive forms (PSP-S) with close Mr values (14,000-19,000) were isolated from normal pancreatic juice. By CM-Trisacryl M chromatography the lowest-Mr form (PSP-S1) was separated from the others and some of its molecular characteristics were investigated. The Mr of the PSP-S1 polypeptide chain calculated from the amino acid composition was about 16,100. The N-terminal sequences (40 residues) of PSP and PSP-S1 are identical, which suggests that the peptide backbone is the same for both of these polypeptides. The PSP-S1 sequence was determined up to residue 65 and was found to be different from all other known protein sequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonicel J., Couchoud P., Foglizzo E., Desnuelle P., Chapus C. Amino acid sequence of horse colipase B. Biochim Biophys Acta. 1981 Jun 29;669(1):39–45. doi: 10.1016/0005-2795(81)90221-x. [DOI] [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Charles M., Erlanson C., Bianchetta J., Joffre J., Guidoni A., Rovery M. The primary structure of porcine colipase II. I. The amino acid sequence. Biochim Biophys Acta. 1974 Jul 7;359(1):186–197. doi: 10.1016/0005-2795(74)90142-1. [DOI] [PubMed] [Google Scholar]
- De Caro A., Bonicel J., Pieroni G., Guy O. Comparative studies of human and porcine pancreatic lipases : N-terminal sequences, sulfhydryl groups and interfacial activity. Biochimie. 1981 Oct;63(10):799–801. doi: 10.1016/s0300-9084(81)80041-7. [DOI] [PubMed] [Google Scholar]
- De Caro A., Figarella C., Guy O. The two human chymotrypsinogens. Purification and characterization. Biochim Biophys Acta. 1975 Feb 27;379(2):431–443. doi: 10.1016/0005-2795(75)90150-6. [DOI] [PubMed] [Google Scholar]
- De Caro A., Lohse J., Sarles H. Characterization of a protein isolated from pancreatic calculi of men suffering from chronic calcifying pancreatitis. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1176–1182. doi: 10.1016/s0006-291x(79)80031-5. [DOI] [PubMed] [Google Scholar]
- De Caro A., Multigner L., Lafont H., Lombardo D., Sarles H. The molecular characteristics of a human pancreatic acidic phosphoprotein that inhibits calcium carbonate crystal growth. Biochem J. 1984 Sep 15;222(3):669–677. doi: 10.1042/bj2220669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caro A., Multigner L., Vérine H. Identification of two major proteins of bovine pancreatic stones as immunoreactive forms of trypsinogens. Biochem J. 1982 Sep 1;205(3):543–549. doi: 10.1042/bj2050543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaage M. Sur la recherche du poids moléculaire le plus cohérent avec l'analyse des acides aminés d'une protéine. Biochim Biophys Acta. 1968 Dec 3;168(3):573–575. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Figarella C., Amouric M., Guy-Crotte O. Proteolysis of human trypsinogen 1. Pathogenic implication in chronic pancreatitis. Biochem Biophys Res Commun. 1984 Jan 13;118(1):154–161. doi: 10.1016/0006-291x(84)91080-5. [DOI] [PubMed] [Google Scholar]
- Giorgi D., Bernard J. P., De Caro A., Multigner L., Lapointe R., Sarles H., Dagorn J. C. Pancreatic stone protein. I. Evidence that it is encoded by a pancreatic messenger ribonucleic acid. Gastroenterology. 1985 Aug;89(2):381–386. doi: 10.1016/0016-5085(85)90340-3. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Gross J., Brauer A. W., Bringhurst R. F., Corbett C., Margolies M. N. An unusual bovine pancreatic protein exhibiting pH-dependent globule-fibril transformation and unique amino acid sequence. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5627–5631. doi: 10.1073/pnas.82.17.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Crotte O., Amouric M., Figarella C. Characterization and N-terminal sequence of a degradation product of 14,000 molecular weight isolated from human pancreatic juice. Biochem Biophys Res Commun. 1984 Dec 14;125(2):516–523. doi: 10.1016/0006-291x(84)90570-9. [DOI] [PubMed] [Google Scholar]
- Guy O., Robles-Diaz G., Adrich Z., Sahel J., Sarles H. Protein content of precipitates present in pancreatic juice of alcoholic subjects and patients with chronic calcifying pancreatitis. Gastroenterology. 1983 Jan;84(1):102–107. [PubMed] [Google Scholar]
- Hauschka P. V. Quantitative determination of gamma-carboxyglutamic acid in proteins. Anal Biochem. 1977 May 15;80(1):212–223. doi: 10.1016/0003-2697(77)90640-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Montalto G., Lusher M., De Caro A., Multigner L., Sarles H., Delaage M. Analyse au moyen d'un anticorps monoclonal des composants du suc pancréatique humain apparentés à la protéine des calculs pancréatiques. C R Acad Sci III. 1985;300(6):199–202. [PubMed] [Google Scholar]
- Multigner L., De Caro A., Lombardo D., Campese D., Sarles H. Pancreatic stone protein, a phosphoprotein which inhibits calcium carbonate precipitation from human pancreatic juice. Biochem Biophys Res Commun. 1983 Jan 14;110(1):69–74. doi: 10.1016/0006-291x(83)91261-5. [DOI] [PubMed] [Google Scholar]
- Multigner L., Sarles H., Lombardo D., De Caro A. Pancreatic stone protein. II. Implication in stone formation during the course of chronic calcifying pancreatitis. Gastroenterology. 1985 Aug;89(2):387–391. doi: 10.1016/0016-5085(85)90341-5. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Scheele G. A. Two-dimensional gel analysis of soluble proteins. Charaterization of guinea pig exocrine pancreatic proteins. J Biol Chem. 1975 Jul 25;250(14):5375–5385. [PubMed] [Google Scholar]
- Turner J. D., Rouser G. Precise quantitative determination of human blood lipids by thin-layer and triethylaminoethylcellulose column chromatography. I. Erythrocyte lipids. Anal Biochem. 1970 Dec;38(2):423–436. doi: 10.1016/0003-2697(70)90467-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson M., Iacobucci G. A., Myers D. V. 5-Methyltryptophan: an internal strandard for tryptophan determination by ion-exchange chromatography. Anal Biochem. 1976 Feb;70(2):470–478. doi: 10.1016/0003-2697(76)90472-3. [DOI] [PubMed] [Google Scholar]



