Highlights
-
•
This is the first evidence of triple infection, by hepatitis B virus (HBV), hepatitis Delta virus (HDV), and Plasmodium falciparum.
-
•
HBV, HDV and P. falciparum, are circulating in Gabon.
-
•
In patients coinfected with HBV and HDV, HDV seems to dominate significantly.
-
•
Leukocytes and lymphocytes were significantly low to triple-infected pregnant women.
-
•
Triple burden appears to have severe consequences on both pregnant women and newborns.
Keywords: Triple infection, Hepatitis B, Hepatitis Delta, Plasmodium falciparum, Prevalence, Pregnant women
Abstract
Objectives
Chronic hepatitis B virus (HBV) infection remains a major health problem worldwide. This infection is more severe when combined with hepatitis Delta virus (HDV). Moreover, Plasmodium falciparum (Pf) malaria infection during pregnancy can have severe consequences for the mother and the newborn. Importantly, the manifestation of these three infections has never been described to date.
Methods
Thus, we conducted a prospective study, between May 27, 2022, and April 15, 2023, and we investigated these three infections in 260 pregnant women aged 24 to 46 years, in Gabon and evaluated the impact on newborns. The sera were used to screen hepatitis B surface antigen (HBsAg) and Pf by determining HBsAg® ALERE rapid diagnostic test and malaria rapid diagnostic test kits. The positive sample was confirmed using MINI VIDAS® for HBV and Lambaréné method for “Pf”. The real-time-polymerase chain reaction assay was used to amplify HBV DNA and HDV RNA on Roche instrument.
Results
Our results showed that the prevalences of HBV and (Pf) infection were 4.23% (n = 11) and 34.62% (n = 90), respectively. Moreover, we found that 3.46 % (n = 9) of pregnant women infected with HBV were coinfected with HDV. The prevalence of triple infection was 1.15% (n = 3). In addition, the leukocytes and lymphocytes absolute count were significantly lower for the triple-infected pregnant women.
Conclusions
We describe for the first time the triple coinfection by HBV, HDV, and Pf, which could induce a great inflammatory reaction and high liver disorder in newborns.
Introduction
Hepatitis is an inflammation of the liver often caused by several hepatitis viruses, such as hepatitis B virus (HBV) and hepatitis Delta virus (HDV). Viral hepatitis is a major health problem worldwide. Indeed, hepatitis B infection affects 257 million people worldwide [1]. Also, about 70% of global hepatitis B cases are concentrated in Africa [2], and in Sub-Saharan Africa, HBV infection affects between 5-10% of the population. In Gabon, the prevalence of chronic HBV infection is estimated at 9.3% [3].
Moreover, HDV affects approximately 12 million people worldwide, predominating in Sub-Saharan Africa, an endemic area [4]. Concerning Gabon, no data is available to date.
Also, the HBV is a double-stranded DNA virus belonging to the Hepadnaviridae family, and hepatitis D is a negative-sense single-stranded RNA virus belonging to the genus Delta virus. HDV is a satellite virus, which depends on HBV for its replication. Notably, combined HBV-HDV infection induces more severe liver affection than HBV alone. Indeed, with chronic hepatitis B and hepatitis D infection, people have a twice-higher risk of developing cirrhosis and a thrice-higher risk of developing hepatocellular carcinoma.
The screening for hepatitis B and Delta in pregnant women is crucial because vertical transmission of HBV and HDV from infected mothers to their newborns remains a primary source of new infections. Moreover, these infected newborns have a 90% higher risk of developing chronic hepatitis.
Plasmodium falciparum (Pf), is a unicellular protozoan parasite, which is responsible for severe forms of malaria. This infection occurs mainly in Sub-Saharan Africa. Indeed, 247 million cases and 619,000 deaths were recorded [5].
Pf malaria infection during pregnancy varies from asymptomatic to severe anemia and is associated with serious maternal-fetal complications such as spontaneous abortion or prematurity. Moreover, 20% of stillbirths in Sub-Saharan Africa, have malaria or malaria-induced anemia due to gestational malaria of their mother. In Gabon, despite prevention measures, Malaria remains frequent among Gabonese populations and 28.3% of child deaths are due to malaria [6].
Currently, studies on hepatitis coinfections are limited to hepatitis B and hepatitis Delta [7,8] or hepatitis B and malaria [[9], [10], [11]]. In these latest studies, it was reported that hepatitis B and Pf coinfection could cause an inflammatory reaction in pregnant women and jaundice associated with maternal mortality. However, no impact on the newborn baby has been reported.
Interestingly, triple infections of hepatitis B, hepatitis Delta, and Pf have not yet been investigated to date. Hence, a good knowledge of this triple infection is needed to improve pregnant women's and their newborns’ health. In other words, understanding the triple burden of HBV, HDV, and Pf on pregnant women is crucial in caring for them and their babies.
In the present study, because Pf, HBV, and HDV share a common replicative tropism, we believe that the presence of Pf and HDV could regulate HBV replication. We aimed to investigate the triple burden of these pathogens in pregnant women and the consequences on newborn babies.
To do this, several biological analyses, including HBsAg, HDV RNA, and Pf antigen were carried out.
Methods
Study site and population
This study was carried out at the laboratory of the mother-child university hospital center, Fondation Jeanne Ebori (CHUMEFJE) in Libreville. Pregnant women aged 24 to 46 were recruited on their first antenatal biological check-up between May 27, 2022, and April 15, 2023. Blood samples were collected from 260 pregnant women in a dry tube and an ethylene diamine tetra-acetic (EDTA) tube, which was used to perform serological tests.
Importantly, concerning the data collection tool, a simplified and pre-established data collection sheet was used. It included patient identification (Last name(s) and first name(s); date of birth; telephone number) and gestation (age of pregnancy).
Ethical consideration
Informed consent was obtained from each pregnant woman after an explanation of the purpose, benefits, and risks of the study was provided. The scientist committee of the Mother-Child University Hospital Center Foundation, Jeanne Ebori, approved the study (Approval ID: CHUMEFJE/007/22/05/20). In addition, we declare that all methods were performed in accordance with the relevant guidelines and that individual information is strictly stored and protected. Indeed, the protocol submitted to the scientist committee of the Mother-Child University Hospital Center Foundation, Jeanne Ebori, complies with the Declaration of Helsinki, which governs the ethical principles for medical research involving human subjects, including research on identifiable human material and data.
Serological assays
The blood was collected from each of the 260 pregnant women by the venepuncture method into dry or EDTA tubes and spun at 4000 rpm for 10 minutes to separate sera from whole blood. The sera were used to screen hepatitis B surface antigen (HBsAg) and Pf parasite using rapid diagnostic test kits (RDTs). Both tests were performed following the manufacturer's recommendations. We used Determine HBsAg® ALERE RDTs with 96.4% sensitivity and 100% specificity for HBsAg. The results were confirmed using MINI VIDAS®, a compact automated immunoassay system based on the enzyme-linked fluorescent assay principle. Concerning Pf, the parasitemia of the positive sample was determined using the Lambaréné method [12]. Briefly, 10 µl of total blood was spread into areas of 10×18 mm on a microscope slide. Additionally, a slide was dried and stained with Giemsa for 15 minutes and read under a light microscope (x100). Parasitemia was determined as the number of Pf parasites per microliter of blood (p/µl).
Hemogram analyses
Blood from pregnant women was collected into the EDTA tube for a hemogram analysis via a Yumezen H550. The Yumizen H550 is a low-range 5-dif automated analyzer embedding a mini flow cell for different hematology parameter counting. Thus, leukocytes, lymphocytes, monocytes, and neutrophils were analyzed.
Molecular analysis
Polymerase chain reaction (PCR) for quantification of HBV DNA
A PCR assay was used to amplify HBV DNA. Indeed, we performed sensitive and reliable quantitative PCR (qPCR) for HBV DNA from pregnant women's serum or plasma. HBV DNA was extracted manually from serum using the QIAamp DNA Blood kit (Qiagen Hilden, Germany). Extracted viral DNA was quantified using the real-time PCR (RT-PCR) quantification method. The PCR kit includes iQTM, Sybr® Green and Supermix (BioRad) and using primers located in the HBV S gene (sense [Ss: 5′-GTG TCT GCG GCG TTT TAT CA-3′, nts 379-398 bp] and antisense [Sas: 5′-GAC AAA CGG GCA ACA TAC CTT-3′, nts 456-476 bp]) [13]. To guarantee the specificity of qPCR, the standard HBV DNA, and positive control samples were included. RT-PCR can be performed using a 96-well plate on a Roche instrument (Light Cycler 480). We tested all samples from HBsAg-positive patients.
On a 96-well plate, 20 µl of the master mix was placed and mixed in each well. Standard HBV DNA samples were included at seven points from 107 to 10 copies of HBV. This standard curve is produced with a cloned HBV genome (serotype ayw, genotype D) of known concentration determined by an optical density assay (nanodrop). In addition, three control samples were included in the assay. The plate was then sealed with an adhesive film to prevent sample evaporation during PCR processing and centrifuged at 300 rpm for 5 minutes before analysis. Finally, the plate was placed in the Light Cycler and run under thermal cycler conditions. The RT-PCR is a 40-cycle program with two steps after activation of polymerase.
Importantly, the fluorescence emission recorded at the end of each PCR cycle allows us to define a threshold cycle (Ct). Indeed, the Ct is the intersection between an amplification curve and a threshold line, it is a relative measure of the amount of DNA template in the amplification reaction. Thus, the Ct value increases with a decreasing concentration of template.
RT-PCR for quantification of HDV RNA
An RT-PCR assay was used to amplify HDV RNA. HDV RNA from pregnant women's serum or plasma was extracted using the QIAamp RNA Blood kit (Qiagen Hilden, Germany). Extracted viral RNA was quantified by nested RT-PCR using the following primers: 5413: 5′-GCC CAG GTC GGA CCG CGA GGA GGT-3′ (nts 858-881), 8276: 5′-ACA AGG AGA GGC AGG ATC ACC GAC-3′ (nts 1312-1289), 5414: 5′-GAG ATG CCA TGC CGA CCC GAA GAG-3′ (nts 883-906), and 5415: 5′-GAA GGA AGG CCC TCG AGA ACA AGA-3′ (nts 1288-1265) [13].
Statistical analysis
Statistical analysis was conducted using GraphPad Prism software version 8. The Mann-Whitney U-test was performed to compare two groups and for three groups we used the analysis of variance one-way non-parametric multiple comparisons test (Kruskal-Wallis test) coupled with the Dunn's multiple comparisons test. The threshold of significance was a P-value of 0.05.
Results
Prevalence of HBV, of Plasmodium falciparum, and their coinfection
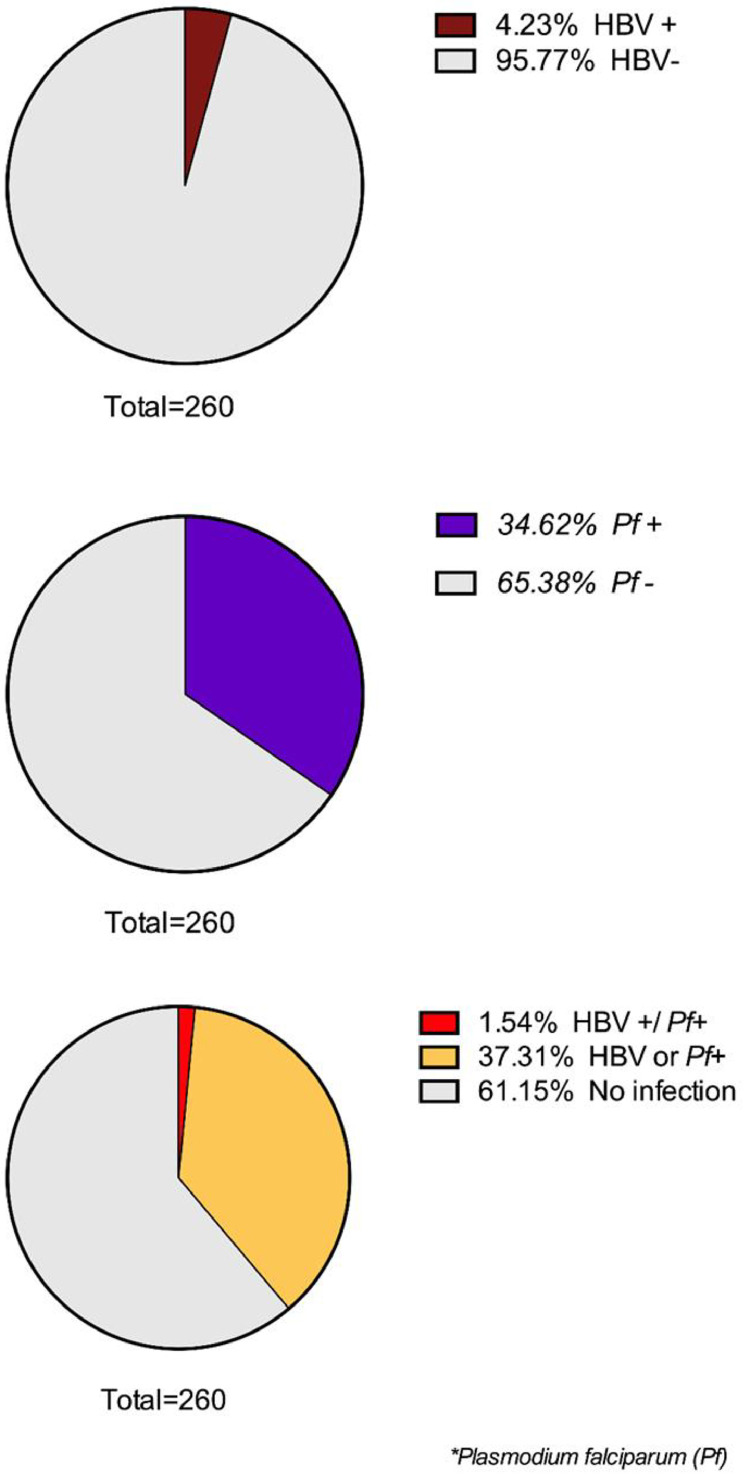
Of the 260 study pregnant women screened for HBV (using HBsAg-based tests) and malaria Pf, 11 (4.23%) were HBsAg positive, and 90 (34.62%) were positive for malaria Pf, while four women (1.54%) had a coinfection, with both hepatitis B virus and Pf (Figure 1).
Figure 1.
Proportion of HBV and Pf to pregnant woman. Brown color indicates the rate of positive hepatitis B; violet color indicates the rate of positive Pf; red color indicates HBV and Pf coinfection and yellow color indicates HBV (+) or Pf (+). HBV, hepatitis B virus; Pf, Plasmodium falciparum.
Prevalence of HBV and HDV coinfections
To evaluate the prevalence of HDV, sera from eleven (n = 11) pregnant women who were infected with HBV have been analyzed by PCR. We firstly confirmed the HBV infection by PCR to these pregnant women with HBsAg positive, and secondly, we analyzed HDV RNA by PCR assay on the same HBV-positive samples. Interestingly, we found that 3.46% (n = 9) of these pregnant women infected with hepatitis B were coinfected with hepatitis Delta (Table 1 in Supplemental files).
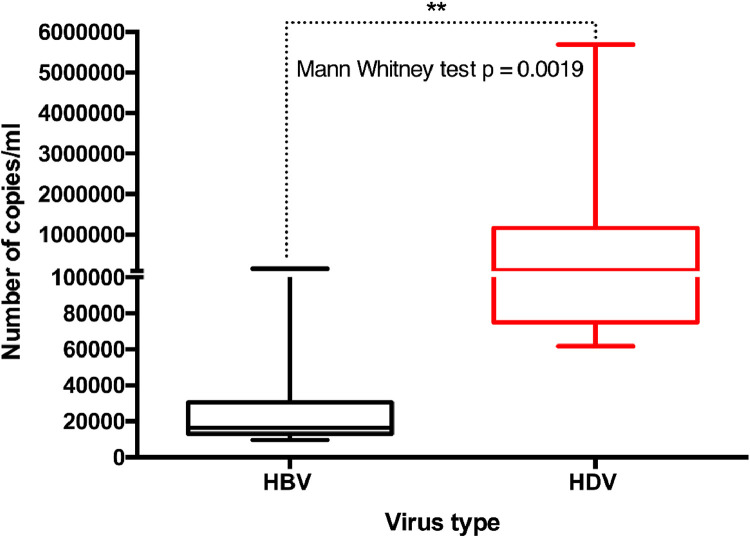
In HBV-HDV-coinfected patients, HDV seems to dominate with a significantly higher number of viral copies than HBV (Mann-Whitney test P = 0.0019) (Figure 2).
Figure 2.
Quantification of HBV and HDV viruses. Polymerase chain reaction indicates the HBV and HDV levels in pregnant women. Mann-Whitney test was performed and a significant P-value was observed (P = 0.0019). Horizontal bar with error bars represents the median and the interquartile range, respectively. HBV, hepatitis B virus; HDV, hepatitis Delta virus.
Prevalence of HBV-HDV-Plasmodium falciparum coinfection
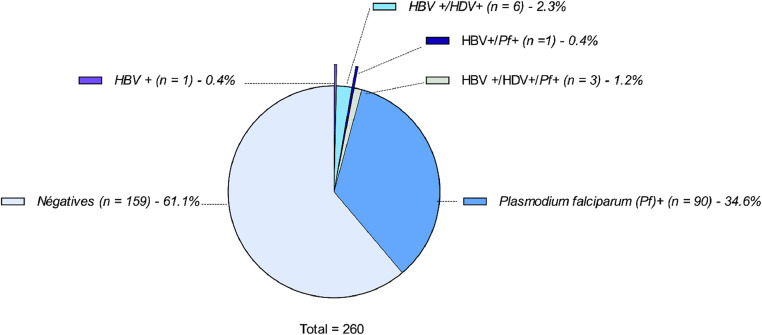
The analysis of Pf from the 9 HBV-HDV-infected pregnant women was conducted. Our results show that the prevalence of this parasite was 1.15% (n = 3) in HBV-HDV-coinfected pregnant women (Table 2 in supplemental files). Moreover, the parasitemia rate of Pf was high in two of the three triple-infected pregnant women (6930 parasites/µl and 11550 parasites/µl), respectively termed MP00003 and MN00004. Taken together, the results of screening of these three pathogens, HBV, HDV, and Pf, to 260 pregnant women are summarized in Figure 3 and indicate that (Figure 3): 90 (33.1%) were positive for Pf; six (2.3%) were positive for HBV and HDV; three (1.2%) were triple-infected with HBV, HDV, and Pf; one (0.4%) was infected by HBV and Pf; one (0.4%) was infected by HBV only.
Figure 3.
The proportions of different combinations of HBV, HDV, and Pf. Clockwise direction, the different colors indicate uninfected pregnant women; HBV (+) only; HBV (+)/HDV (+); HBV (+)/Pf (+); HBV (+)/HDV (+)/Pf (+); Pf (+) only. HBV, hepatitis B virus; HDV, hepatitis Delta virus; Pf, Plasmodium falciparum.
Thus, these results confirm that HBV, HDV, and Pf, are three pathogens circulating in Gabon (Figure 1 in Supplementary files).
Impact of this triple infection on pregnant women
Leukocytes
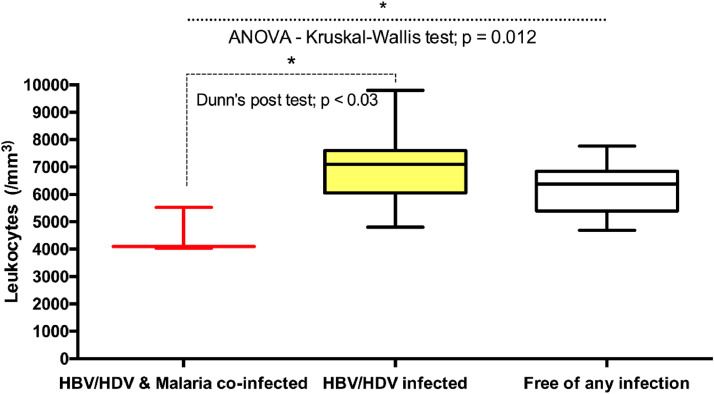
The leukocyte absolute count was significantly lower in triple-infected pregnant women than in HBV-HDV-coinfected pregnant women (P ˂0.03) and pregnant women free of any infection (P = 0.012). No significant differences in the absolute count of leukocytes were observed between the HBV-HDV-coinfected pregnant women and the pregnant women free of any infection (Figure 4).
Figure 4.
Comparison levels of Leukocytes across the different categories of pregnant women. ANOVA-Kruskal-Wallis and Dunn's post-tests were performed. Significant P-value were observed (P = 0.012 and P ˂ 0.03, respectively). Horizontal bar with error bars represents the median and the interquartile range, respectively. ANOVA, analysis of variance; HBV, hepatitis B virus; HDV, hepatitis Delta virus.
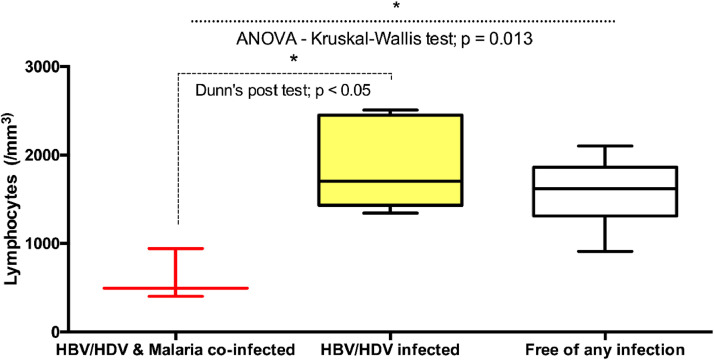
Lymphocytes
Lymphocyte absolute count was significantly lower for triple-infected pregnant women than for HBV-HDV-coinfected pregnant women (P ˂ 0.05) and pregnant women free of any infection (P = 0.013). No significant differences in the absolute count of lymphocytes were observed between the HBV-HDV-coinfected pregnant women and the pregnant women free of any infection (Figure 5).
Figure 5.
Comparison levels of Lymphocytes across the different categories of pregnant women. ANOVA-Kruskal-Wallis and Dunn's post-tests were performed. Significant P-values were observed (P = 0.013 and P ˂0.05, respectively). Horizontal bar with error bars represents the median and the interquartile range, respectively. ANOVA, analysis of variance; HBV, hepatitis B virus; HDV, hepatitis Delta virus.
Monocytes and neutrophils
Monocytes and neutrophils analysis were also carried out. No significant differences in the absolute count of monocytes and neutrophils were observed between these different groups of pregnant women (Figures 2 & 3 in Supplementary files).
Impact of this triple burden on newborn babies
The study of hemogram profiles showed that newborn babies from HBV-HDV-Pf-infected pregnant women present a higher rate of white blood cells, neutrophils, basophils, and lymphocytes than unexposed newborn babies. Additionally, the red blood cell rate was lowered in newborn babies from double and triple-infected pregnant women. Moreover, the C-reactive protein (CRP) and D Bilirubin (D Bili) were higher for these exposed newborns than for unexposed newborns (data not shown).
Additionally, this triple infection increases the burden of newborn mortality. Indeed, 2/3 (66.7%) of newborn babies from HBV-HDV-Pf-infected pregnant women were dead at birth.
Importantly, our results show no obvious link between the stage of pregnancy and the severity of these coinfections; HBV-Pf; HBV-HDV; HBV-HDV-Pf, in both the mother and the child.
Discussion
In this study, we provide the first evidence of the triple burden of HBV, HDV, and Pf in pregnant women and the consequences on newborn babies of the diseases they cause in their mothers. Indeed, no studies to date have considered these three pathogens at the same time in pregnant women.
We evaluated the prevalence of hepatitis B, hepatitis Delta, and Pf in pregnant women. Our results show that 4.23% were infected with HBV, 34.62% were positive for Pf, and 1.54% were coinfected with both HBV and Pf. These results are not surprising because Gabon is an endemic area for these pathogens, which remain a real public health problem.
Additionally, the hepatitis B prevalence in our study is comparable to that obtained by other authors who found 4.2% of pregnant women in Ghana and 3.9% of pregnant women in southern Gabon [9,14]. The most prevalent infection was Pf malaria, with 34.62% as in other studies conducted in Ghana in Africa [9,10]. The prevalence of Pf malaria was higher than that obtained in other African studies [15,16].
Furthermore, this prevalence was low compared to a previous study on pregnant women in Gabon [17]. This decrease could be due to an increase in pregnant women's use of preventive measures such as insecticides and impregnated mosquito nets. Moreover, the HBV and Pf (1.54%) coinfection is unsurprising because Gabon is an endemic area for both pathogens.
Significantly, HDV infection is screened in HBV-positive patients because HDV is a satellite virus that depends on HBV for its replication [18]. However, testing for HDV virus is limited, and little data on hepatitis Delta for several regions is available. Moreover, molecular detection of HDV virus is performed by RT-PCR, a technique that is not easily accessible.
We found that 2.3% of HBV-infected pregnant women were coinfected with hepatitis Delta virus. Additionally, in HBV-HDV-coinfected patients, HDV seems to dominate HBV.
This high prevalence found in this study may be misleading because the present study was performed on a small number of HBV-infected pregnant women (n = 11). Furthermore, this high prevalence of HDV means that this pathogen strongly circulates in Gabon, and it remains higher than that found in other regions such as in Israel at 18% (n = 4/22), in Burkina Faso at 3.4% (n = 4/117) [7,19].
Interestingly, our molecular analyses show a significant predominance of HDV in the three HBV-infected pregnant women. The Hepatitis Delta virus seems to take over the HBV into the same infected hepatocyte, certainly due to the great virulence of the HDV. Indeed, an old study already suggested that HDV acts as a dominant virus in HBV [20].
In addition, our results show that, among these pregnant women infected, only three were triple coinfected with HBV, HDV, and Pf. Our analysis of white blood cells revealed that triple-infected women have low leukocytes (leukopenia) and lymphocytes (lymphopenia), compared to other infection groups. These results on leukopenia and lymphopenia were similar to those obtained by Zehentmeier et al. [21]. Also, a non-significant difference was observed in the monocytes and neutrophils of all groups. Importantly, this triple infection seems to induce a great inflammatory reaction (increased CRP) and high liver disorder (increased D Bili) in newborns from HBV-HDV-Pf-infected women. Indeed, CRP was 64 times above average for the triple-exposed newborn baby, and D Bili was 34 times higher for the HBV-HDV-Pf-exposed newborn than for the HBV-Pf-exposed newborn.
On the other hand, the CRP and D Bili levels were negatively correlated with HBV-HDV and Pf load. Thus, this triple infection increases the burden of newborn mortality.
Importantly, we provide the first evidence that HBV-HDV-Pf pathogens can infect a single individual simultaneously.
This is the first study describing the triple burden of these three pathogens on pregnant women and its consequences on newborn babies. Also, these three pathogens seem to induce a great inflammatory reaction and high liver disorder, contributing to the newborn's death.
Conclusion
Our results show a high HDV prevalence in HBV-HDV-coinfected pregnant women in Gabon. We also found the first evidence of triple infection, by HBV, HDV, and Pf in pregnant women. This triple burden appears to have severe consequences on the survival of newborns. Based on our results, we invite the government to implement a policy for diagnosing this triple infection in pregnant women to guarantee their health and that of their newborns.
Declarations of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval and consent to participate
Informed consent was obtained from each pregnant woman before their enrollment in the study. The hepatitis B virus-infected pregnant women were referred to the hepatologist for treatment. The institutional scientist committee of the Mother-Child University Hospital Center Foundation Jeanne Ebori, approved the study (Approval ID: CHUMEFJE/007/22/05/20).
Acknowledgments
This work was supported by CHU Mère-Enfant Fondation Jeanne Ebori and INSERM.
Author contributions
ASAE, OMK, CC, and IPMB, collected samples, conducted the experiment, and interpreted the results. LM, KMWAM, EIB, OMN, and ACMS collected samples and contributed to the interpretation of the data. PEIB, IC, and JFDS contributed to the interpretation of the data and a critical discussion of the paper, ASAE participated in the writing of the paper, and BN designed the experiment and wrote the paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2024.100447.
Appendix. Supplementary materials
References
- 1.Reardon JM, O'Connor SM, Njau JD, Lam EK, Staton CA, Cookson ST. Cost-effectiveness of birth-dose hepatitis B vaccination among refugee populations in the African region: a series of case studies. Confl Health. 2019;13:5. doi: 10.1186/s13031-019-0188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Word Health Organization . World Health Organization; Geneva: 2022. report 2022. [Google Scholar]
- 3.Bivigou-Mboumba B, Rouet F, Mouinga-Ondeme A, Deleplancque L, Sica J, Ndjoyi-Mbiguino A, et al. Hepatitis B, C, and E infection among HIV-infected patients in Franceville, Gabon: retrospective cross-sectional study. Med Sante Trop. 2017;27:274–280. doi: 10.1684/mst.2017.0698. [DOI] [PubMed] [Google Scholar]
- 4.Miao Z, Pan Q. Revisiting the estimation of hepatitis D global prevalence. J Hepatol. 2020;73:1279–1280. doi: 10.1016/j.jhep.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; Geneva: 2022. World Malaria Report 2022. [Google Scholar]
- 6.Word Health Organization . World Health Organization; Geneva: 2023. report 2023. [Google Scholar]
- 7.Shirazi R, Ram D, Rakovsky A, Bucris E, Gozlan Y, Lustig Y, et al. Characterization of hepatitis B and delta coinfection in Israel. BMC Infect Dis. 2018;18:97. doi: 10.1186/s12879-018-3008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razavi HA, Buti M, Terrault NA, Zeuzem S, Yurdaydin C, Tanaka J, et al. Hepatitis D double reflex testing of all hepatitis B carriers in low-HBV- and high-HBV/HDV-prevalence countries. J Hepatol. 2023;79:576–580. doi: 10.1016/j.jhep.2023.02.041. [DOI] [PubMed] [Google Scholar]
- 9.Helegbe GK, Aryee PA, Mohammed BS, Wemakor A, Kolbila D, Abubakari AW, et al. Seroprevalence of malaria and hepatitis B coinfection among pregnant women in tamale metropolis of Ghana: a cross-sectional study. Can J Infect Dis Med Microbiol. 2018;2018 doi: 10.1155/2018/5610981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anabire NG, Aryee PA, Abdul-Karim A, Abdulai IB, Quaye O, Awandare GA, et al. Prevalence of Malaria and hepatitis B among pregnant women in Northern Ghana: comparing RDTs with PCR. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omatola CA, Okolo MO. Hepatitis B and asymptomatic malaria infection among pregnant women in a Semiurban Community of North-Central Nigeria. J Environ Public Health. 2021;2021 doi: 10.1155/2021/9996885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Planche T, Krishna S, Kombila M, Engel K, Faucher JF, Ngou-Milama E, et al. Comparison of methods for the rapid laboratory assessment of children with Malaria. Am J Trop Med Hyg. 2001;65:599–602. doi: 10.4269/ajtmh.2001.65.599. [DOI] [PubMed] [Google Scholar]
- 13.Chemin I, Pujol FH, Scholtès C, Loureiro CL, Amirache F, Levrero M, et al. Preliminary evidence for hepatitis delta virus exposure in patients who are apparently not infected with hepatitis B virus. Hepatology. 2021;73:861–864. doi: 10.1002/hep.31453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koumba Mavoungou DS, N'dilimabaka N, Elguero E, Kombila LB, Diane A, Koumba Moukouama SE, et al. Burden of hepatitis B virus infection in pregnant women attending antenatal clinics in the southern Gabon. IJID Reg. 2023;9:32–37. doi: 10.1016/j.ijregi.2023.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valente B, Campos PA, do Rosário VE, Varandas L, Silveira H. Prevalence and risk factors of Plasmodium falciparum infections in pregnant women of Luanda, Angola. Trop Med Int Health. 2011;16:1206–1214. doi: 10.1111/j.1365-3156.2011.02830.x. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Pérez G, Lansana DP, Omeonga S, Gupta H, Breeze-Barry B, González R, et al. Prevalence of Plasmodium falciparum infection among pregnant women at first antenatal visit in post-Ebola Monrovia, Liberia. Malar J. 2018;17:357. doi: 10.1186/s12936-018-2506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouyou-Akotet MK, Ionete-Collard DE, Mabika-Manfoumbi M, Kendjo E, Matsiegui PB, Mavoungou E, et al. Prevalence of Plasmodium falciparum infection in pregnant women in Gabon. Malar J. 2003;2:18. doi: 10.1186/1475-2875-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turon-Lagot V, Saviano A, Schuster C, Verrier ÉR. Hepatitis D virus: viral cycle and new therapeutic approaches. Virologie (Montrouge) 2019;23:149–159. doi: 10.1684/vir.2019.0776. [DOI] [PubMed] [Google Scholar]
- 19.Sanou AM, Benkirane K, Tinto B, Cissé A, Sagna T, Ilboudo AK, et al. Prevalence of hepatitis B virus and hepatitis D virus Coinfection in Western Burkina Faso and molecular characterization of the detected virus strains. Int J Infect Dis. 2018;70:15–19. doi: 10.1016/j.ijid.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Mathurin P, Thibault V, Kadidja K, Ganne-Carrié N, Moussalli J, El Younsi M, et al. Replication status and histological features of patients with triple (B, C, D) and dual (B, C) hepatic infections. J Viral Hepat. 2000;7:15–22. doi: 10.1046/j.1365-2893.2000.00195.x. [DOI] [PubMed] [Google Scholar]
- 21.Zehentmeier S, Lim VY, Ma Y, Fossati J, Ito T, Jiang Y, et al. Dysregulated stem cell niches and altered lymphocyte recirculation cause B and T cell lymphopenia in WHIM syndrome. Sci Immunol. 2022;7:eabo3170. doi: 10.1126/sciimmunol.abo3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.