Abstract
Viral infection often perturbs host cell signaling pathways including those involving mitogen-activated protein kinases (MAPKs). We now show that reovirus infection results in the selective activation of c-Jun N-terminal kinase (JNK). Reovirus-induced JNK activation is associated with an increase in the phosphorylation of the JNK-dependent transcription factor c-Jun. Reovirus serotype 3 prototype strains Abney (T3A) and Dearing (T3D) induce significantly more JNK activation and c-Jun phosphorylation than does the serotype 1 prototypic strain Lang (T1L). T3D and T3A also induce more apoptosis in infected cells than T1L, and there was a significant correlation between the ability of these viruses to phosphorylate c-Jun and induce apoptosis. However, reovirus-induced apoptosis, but not reovirus-induced c-Jun phosphorylation, is inhibited by blocking TRAIL/receptor binding, suggesting that apoptosis and c-Jun phosphorylation involve parallel rather than identical pathways. Strain-specific differences in JNK activation are determined by the reovirus S1 and M2 gene segments, which encode viral outer capsid proteins (ς1 and μ1c) involved in receptor binding and host cell membrane penetration. These same gene segments also determine differences in the capacity of reovirus strains to induce apoptosis, and again a significant correlation between the capacity of T1L × T3D reassortant reoviruses to both activate JNK and phosphorylate c-Jun and to induce apoptosis was shown. The extracellular signal-related kinase (ERK) is also activated in a strain-specific manner following reovirus infection. Unlike JNK activation, ERK activation could not be mapped to specific reovirus gene segments, suggesting that ERK activation and JNK activation are triggered by different events during virus-host cell interaction.
Mitogen-activated protein kinases (MAPKs) play a critical role in the transduction of a wide variety of extracellular signals (22). MAPKs include the extracellular signal-related kinases (ERKs), which are activated by growth factors and many other mitogenic stimuli (11) and are generally thought to have antiapoptotic properties, and the c-Jun N-terminal kinases (JNKs, also called stress-activated protein kinases, SAPKs) (16, 39) and p38 MAPKs (23, 40, 53), which are activated by stress stimuli and function to communicate growth-inhibitory and apoptotic signals within cells. It is thought that the commitment to apoptosis and determination of cell fate may involve the balance between the activity of the JNK and p38 kinases and that of ERK (7). For example, the inhibition of ERK activity and the coordinate activation of JNK and p38 kinase correlate with the induction of apoptosis in nerve growth factor-deprived PC12 pheochromocytoma cells (61), Fas-treated Jurkatt cells (37, 60), and UV-irradiated mouse fibroblasts (3).
Infection with a wide variety of viruses can result in perturbation of host cell signaling pathways including MAPK cascades. Some viruses show a dependence on the ERK signaling cascades for replication, and viral proteins that induce ERK activation have been identified (34, 36, 38, 43, 57). Virus-induced MAPK activation, including JNK and p38, has also been described (19, 31, 32, 42, 44, 49, 54, 63), as has the activation of MAPK-associated transcription factors (33, 42, 54, 63). However, the reason for their activation following infection remains largely unclear.
Reovirus is a double-stranded RNA virus that induces apoptosis in cultured cells in vitro (46, 50, 58) and in target tissues in vivo including the central nervous system and heart (14, 46, 47). Reovirus-induced apoptosis correlates with pathology in vivo and is a critical mechanism by which disease is triggered in the host (14, 47). Strain-specific differences in the capacity of reoviruses to induce apoptosis are determined by the viral S1 and M2 gene segments (58, 59). Reovirus-induced apoptosis requires viral binding to cell surface receptors, including junctional adhesion molecule (2), but not completion of the full viral replication cycle (50, 58). Reovirus induces apoptosis by a p53-independent mechanism that involves cellular proteases including calpains (15) and caspases (10), is dependent on reovirus-induced NF-κB activation (2, 12), and is inhibited by overexpression of Bcl-2 (50).
We have previously shown that reovirus-induced apoptosis is mediated by tumor necrosis factor (TNF) related apoptosis-inducing ligand (TRAIL) (10), which is released from infected cells, and can be inhibited by antibodies against TRAIL or by treatment of infected cells with soluble forms of TRAIL receptors. TRAIL interacts with several members of the TNF receptor superfamily including the apoptosis-associated receptors DR4 (TRAIL-R1) and DR5 (TRAIL-R2/TRICK2/KILLER). These receptors contain an intracellular 80-amino-acid “death domain” (reviewed in reference 1), which is indispensable for apoptosis since it interacts with death domains found in cytoplasmic adapter proteins such as TNF-R1-associated death domain protein (29) and Fas-associated protein with death domain (5, 8). Adapter proteins have additional domains that enable interaction with the prodomains of apoptotic caspases (4, 17, 45) and with members of the TNF receptor-associated factor family (27, 28) of molecules involved in the activation of NF-κB and JNK (52, 56). In addition to activating apoptotic caspases, TRAIL-receptor activation in reovirus-infected cells is thus also likely to result in the activation of NF-κB (35) and JNK (30).
The capacity of reovirus to induce apoptosis through a TRAIL-dependent pathway in infected cells suggests that proapoptotic MAPKs, including JNK, might be activated in reovirus-infected cells. Reovirus infection also disrupts cell cycle regulation by inducing a G2/M arrest (48), suggesting that ERK, which promotes cell cycle progression, might also be inhibited following reovirus infection. This study investigated the activation of MAPKs in reovirus-infected cells. We showed that reovirus infection causes the selective activation of both the JNK and ERK MAPK cascades. Strain-specific differences in JNK, but not ERK, activation are determined by the viral S1 and M2 gene segments, suggesting that different mechanisms are involved in the activation of these kinases in reovirus-infected cells. The viral S1 and M2 gene segments also determine differences in the capacity of reoviruses to induce apoptosis, and we now show that there is a significant correlation between the capacity of reassortant reoviruses to activate JNK and to induce apoptosis. Blocking TRAIL-receptor interaction does not prevent the early activation of c-Jun by reovirus, indicating that death receptor-independent signaling pathways are required for reovirus-induced JNK activation.
MATERIALS AND METHODS
Cells and virus.
Mouse L929 cells (ATCC CCL1) were grown in Joklik's modified Eagle's medium supplemented to contain 5% fetal bovine serum and 2 mM l-glutamine (Gibco BRL). Reovirus strains Type 3 Abney (T3A), Type 3 Dearing (T3D), and Type 1 Lang (T1L) are laboratory stocks, which have been plaque purified and passaged (twice) in L929 (ATCC CCL1) cells to generate working stocks (59). T1L × T3D reassortant viruses were grown from stocks originally isolated by Kevin Coombs, Max Nibert, and Bernard Fields (6, 13). Virus infections were performed at a multiplicity of infection (MOI) of 100 to ensure that 100% of susceptible cells were infected and to maximize the synchrony of virus replication.
Western Blot analysis and antibodies.
Following infection with reovirus, cells were pelleted by centrifugation, washed twice with ice-cold phosphate-buffered saline, and lysed by sonication in 200 μl of a buffer containing 15 mM Tris (pH 7.5), 2 mM EDTA, 10 mM EGTA, 20% glycerol, 0.1% NP-40, 50 mM β-mercaptoethanol, 100 μg of leupeptin per ml, 2 μg of aprotinin per ml, 40 μM Z-Asp-2,6-dichlorobenzoyloxime, and 1 mM phenylmethylsulfonyl fluoride. The lysates were then cleared by centrifugation at 16,000 × g for 5 min, normalized for protein amount, mixed 1:1 with SDS sample buffer (100 mM Tris [pH 6.8], 2% sodium dodecyl sulfate [SDS], 300 mM β-mercaptoethanol, 30% glycerol, 5% pyronine Y), boiled for 5 min and stored at −70°C. Proteins were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) (10% polyacrylamide gels) and probed with antibodies directed against phospho-ERK, phospo c-Jun, and total c-Jun (New England Biolabs, Beverly, Mass.). All lysates were standardized for protein concentration with antibodies directed against actin (no. CP01; Oncogene, Cambridge, Mass.). Autoradiographs were quantitated by densitometric analysis using a Fluor-S MultiImager (Bio-Rad Laboratories, Hercules, Calif.).
In vitro kinase assays.
L929 cells were solubilized in TX-100 lysis buffer (70 mM β-glycerophosphate, 1 mM EGTA, 100 μM Na3VO4, 1 mM dithiothreitol, 2 mM MgCl2, 0.5% Triton X-100, 20 μg of aprotinin per ml). Cellular debris was removed by centrifugation at 8,000 × g for 5 min. The protein concentration was determined by a Bradford assay using bovine serum albumin as a standard. JNK activity was measured using a solid-phase kinase assay in which glutathione S-transferase–c-Jun bound to glutathione-Sepharose 4B beads was used to affinity purify JNK from cell lysates as described previously (20, 25). The phosphorylation of glutathione S-transferase–c-Jun was quantitated with a Phosphorimager instrument (Molecular Dynamics). ERK activation was measured by first incubating the lysate with 2 μg of an anti-ERK2 antibody (Santa Cruz Biotechnology, Inc.) per ml for 1 hr at 4°C with agitation followed by the addition of 15 μl of a slurry of protein A-Sepharose beads (no. P-3391; Sigma) and a further 20-min incubation at 4°C. The beads were washed twice with 1 ml of lysis buffer and twice with 1 ml of lysis buffer without Triton X-100. A 35-μl volume of the last wash was left in the tube, mixed with 20 μl of ERK reaction mix (50 mM β-glycerophosphate, 100 μM Na3VO4, 20 mM MgCl2, 200 μM ATP, 0.5 μCi of [γ-32P]ATP per μl 400 μM epidermal growth factor receptor peptide 662–681, 100 μg of IP-20 per μl, 2 mM EGTA), and incubated for 20 min at 20°C. The reaction was stopped with 10 μl of 25% trichloroacetic acid, and the reaction product was spotted on P81 Whatman paper. The samples were washed three times for 5 min each in 75 mM phosphoric acid and once for 2 min in acetone. They were then air dried, and their radioactivity was measured in a β-counter. The activity of p38 was measured as described by Gerwins et al. (21).
Apoptosis assays and reagents.
At 48 h after infection with reovirus, the cells were harvested and stained with acridine orange, for determination of nuclear morphology and ethidium bromide in order to distinguish cell viability, at a final concentration of 1 μg/ml (18). Following staining, the cells were examined by epifluorescence microscopy (Nikon Labophot-2; B-2A filter; excitation, 450 to 490 nm; barrier, 520 nm; dichroic mirror, 505 nm). The percentage of cells containing condensed nuclei and/or marginated chromatin in a population of 100 cells was recorded. The specificity of this assay has been previously established in reovirus-infected cells by using DNA-laddering techniques and electron microscopy (10, 58). Soluble death receptors (Fc:DR5 and Fc:DR4) were obtained from Alexis Corp. (Pittsburgh, Pa.).
RESULTS
Reovirus activates JNK in infected cells.
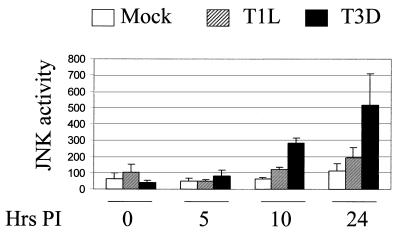
We first investigated whether JNK was activated in reovirus-infected cells. L929 cells were infected (MOI, 100) with two prototype strains of reovirus, T3D and T1L. At 0, 5, 10 and 24 h postinfection (p.i.), the cells were harvested and the presence of JNK activity was detected by in vitro kinase assays (Fig. 1). In three independent experiments, JNK activity was significantly increased (P < 0.01) at 24 h p.i. in T3D-infected cells compared to mock-infected cells. However, JNK activity was not significantly increased (P > 0.05) at 24 h p.i. in T1L-infected cells compared to mock-infected cells. An increase in JNK activity was apparent in T3D-infected cells at 10 h p.i. Although statistically significant, variability in JNK activity was greater in the T3D-infected cells at 24 h p.i. than for other times and conditions, reflecting the increase in the mean value.
FIG. 1.
Reovirus activates JNK infected cells. L929 cells were infected with two different serotypes of reovirus, T1L and T3D (MOI, 100) or were mock infected. At various times p.i., lysates were prepared and JNK activity was determined by in vitro kinase assays. The graph shows the mean JNK activity, as measured by c-Jun phosphorylation (arbitrary imager units), of three independent experiments. Error bars represent standard errors of the mean.
Levels of phosphorylated c-Jun are increased in reovirus-infected cells.
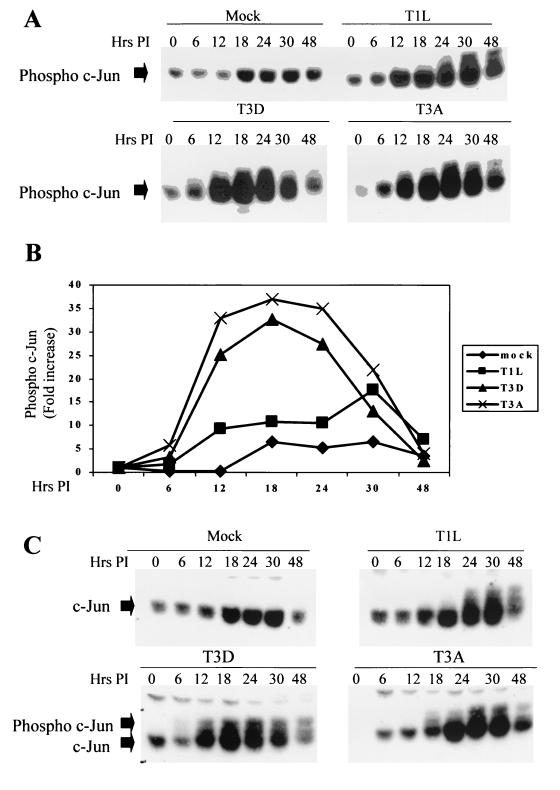
The activation of JNK results in the phosphorylation and activation of the transcription factor c-Jun, which in turn regulates the transcription of a multitude of cellular genes. Having shown that JNK was activated in reovirus-infected cells, we wished to determine whether the JNK-dependent transcription factor c-Jun was also activated following reovirus infection. Cells were infected with three different strains of reovirus, T1L, T3D, and T3A, the second prototypic T3 strain. The cells were then harvested at various times p.i., and the activation state of c-Jun was determined by Western blot analysis using an antibody directed against the phosphorylated, activated form of c-Jun. Levels of phosphorylated c-Jun were increased in cells infected with the T1L, T3D, and T3A strains compared to those in mock-infected cells (Fig. 2). Increased levels of activated c-Jun were present 12 h p.i., which closely parallels the activation of JNK (Fig. 1). There were serotype-specific differences in the ability of reovirus to phosphorylate c-Jun with T3 strains (T3D and T3A), inducing higher levels of phosphorylated c-Jun at earlier times p.i., and T1L strains. For example, at 12 h p.i., the levels of phosphorylated c-Jun were increased 8-fold in T1L-infected cells compared to those seen in mock-infected cells whereas the levels of phosphorylated c-Jun were increased 24-fold in T3D-infected cells and 33-fold in T3A-infected cells. Some increase in c-Jun phosphorylation was observed in mock-infected cells, which may be due to increasing cell confluence. We probed the same lysates with an antibody that detects total c-Jun (both phosphorylated and unphosphorylated forms [Fig. 2C]). These blots show that there was also an increase in the levels of total c-Jun in both mock and reovirus-infected cells. Also visible on these blots are bands corresponding to the phosphorylated form of c-Jun (Fig. 2C). Once again, serotype-specific differences in the ability of reovirus to phosphorylate c-Jun were observed, with T3D and T3A inducing higher levels of phosphorylated c-Jun than T1L did.
FIG. 2.
c-Jun is activated following infection with reovirus. Cells were infected with different strains of reovirus (MOI, 100) and were harvested at various times p.i. (A and C) Extracts were standardized for protein concentration, using an anti-actin antibody, and equal amounts of protein were separated by SDS-PAGE and probed with antibodies directed against phosphorylated (A) or total (C) c-Jun. Bands corresponding to phosphorylated and unphosphorylated c-Jun are shown. The gels are representative of at least two independent experiments. (B) Graphical representation of the Western blot shown in panel A, showing the fold increase in the levels of phophorylated c-Jun over time.
In support of the association between JNK activation and c-Jun phosphorylation, there was a high correlation between the ability of reovirus to induce JNK activation and to phosphorylate c-Jun (Pearson parametric correlation R2 = 0.99, P = 0.0217).
Reovirus-induced JNK activation is determined by the S1 and M2 gene segments.
Having shown that T3D activates JNK to a greater extent than T1L does, we wished to identify whether specific viral genes determined these differences. L929 cells were infected with a panel of T1L × T3D reassortant reoviruses (MOI, 100). At 24 h p.i., the cells were harvested and the JNK activity was determined by the in vitro kinase assay. JNK activation following infection with different reassortant viruses, as well as with parental strains, and the derivation of the various genome segments of each virus are shown in Table 1. The results were analyzed using both parametric (t test) and nonparametric (Mann-Whitney [M-W] test) methods. The reovirus S1 (t test, P = 0.04; M-W test, P = 0.008) and M2 (t test, P = 0.026; M-W test, P = 0.014) gene segments were both significantly associated with strain-specific differences in virus-induced apoptosis. Using linear-regression analysis, we obtained R2 values of 48.6% (P = 0.017) for the S1 gene segment and 42.5% (P = 0.03) for the M2 gene segment. These results indicate that both the S1 and M2 gene segments contribute to strain-specific differences in the capacity of reovirus to activate JNK in infected cells. The nature of the reassortant pool tested, in which eight of nine viruses were concordant for the parental origin of their S1 and M2 segments, prevented us from more accurately defining the relative contributions of these two segments to JNK activation.
TABLE 1.
Reovirus-induced JNK activation is determined by the S1 and M2 gene segments
| Virus straina | JNK activity (imager units) | Reovirus gene segment
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | M1 | M2 | M3 | S1 | S2 | S3 | S4 | ||
| EB97 | 189,000 | T3D | T3D | T1L | T3D | T3D | T3D | T3D | T3D | T3D | T1L |
| T3D | 108,000 | T3D | T3D | T3D | T3D | T3D | T3D | T3D | T3D | T3D | T3D |
| EB13 | 108,000 | T3D | T3D | T3D | T3D | T3D | T3D | T3D | T3D | T3D | T1L |
| H15 | 102,000 | T1L | T3D | T3D | T1L | T3D | T3D | T3D | T3D | T3D | T1L |
| KC150 | 84,000 | T3D | T1L | T1L | T1L | T3D | T1L | T3D | T3D | T1L | T3D |
| EB121 | 81,000 | T3D | T3D | T1L | T3D | T1L | T3D | T1L | T3D | T3D | T3D |
| EB1 | 78,000 | T1L | T3D | T1L | T1L | T3D | T1L | T1L | T1L | T3D | T1L |
| EB85 | 75,000 | T1L | T1L | T1L | T1L | T1L | T3D | T1L | T3D | T1L | T1L |
| EB31 | 63,000 | T1L | T1L | T1L | T3D | T1L | T1L | T1L | T3D | T3D | T1L |
| T1L | 54,000 | T1L | T1L | T1L | T1L | T1L | T1L | T1L | T1L | T1L | T1L |
| H41 | 24,000 | T3D | T3D | T1L | T1L | T1L | T3D | T1L | T3D | T3D | T1L |
| None (mock) | 20,000 | ||||||||||
| t test | 0.33 | 0.18 | 0.19 | 0.15 | 0.026 | 0.20 | 0.04 | 0.24 | 0.19 | 0.83 | |
| M-W test | 0.17 | 0.16 | 0.08 | 0.12 | 0.014 | 0.22 | 0.008 | 0.29 | 0.24 | 0.41 | |
t test and M-W values are P values, with significant values in bold type.
It is important to note that although statistical analysis identifies the S1 and M2 gene segments as important determining factors in the ability of reoviruses to induce JNK activation, some viruses with differing genotypes (e.g., KC150 and EB121) have closely related JNK activity levels. This suggests that nongenetic factors also contribute to reovirus-induced JNK activation.
Reovirus-induced c-Jun phosphorylation and reovirus-induced apoptosis are correlated.
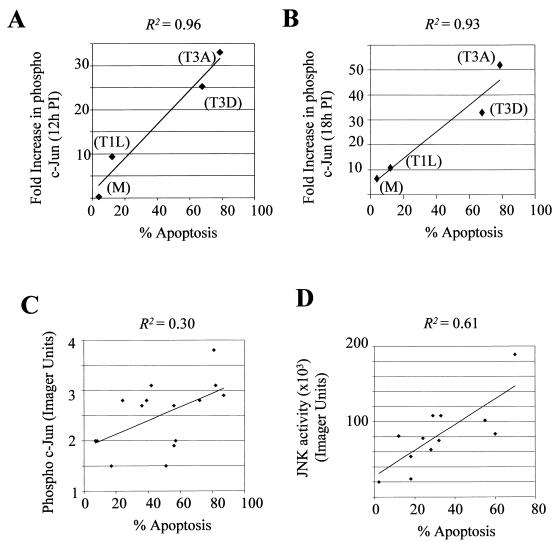
Reovirus prototypic strains T3D and T3A induce more apoptosis in infected cells than T1L (58, 59). Since our results indicate that Type 3 reoviruses also induce higher levels of phosphorylated c-Jun and JNK activity, we compared the ability of the prototypic strains of reovirus to phosphorylate c-Jun at 12 and 18 h p.i. (Fig. 2) with their ability to induce apoptosis (Fig. 3A and B). Apoptosis was measured at 48 h p.i., and the apoptosis experiments were set up in parallel to the c-Jun experiments to ensure that the experimental conditions were as similar as possible. A significant association between the capacity of reoviruses to induce apoptosis and phosphorylate c-Jun was found (Pearson parametric correlation R2 = 0.964 using c-Jun values obtained at 12 h p.i. and R2 = 0.9330 using c-Jun values obtained at 18 h p.i.). We also investigated whether there was a correlation between the capacity of T1L × T3D reassortants to phosphorylate c-Jun (Fig. 3C) or activate JNK (Fig. 3D) and to induce apoptosis. Significant associations between the capacity of reovirus reassortants to induce apoptosis and both activate JNK (Pearson parametric correlation R2 = 0.6077, P = 0.0028) and phosphorylate c-Jun (Pearson parametric correlation R2 = 0.30, P = 0.0354) were found. The larger pool of viruses used to generate the reassortant data enabled us to derive P values for these correlations.
FIG. 3.
There is a correlation between the capacities of different prototype strains of reovirus to phosphorylate c-Jun and induce apoptosis and between the capacities of T1L × T3D reassortant viruses to induce JNK activation or c-Jun phosphorylation and apoptosis. The abilities of different strains of reovirus (T3A, T3D, and T1L) and mock (M) infection to induce increased levels of phosphorylated c-Jun, at 12 h (A) and 18 h (B) p.i. (values taken from Fig. 2) and apoptosis are shown. Experiments to determine c-Jun phosphorylation and apoptosis were set up in parallel. (C) The capacity of reovirus reassortants (MOI, 100) to induce phosphorylated c-Jun and apoptosis was plotted. Each point represents a single reassortant virus. Lysates were harvested at 18 h p.i., standardized for protein concentration, and analyzed by Western blotting using an antibody directed against phospho c-Jun. Apoptosis values were obtained in a parallel experiment and represent the mean value from three separate wells (24-well tissue culture plate) of the same experiment. (D) The capacities of reovirus reassortants (MOI, 100) to induce JNK activity and apoptosis were plotted. Each point represents a single reassortant virus. JNK activity values were taken from Table 1. Apoptosis values were obtained in a parallel experiment and represent the mean value from three separate wells (24-well tissue culture plate) of the same experiment.
Soluble TRAIL receptors block reovirus-induced apoptosis but not reovirus-induced c-Jun activation.
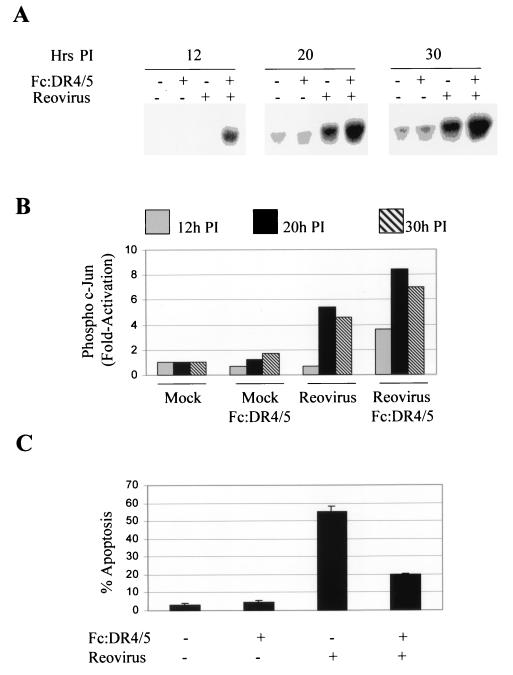
TRAIL receptor ligation results in the activation of JNK (30). We have previously shown that reovirus-induced apoptosis requires TRAIL receptor ligation (10). We next investigated whether TRAIL receptor activation was required for the activation of c-Jun in reovirus-infected cells. A combination of the soluble TRAIL receptors Fc:DR5 and Fc:DR4 was used to inhibit the binding of TRAIL to its functional cellular receptors. L929 cells were infected with T3D (MOI, 50) in the presence or absence of Fc:DR5 and Fc:DR4 (final concentration, 100 ng/ml each) and were harvested after 18 h. Lysates were then analyzed by Western blot analysis using an anti-phospho-c-Jun antibody. In parallel, cells were infected with reovirus in the presence of a combination of Fc:DR5 and Fc:DR4 and were assayed for reovirus-induced apoptosis after 48 h (Fig. 4). The presence of soluble TRAIL receptors did not inhibit c-Jun activation in T3D-infected cells (Fig. 4A and B). In fact, there seemed to be an increase in levels of phosphorylated c-Jun when cells were infected with reovirus in the presence of soluble TRAIL receptors compared to levels seen in untreated, infected cells. Conversely, the presence of soluble TRAIL receptors markedly reduced the ability of T3D to induce apoptosis (Fig. 4C), indicating that inhibition of TRAIL receptor ligation inhibits apoptosis but fails to reduce the activation of c-Jun in reovirus-infected cells. This suggests that pathways other than those initiated by TRAIL contribute to reovirus-induced JNK activation.
FIG. 4.
Apoptosis but not c-Jun phosphorylation is inhibited in T3D-infected cells in the presence of the soluble TRAIL receptors Fc:DR5 and Fc:DR4. In parallel experiments, cells were infected with T3D (MOI, 50) and were assayed for c-Jun activation at various times p.i. and for apoptosis after 48 h. (A) Representative autoradiograph showing levels of phosphorylated c-Jun following infection with reovirus (T3D), in the presence or absence of Fc:DR5 and Fc:DR4 (final concentrations, 100 ng/ml each). Equal amounts of protein, as determined by actin concentration (data not shown), were loaded. (B) Graphical analysis of the results shown in panel A showing the fold increase in c-Jun phosphorylation compared to that in mock-infected, untreated cells, at 12 h, 20 h, and 30 h p.i. (C) Graph showing the percentage of cells with apoptotic nuclear morphology in reovirus (T3D)- or mock-infected cells in the presence or absence of soluble TRAIL receptors (final concentration, 100 ng/ml each). Error bars represent standard errors of the mean from three separate wells (24-well tissue culture plate) of the same experiment.
ERK, but not p38 MAPK, is activated following reovirus infection.
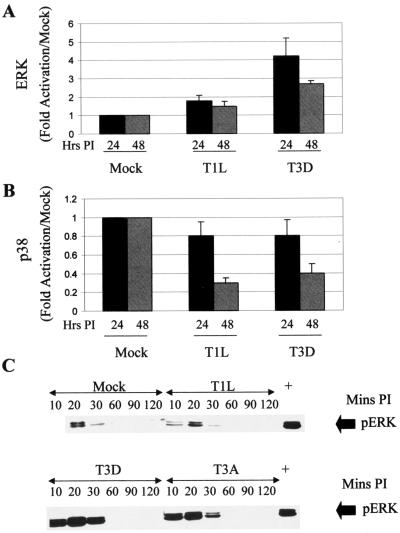
Having shown that JNK is activated in reovirus-infected cells we wished to determine whether other MAPK pathways are also activated following reovirus infection. The activities of p38 and ERK were thus investigated in reovirus-infected L929 cells (MOI, 100) at 24 and 48 h p.i. by in vitro kinase assays. T3D, but not T1L, infection resulted in the activation of ERK. ERK activation peaked at 24 h p.i., with a four fold increase in the levels of ERK activation compared to those in mock-infected cells (Fig. 5A). There was a slight decrease in p38 activity following infection with the T1L and T3D strains of reovirus (Fig. 5B).
FIG. 5.
Reovirus activates ERK but not p38 in infected cells. (A and B) The activities of ERK (A) and p38 (B) were investigated in reovirus-infected L929 cells (MOI, 100) at 24 and 48 h p.i. by in vitro kinase assays. The graphs show fold activation compared to that in mock-infected cells. Error bars represent standard errors of the mean from three independent experiments. (C) ERK is activated at early times after reovirus infection. L929 cells were infected with reovirus (MOI, 100) and harvested at various times p.i. Proteins were separated by SDS-PAGE and subjected to Western blot analysis using antibodies directed against phospho-ERK. Actin was used to standardize for protein loading (data not shown).
Since MAPKs can be activated rapidly in some systems, we also looked at MAPK activation at very early times (less than 2 h) after reovirus infection. Using antibodies directed against the phosphorylated, active form of ERK, we were able to show that ERK had an additional activation peak at around 20 min p.i. (Fig. 5C). This activation peak was also strain specific, with T3D and T3A inducing more activity than T1L did. JNK and p38 were not activated within 2 h of infection (results not shown). Taken together with our above-described data, these results indicate that reovirus preferentially and selectively activates the JNK and ERK MAPK pathways.
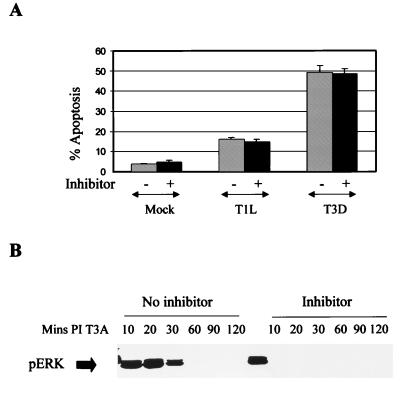
Although we were able to show strain-specific differences in the activation of ERK in infected cells, we have been unable to map this phenomenon to a specific reovirus gene segment (results not shown), suggesting that ERK and JNK activation involve different types of virus-host cell interactions. The activation of ERK is generally associated with antiapoptotic effects. Conversely, the inhibition of ERK activity often correlates with the activation of JNK and p38 kinase and the resultant induction of apoptosis. We therefore wished to investigate whether reovirus-induced apoptosis would be enhanced by treatment with PD 98059, a chemical inhibitor of ERK activation. Cells were pretreated with 10 μM PD 98059 for 2 h prior to infection with reovirus and were maintained in medium in the presence of inhibitor for 48 h following infection. There was no change in reovirus-induced apoptosis in cells treated with PD 98059 (Fig. 6A), even though ERK activation following reovirus infection was blocked (Fig. 6B). The activity of MAPK p38 is decreased in reovirus-infected cells, and, as expected, chemical inhibitors of p38 (PD 169316 and SB 202190) did not affect reovirus-induced apoptosis (results not shown).
FIG. 6.
Inhibition of ERK activation does not affect reovirus-induced apoptosis. (A) Reovirus-induced apoptosis (MOI, 100) was measured in the presence of a chemical inhibitor of ERK activation (PD 98059). L929 cells were pretreated with inhibitor (10 μM) for 2 h prior to infection with reovirus and were maintained in medium in the presence of inhibitor for 48 h following reovirus infection. They were then harvested and assayed for apoptosis. The graph shows percent apoptosis. Error bars represent standard errors of the mean. (B) Activation-phosphorylation of ERK following reovirus (T3D) infection (MOI, 100) in the presence of PD 98059 (10 μM) as determined by Western blot analysis using antibodies directed against phospho-ERK.
DISCUSSION
Our results indicate that reovirus infection selectively induces MAPK activation in infected cells. JNK is activated following reovirus infection in a strain-specific manner, with the type 3 prototype reovirus strain (T3D) inducing more JNK activation than the type 1 prototype reovirus strain (T1L) does. Reovirus-induced JNK activation is associated with phosphorylation, and hence activation, of the JNK-dependent transcription factor c-Jun. The phosphorylation of c-Jun is also a strain-specific event, with the prototype 3 strains (T3D and T3A) inducing more JNK activation than T1L does.
Strain-specific differences in JNK activation are determined by the S1 and M2 reovirus gene segments, which both encode reovirus capsid proteins. The reovirus S1 gene is bicistronic, encoding both the viral attachment protein ς1 and a non-virion-associated protein, ς1s, that is required for reovirus-induced G2/M cell cycle arrest (48) but is not required for reovirus growth in cell culture or for the induction of apoptosis (51, 59). Reovirus-induced c-Jun activation is not blocked following infection by a ς1s-deficient virus strain (results not shown), indicating that ς1s is not required for c-Jun activation in reovirus-infected cells. The M2 segment encodes the main reovirus outer capsid protein μ1c, which plays a key role in membrane penetration and in the transmembrane transport of virions (24, 26, 41).
The S1 and M2 gene segments also determine the ability of reovirus to induce apoptosis (59), and there is a correlation between the ability of different prototype reovirus strains, and T3D × T1L reassortant reoviruses to induce apoptosis and to activate JNK and/or phosphorylate c-Jun. This suggests that JNK activation and c-Jun phosphorylation and apoptosis either are components of the same pathway that induces apoptosis following reovirus infection or are components of distinct, parallel pathways induced by the same viral factors.
Reovirus-induced apoptosis is mediated by TRAIL-induced activation of death receptors and is associated with the release of TRAIL from infected cells (10). TRAIL receptor activation can also result in the activation of JNK (30), suggesting that this may be the mechanism by which reovirus induces JNK activation. However, reovirus-induced TRAIL release and TRAIL receptor activation do not occur until 24 to 48 h p.i. (10), suggesting that death receptor-independent signaling pathways are responsible for the earlier (10-h p.i.) activation of JNK and c-Jun that occurs following reovirus infection. The fact that reovirus-induced apoptosis can be inhibited by soluble TRAIL receptors, without affecting reovirus-induced c-Jun phosphorylation, indicates that pathways leading to apoptosis and JNK activity can be disassociated and also suggests that these pathways are distinct rather than identical. Both these observations are consistent with our previous studies showing that reovirus-induced JNK activity (62), but not apoptosis (results not shown), is inhibited in MEKK1−/− embryonic stem cells. However, until we can find methods to completely block apoptosis or JNK activity, we cannot rule out the possibility of a common pathway.
Reovirus-induced JNK activation is a specific event. For example, p38 MAPK is not activated following reovirus infection. In addition, although ERK is activated in a serotype-specific manner in infected cells, our inability to map this activation to a specific reovirus gene segment indicates that ERK is activated by a different mechanism from that used for JNK activation. The kinetics of ERK and JNK activation also differ, with ERK showing an early phase of activation that is not seen with JNK, again suggesting that ERK and JNK are activated by different mechanisms. The specificity of reovirus-induced JNK activation suggests that it is important for some aspect of the reovirus life cycle. The role of virus-induced JNK activation is not yet known; however, reovirus growth is not inhibited in MEKK1−/− cells (results not shown) despite a marked reduction in reovirus-induced JNK activation (62). This suggests that JNK activation is not essential for viral replication. Reovirus-induced JNK activation is associated with activation of the JNK-dependent transcription factor c-Jun. We have previously shown (12) that reovirus infection is also associated with activation of the transcription factor NF-κB, suggesting that virus-induced perturbation of gene expression plays a critical role in viral pathogenesis and cytopathicity. This is confirmed by our recent studies showing that reovirus infection is associated with alterations in the expression of a number of host cell genes involved in the regulation of cell cycle, apoptosis, and DNA repair (R. DeBiasi, personal communication). In addition, one of the largest functional groups of genes whose expression is altered following reovirus infection is the group associated with interferon activation, consistent with the known role of c-Jun in the optimal induction of alpha-1 and beta interferon transcription (9) and onset of the antiviral response (55).
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants 1RO1AG14071 and GM30324 from the National Institutes of Health, merit and REAP grants from the Department of Veterans Affairs, and a U.S. Army Medical Research and Material Command grant (DAMD17-98-1-8614).
The University of Colorado Cancer Center provided core tissue culture and medium facilities.
REFERENCES
- 1.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Barton E S, Forrest J C, Connolly J L, Chappell J D, Liu Y, Schnell F J, Nusrat A, Parkos C A, Dermody T S. Junction Adhesion Molecule is a receptor for reovirus. Cell. 2001;104:441–451. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 3.Berra E, Municio M M, Sanz L, Frutos S, Diaz-Meco M T, Moscat J. Positioning atypical protein kinase C isoforms in the UV-induced apoptotic signaling cascade. Mol Cell Biol. 1997;17:4346–4354. doi: 10.1128/mcb.17.8.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 5.Boldin M P, Varfolomeev E E, Pancer Z, Mett I L, Camonis J H, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- 6.Brown E G, Nibert M L, Fields B N. The L2 gene of reovirus serotype 3 controls the capacity to interfere, accumulate deletions and establish persistent infection. In: Compans R W, Bishop D H L, editors. Double-stranded RNA-viruses. New York, N.Y: Elsevier; 1983. pp. 275–287. [Google Scholar]
- 7.Canman C E, Kastan M B. Signal transduction. Three paths to stress relief. Nature. 1996;384:213–214. doi: 10.1038/384213a0. [DOI] [PubMed] [Google Scholar]
- 8.Chinnaiyan A M, O'Rourke K, Tewari M, Dixit V M. FADD, a novel death domain containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 9.Chu W-M, Ostertag D, Li Z-W, Chang L, Chen Y, Hu Y, Williams B, Perrault J, Karin M. JNK2 and IKKβ are required for activating the inate response to viral infection. Immunity. 1999;11:721–731. doi: 10.1016/s1074-7613(00)80146-6. [DOI] [PubMed] [Google Scholar]
- 10.Clarke P, Meintzer S M, Gibson S, Widmann C, Garrington T, Johnson G L, Tyler K L. Reovirus-induced apoptosis is mediated by TRAIL. J Virol. 2000;74:8135–8139. doi: 10.1128/jvi.74.17.8135-8139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobb M H, Boulton T G, Robbins D J. Extracellular signal-related kinases: ERKs in progress. Cell Regul. 1991;2:965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connolly J L, Rodgers S E, Clarke P, Ballard D W, Kerr L D, Tyler K L, Dermody T S. Reovirus-induced apoptosis requires activation of transcription factor NF-κB. J Virol. 2000;74:2981–2989. doi: 10.1128/jvi.74.7.2981-2989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coombs K M, Fields B N, Harrison S C. Crystallization of the reovirus type 3 Dearing core. Crystal packing is determined by the lambda 2 protein. J Mol Biol. 1990;215:1–5. doi: 10.1016/s0022-2836(05)80089-0. [DOI] [PubMed] [Google Scholar]
- 14.DeBiasi R L, Edelstein C L, Sherry B, Tyler K L. Calpain inhibition protects against virus-induced apoptotic myocardial injury. J Virol. 2001;75:351–361. doi: 10.1128/JVI.75.1.351-361.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeBiasi R L, Squier M K T, Pike B, Wynes M, Dermody T S, Cohen J J, Tyler K L. Reovirus-induced apoptosis is preceded by increased cellular calpain activity and is blocked by calpain inhibitors. J Virol. 1999;73:695–701. doi: 10.1128/jvi.73.1.695-701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 17.Duan H, Dixit V M. RAIDD is a new death adaptor molecule. Nature. 1997;385:86–89. doi: 10.1038/385086a0. [DOI] [PubMed] [Google Scholar]
- 18.Duke R C, Cohen J J. Morphological and biochemical assays of apoptosis. In: Coligan J E, editor. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1992. pp. 3.17.1–3.17.6. [Google Scholar]
- 19.Eliopoulos A G, Young L S. Activation of c-Jun N-terminal kinase (JNK) by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP-1) Oncogene. 1988;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 20.Gardner A M, Johnson G L. Fibroblast growth factor 2 suppression of tumor nerosis factor alpha-mediated apoptosis requires Ras and the activation of mitogen-activated protein kinase. J Biol Chem. 1996;271:14560–14566. doi: 10.1074/jbc.271.24.14560. [DOI] [PubMed] [Google Scholar]
- 21.Gerwins P, Blank J L, Johnson G L. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J Biol Chem. 1997;272:8288–8295. doi: 10.1074/jbc.272.13.8288. [DOI] [PubMed] [Google Scholar]
- 22.Graves J D, Campbell J S, Krebs E G. Protein serine/threonine kinases of the MAPK cascade. Ann N Y Acad Sci. 1995;766:320–343. doi: 10.1111/j.1749-6632.1995.tb26684.x. [DOI] [PubMed] [Google Scholar]
- 23.Han J, Lee J D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 24.Hazelton P R, Coombs K M. The reovirus mutant tsA279 has temperature sensitive lesions in the M2 and L2 genes: the M2 gene is associated with decreased viral protein production and blockade in transmembrane transport. Virology. 1995;207:46–58. doi: 10.1006/viro.1995.1050. [DOI] [PubMed] [Google Scholar]
- 25.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 26.Hooper J W, Fields B N. Role of the μ1 protein in reovirus stability and capacity to cause chromium release from host cells. J Virol. 1996;70:459–467. doi: 10.1128/jvi.70.1.459-467.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu H, Huang J N, Shu H B, Baichwal V, Goeddel D V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 28.Hsu H, Shu H B, Pan M G, Goeddel D V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 29.Hsu H, Xiong J, Goeddel D V. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 30.Hu W H, Johnson H, Shu H B. Tumor necrosis factor-related apoptosis-inducing ligand receptors signals NF-kappaB and JNK activation and apoptosis through distinct pathways. J Biol Chem. 1999;274:30603–30610. doi: 10.1074/jbc.274.43.30603. [DOI] [PubMed] [Google Scholar]
- 31.Huttunen P, Hyypia T, Vihinen P, Nissinen L, Heino J. Echovirus 1 infection induces both the stress- and growth-activated mitogen-activated protein kinase pathways and regulates the transcription of cellular immediate-early genes. Virology. 1998;250:85–93. doi: 10.1006/viro.1998.9343. [DOI] [PubMed] [Google Scholar]
- 32.Iordanov M S, Paranjape J M, Zhou A, Wong J, Williams B R G, Meurs E F, Silverman R H, Magun B E. Activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R and alternative pathways. Mol Cell Biol. 2000;20:617–627. doi: 10.1128/mcb.20.2.617-627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwai K, Mori N, Oie M, Yamamoto N, Fujii M. Human T-cell leukemia virus type 1 Tax protein activates transcription through AP-1 site by inducing DNA binding activity in T cells. Virology. 2001;279:38–46. doi: 10.1006/viro.2000.0669. [DOI] [PubMed] [Google Scholar]
- 34.Jacque J M, Mann A, Enslen H, Sharuva N, Brichacek B, Davis R J, Stevenson M. Modulation of HIV-1 infectivity by MAPK, a virion associated kinase. EMBO J. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeremias I, Debatin K M. TRAIL induces apoptosis and activation of NFkappaB. Eur Cytokine Netw. 1998;9:687–688. [PubMed] [Google Scholar]
- 36.Jung J U, Desrosiers R C. Association of viral oncoprotein, STP-C488, with cellular ras. Mol Cell Biol. 1995;15:6506–6512. doi: 10.1128/mcb.15.12.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juo P, Kuo C J, Reynold S E, Konz R F, Raingeaud J, Davis R J, Biemann H-P, Blenis J. Fas activation of the p38 mitogen-activated protein kinase signaling pathway requires ICE/CED-3 family proteases. Mol Cell Biol. 1997;17:24–35. doi: 10.1128/mcb.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King C S, Cooper J V, Moss B, Twardzik D R. Vaccinia virus growth factor stimulates tyrosine protein kinase activity of A431 cell epidermal growth factor receptors. Mol Cell Biol. 1986;6:332–336. doi: 10.1128/mcb.6.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature (London) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 40.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, Strickler J E, McLaughlin M M, Siemens I R, Fisher S M, Livi G P, White J R, Adams J L, Young P R. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature (London) 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 41.Lucia-Jandris P, Hooper J W, Fields B W. Reovirus M2 gene is associated with chromatin release from mouse L cells. J Virol. 1993;67:5339–5345. doi: 10.1128/jvi.67.9.5339-5345.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ludwig S, Ehrhardt C, Neumeier E R, Kracht M, Rapp U R, Pleschka S. Influenza virus-induced AP-1 dependent gene expression requires activation of the Jun-N-terminal kinase (JNK) signaling pathway. J Biol Chem. 2001;276:10990–10998. [PubMed] [Google Scholar]
- 43.Martin P, Vass W C, Schiller J T, Lowry D R, Velu T J. The bovine papilloma virus E5 transforming protein can stimulate the transforming activity of EGF and CSF-1 receptors. Cell. 1989;59:21–32. doi: 10.1016/0092-8674(89)90866-0. [DOI] [PubMed] [Google Scholar]
- 44.McLean T I, Bachenheimer S L. Activation of cJun N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J Virol. 1999;73:8415–8426. doi: 10.1128/jvi.73.10.8415-8426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 46.Oberhaus S M, Dermody T S, Tyler K L. Apoptosis and the cytopathic effects of reovirus. Curr Top Microbiol Immunol. 1998;233:23–49. doi: 10.1007/978-3-642-72095-6_2. [DOI] [PubMed] [Google Scholar]
- 47.Oberhaus S M, Smith R L, Clayton G H, Dermody T S, Tyler K L. Reovirus infection and tissue injury in mouse central nervous system are associated with apoptosis. J Virol. 1997;71:2100–2106. doi: 10.1128/jvi.71.3.2100-2106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poggioli G J, Keefer C, Connolly J L, Dermody T S, Tyler K L. Reovirus-induced G2/M cell cycle arrest requires ς1s and occurs in the absence of apoptosis. J Virol. 2000;74:9562–9570. doi: 10.1128/jvi.74.20.9562-9570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popik W, Pitha P M. Early activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase in response to binding of simian immunodeficiency virus to Jurkat TT cells expressing CCR5 receptor. Virology. 1998;252:210–217. doi: 10.1006/viro.1998.9466. [DOI] [PubMed] [Google Scholar]
- 50.Rodgers S E, Barton E S, Oberhaus S M, Pike B, Gibson C A, Tyler K L, Dermody T S. Reovirus-induced apoptosis of MDCK cells is not linked to viral yield and is blocked by Bcl-2. J Virol. 1997;71:2540–2546. doi: 10.1128/jvi.71.3.2540-2546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodgers S E, Connolly J L, Chappell J D, Dermody T S. Reovirus growth in cell culture does not require a full complement of viral proteins: identification of a ς1s-null mutant. J Virol. 1998;72:8597–8604. doi: 10.1128/jvi.72.11.8597-8604.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothe M, Sarma V, Dixit V M, Goeddel D V. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 53.Rouse J, Cohen P, Trigon S, Morange M, Alonso Llamazares A, Zamanillo D, Hunt T, Nebreda A R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 54.See B H, Shi Y. Adenovirus E1B 19,000-molecular weight protein activates c-Jun N-terminal kinase and c-Jun-mediated transcription. Mol Cell Biol. 1998;18:4012–4022. doi: 10.1128/mcb.18.7.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sen G C, Lengyel P. The interferon system. A birds eye view of its biochemistry. J Biol Chem. 1992;267:5017–5020. [PubMed] [Google Scholar]
- 56.Song H Y, Regnier C H, Kirschning C J, Goeddel D V, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappa B and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the MAP kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 58.Tyler K L, Squier M K T, Rodgers S E, Schneider B E, Oberhaus S M, Grdina T A, Cohen J J, Dermody T S. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein sigma 1. J Virol. 1995;69:6972–6979. doi: 10.1128/jvi.69.11.6972-6979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tyler K L, Squier M K T, Brown A L, Pike B, Willis D, Oberhaus S M, Dermody T S, Cohen J J. Linkage between reovirus-induced apoptosis and inhibition of cellular DNA synthesis: role of the S1 and M2 genes. J Virol. 1996;70:7984–7991. doi: 10.1128/jvi.70.11.7984-7991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson D J, Fortner K A, Lynch D H, Mattingly R R, Macara I G, Posada J A, Budd R C. JNK, but not MAPK, activation is associated with Fas-mediated apoptosis in human T cells. Eur J Immunol. 1996;26:989–999. doi: 10.1002/eji.1830260505. [DOI] [PubMed] [Google Scholar]
- 61.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 62.Yujuri T, Ware M, Widmann C, Oyer R, Russell D, Chan E, Zaitsu Y, Clarke P, Tyler K L, Oka Y, Fanger G R, Henson P, Johnson G L. Cell migration and c-jun NH2-terminal kinase but not NF-κB are regulated by MEK kinase 1. Proc Natl Acad Sci USA. 2000;97:7272–7277. doi: 10.1073/pnas.130176697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zachos G, Clements B, Conner J. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J Biol Chem. 1999;274:5097–5103. doi: 10.1074/jbc.274.8.5097. [DOI] [PubMed] [Google Scholar]