Abstract
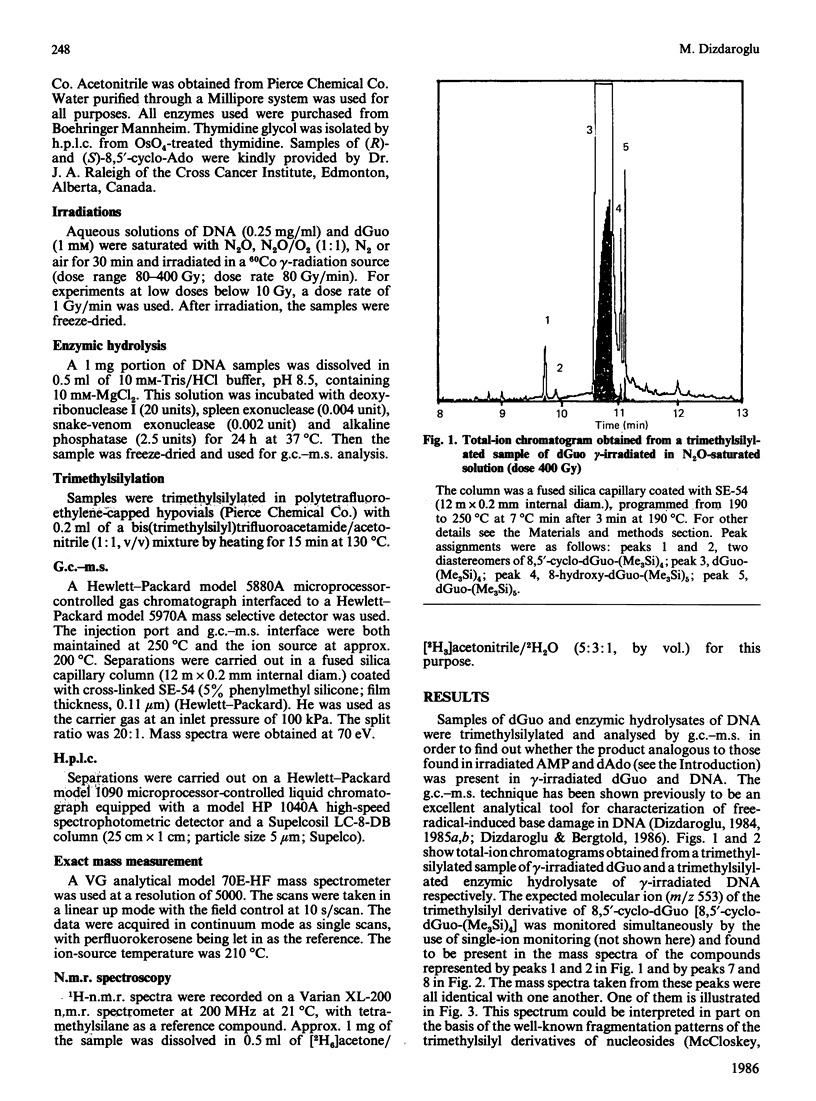
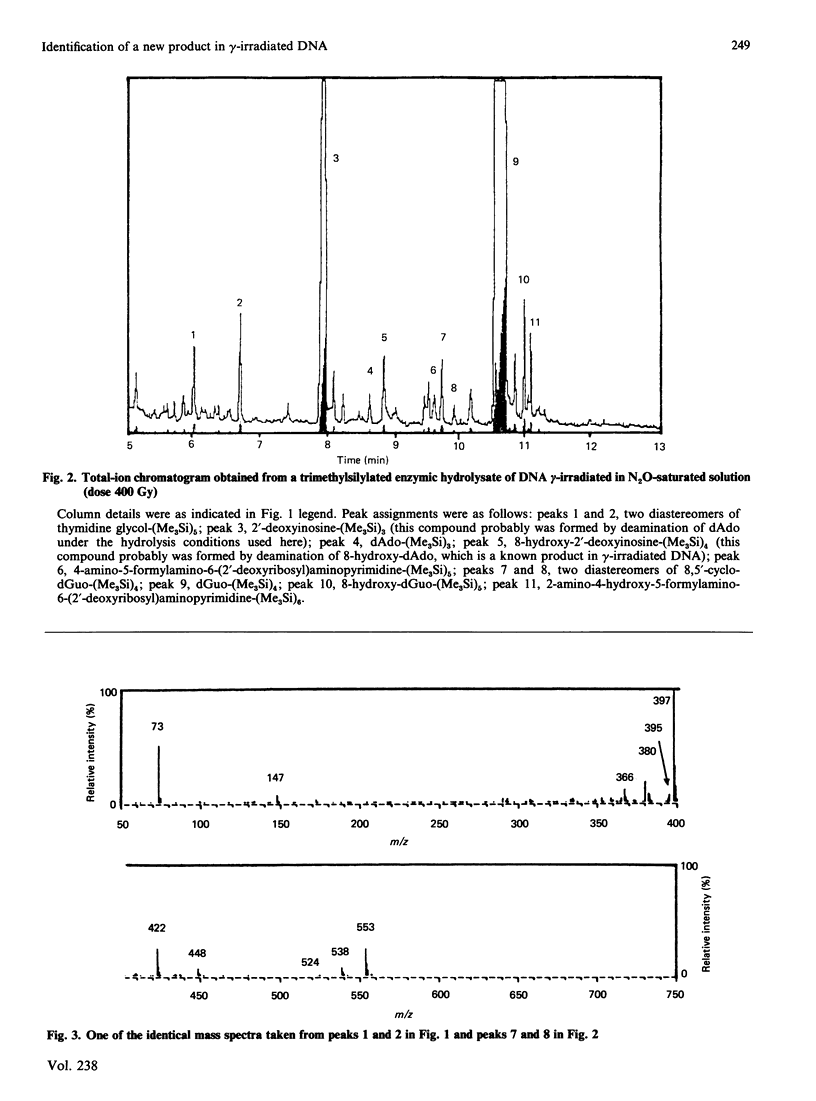
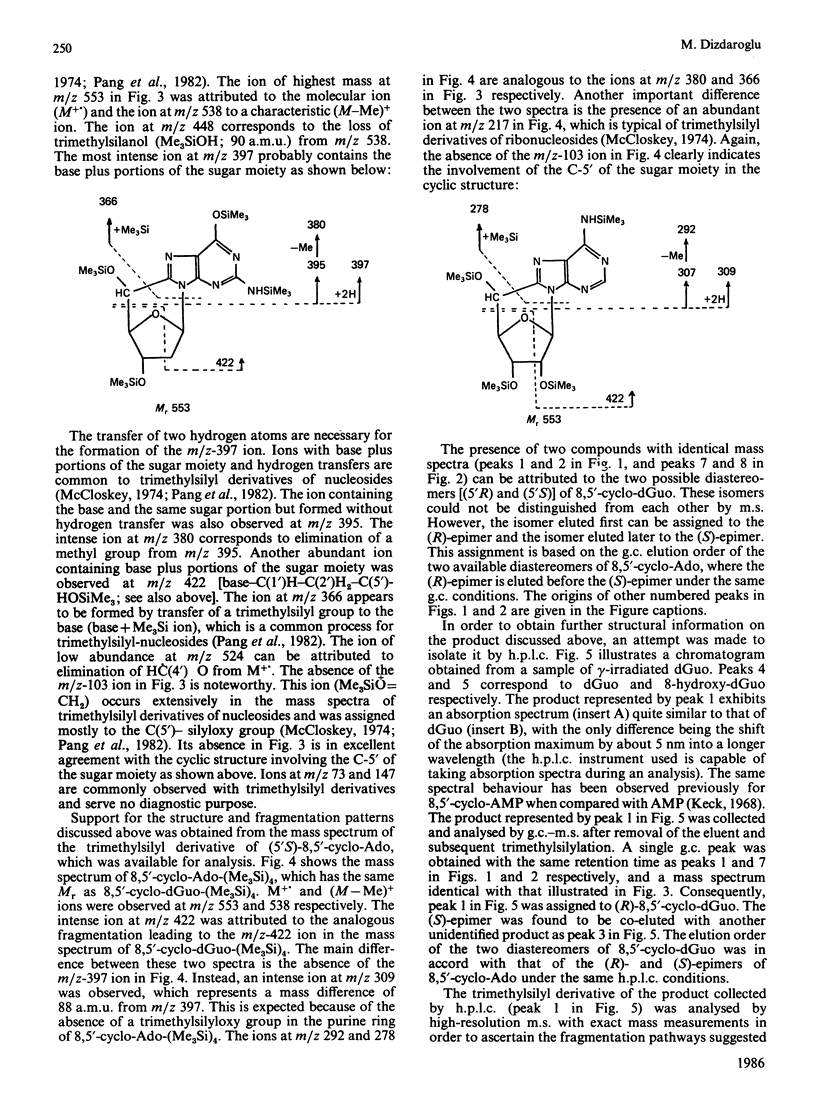
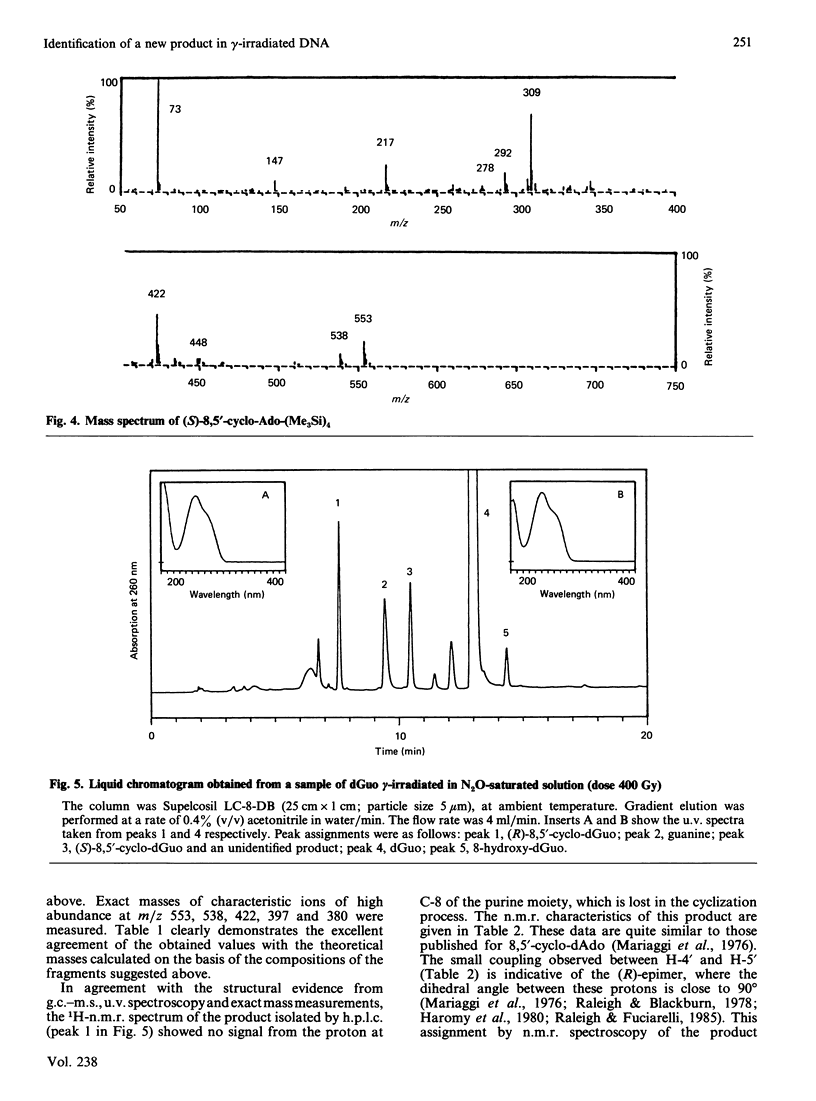
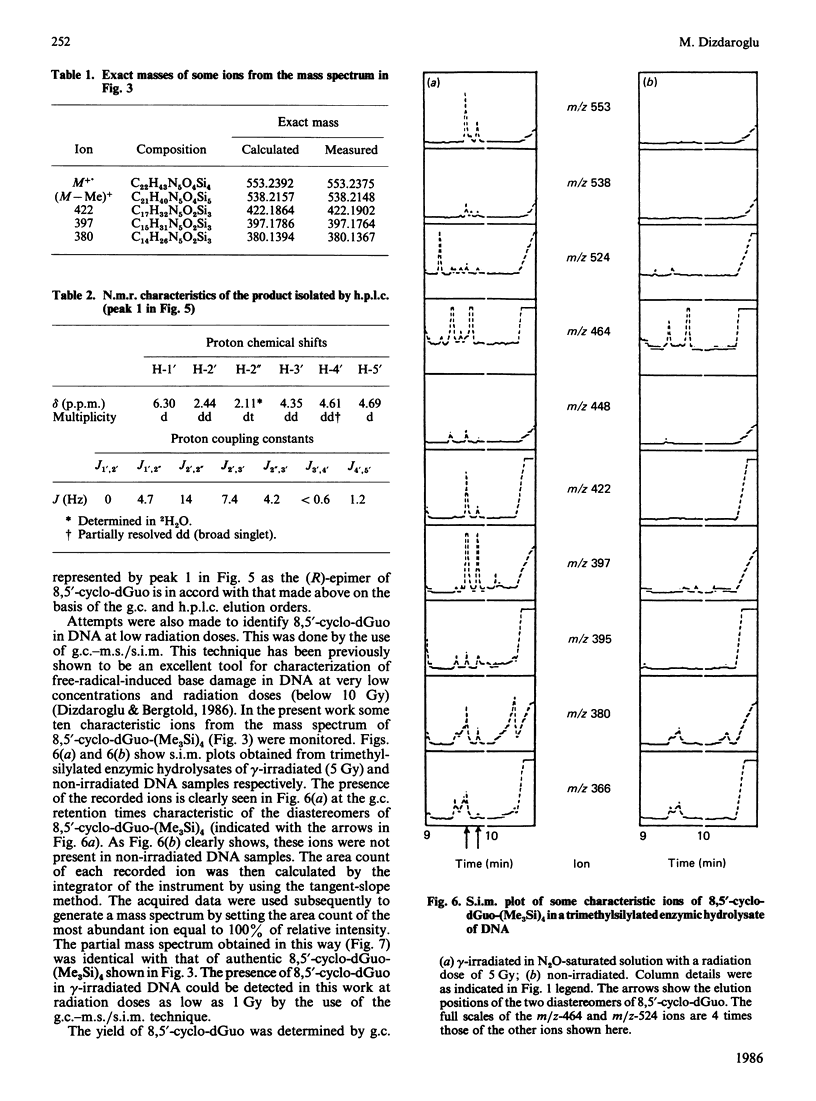
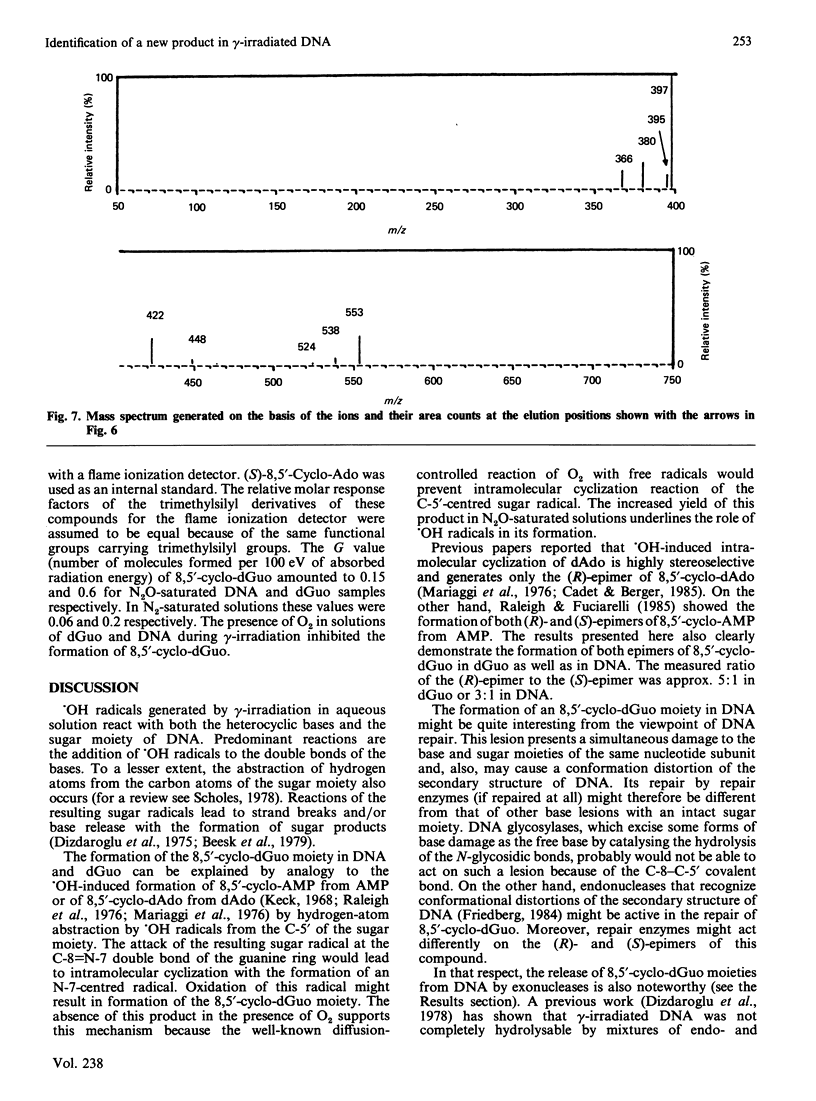
Isolation and identification of a novel .OH-induced product, namely an 8,5'-cyclo-2'-deoxyguanosine moiety, in DNA and 2'-deoxyguanosine are described. .OH radicals were generated in dilute aqueous solutions by gamma-irradiation. Analyses of 2'-deoxyguanosine and enzymic hydrolysates of DNA by gas chromatography-mass spectrometry (g.c.-m.s.) after trimethylsilylation showed the presence of 8,5-cyclo-2'-deoxyguanosine on the basis of its fragment ions. This product was isolated by h.p.l.c. Its u.v. and n.m.r. spectra taken were in agreement with the structure suggested by its mass spectrum. Exact masses of the typical ions from the mass spectrum of the trimethylsilyl derivative of this product were measured by high-resolution m.s. The values found were in excellent agreement with the theoretical mass derived from the suggested fragmentation patterns. Both (5'R)- and (5'S)-epimers of 8,5'-cyclo-2'-deoxyguanosine were observed. These two diastereomers were separated from each other by g.c. as well as by h.p.l.c. The assignment of the epimers was accomplished on the basis of the n.m.r. data. The formation of 8,5'-cyclo-2'-deoxyguanosine was suppressed by the presence of O2 in the solutions. The use of g.c.-m.s. with the selected-ion monitoring technique facilitated the detection of 8,5'-cyclo-2'-deoxyguanosine in DNA at radiation doses as low as 1 Gy. Its mechanism of formation probably involves hydrogen atom abstraction by .OH radicals from the C-5' of the 2'-deoxyguanosine moiety followed by intramolecular cyclization with the formation of a covalent bond between the C-5' and C-8 and subsequent oxidation of the resulting N-7-centred radical.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beesk F., Dizdaroglu M., Schulte-Frohlinde D., von Sonntag C. Radiation-induced DNA strand breaks in deoxygenated aqueous solutions. The formation of altered sugars as end groups. Int J Radiat Biol Relat Stud Phys Chem Med. 1979 Dec;36(6):565–576. doi: 10.1080/09553007914551391. [DOI] [PubMed] [Google Scholar]
- Cadet J., Berger M. Radiation-induced decomposition of the purine bases within DNA and related model compounds. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Feb;47(2):127–143. doi: 10.1080/09553008514550201. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. Application of capillary gas chromatography-mass spectrometry to chemical characterization of radiation-induced base damage of DNA: implications for assessing DNA repair processes. Anal Biochem. 1985 Feb 1;144(2):593–603. doi: 10.1016/0003-2697(85)90158-7. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on gamma-irradiation in aqueous solution. Biochemistry. 1985 Jul 30;24(16):4476–4481. doi: 10.1021/bi00337a032. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Hermes W., Schulte-Frohlinde D., von Sonntag C. Enzymatic digestion of DNA gamma-irradiated in aqueous solution separation of the digests by ion-exchange chromatography. Int J Radiat Biol Relat Stud Phys Chem Med. 1978 Jun;33(6):563–569. doi: 10.1080/09553007814550471. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. The use of capillary gas chromatography-mass spectrometry for identification of radiation-induced DNA base damage and DNA base-amino acid cross-links. J Chromatogr. 1984 Jul 6;295(1):103–121. doi: 10.1016/s0021-9673(01)87602-0. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., von Sonntag C., Schulte-Frohlinde D. Letter: Strand breaks and sugar release by gamma-irradiation of DNA in aqueous solution. J Am Chem Soc. 1975 Apr 16;97(8):2277–2278. doi: 10.1021/ja00841a051. [DOI] [PubMed] [Google Scholar]
- Fuciarelli A. F., Miller G. G., Raleigh J. A. An immunochemical probe for 8,5'-cycloadenosine-5'-monophosphate and its deoxy analog in irradiated nucleic acids. Radiat Res. 1985 Dec;104(3):272–283. [PubMed] [Google Scholar]
- Haromy T. P., Raleigh J., Sundaralingam M. Enzyme-bound conformations of nucleotide substrates. X-ray structure and absolute configuration of 8,5'-cycloadenosine monohydrate. Biochemistry. 1980 Apr 15;19(8):1718–1722. doi: 10.1021/bi00549a031. [DOI] [PubMed] [Google Scholar]
- Keck K. Bildung von Cyclonucleotiden bei Bestrahlung vässriger Lösungen von Purinnucleotiden. Z Naturforsch B. 1968 Aug;23(8):1034–1043. [PubMed] [Google Scholar]
- Mee L. K., Adelstein S. J. Predominance of core histones in formation of DNA--protein crosslinks in gamma-irradiated chromatin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2194–2198. doi: 10.1073/pnas.78.4.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh J. A., Blackburn B. J. Substrate conformation in 5'-AMP-utilizing enzymes: 8,5'-cycloadenosine 5'-monophosphate. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1061–1066. doi: 10.1016/0006-291x(78)91503-6. [DOI] [PubMed] [Google Scholar]
- Raleigh J. A., Kremers W., Whitehouse R. Radiation chemistry of nucleotides: 8,5'-cyclonucleotide formation and phosphate release initiated by hydroxyl radical attack on adenosine monophosphates. Radiat Res. 1976 Mar;65(3):414–422. [PubMed] [Google Scholar]
- Scholes G. Radiation effects on DNA. The Silvanus Thompson Memorial Lecture, April 1982. Br J Radiol. 1983 Apr;56(664):221–231. doi: 10.1259/0007-1285-56-664-221. [DOI] [PubMed] [Google Scholar]


