Abstract
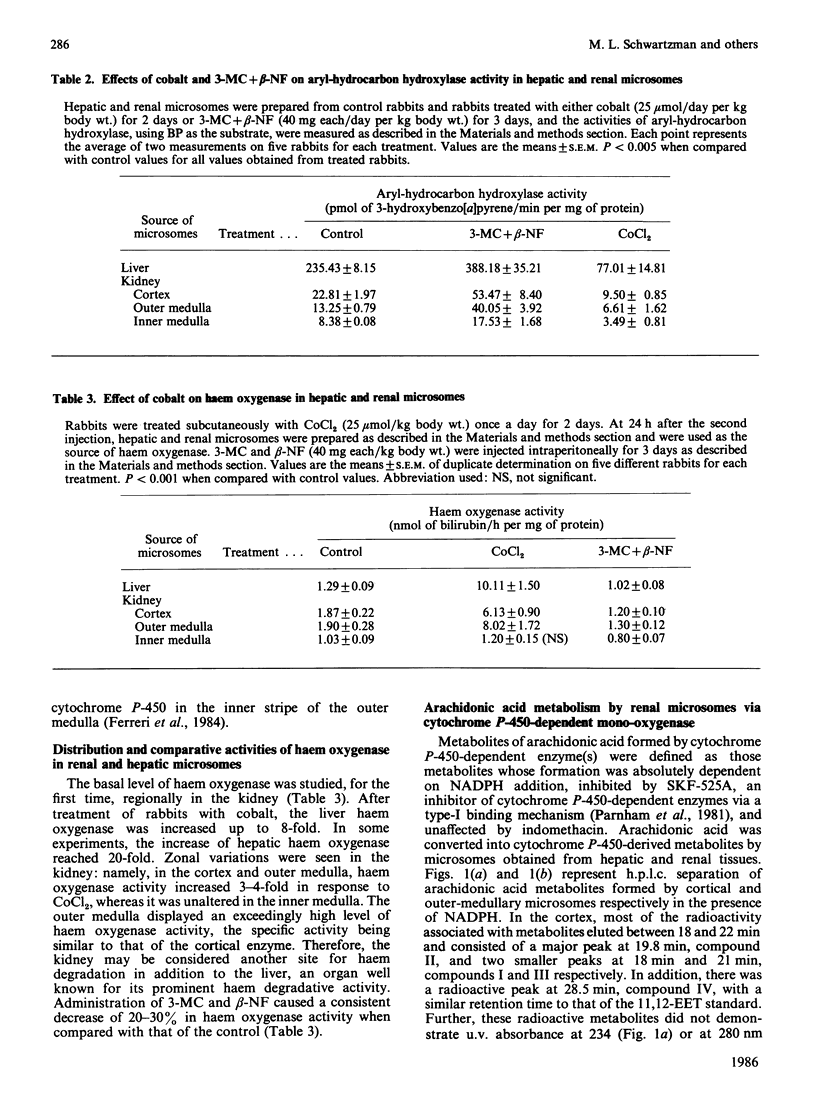
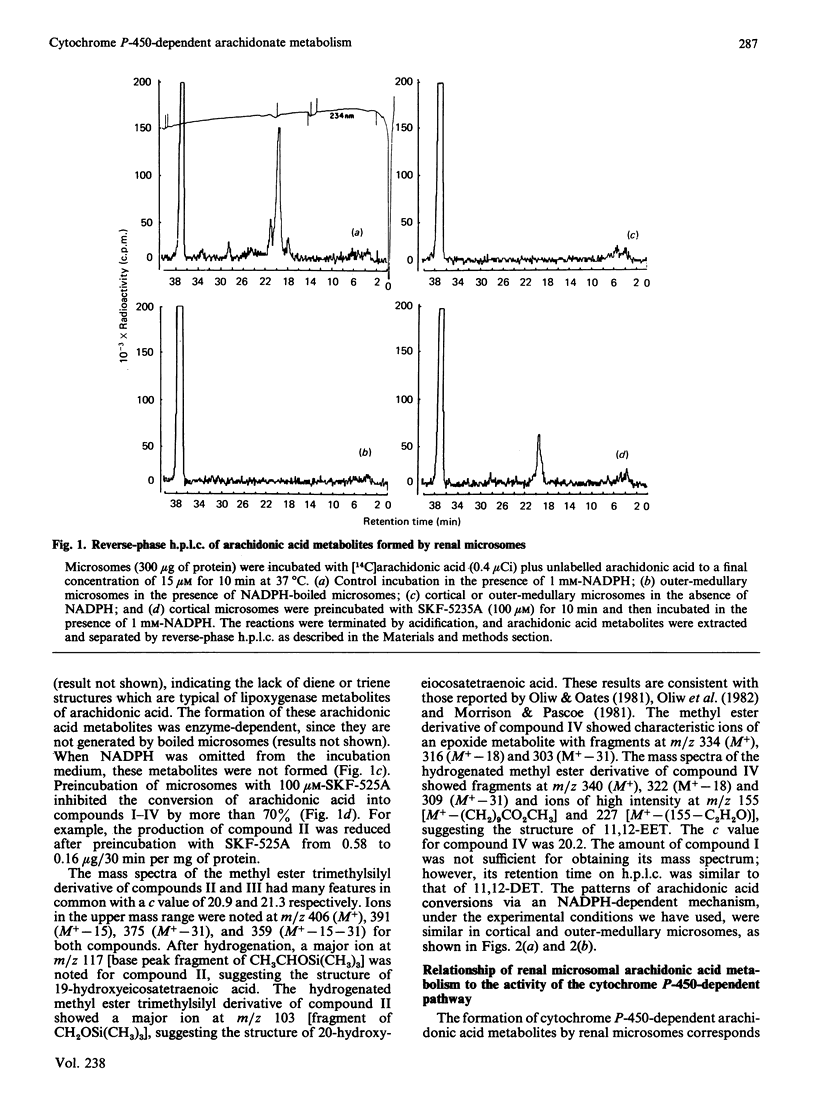
Renal microsomal cytochrome P-450-dependent arachidonic acid metabolism was correlated with the level of cytochrome P-450 in the rabbit kidney. Cobalt, an inducer of haem oxygenase, reduced cytochrome P-450 in both the cortex and medulla in association with a 2-fold decrease in aryl-hydrocarbon hydroxylase, an index of cytochrome P-450 activity, and a similar decrease in the formation of cytochrome P-450-dependent arachidonic acid metabolites by renal microsomes (microsomal fractions). Formation of the latter was absolutely dependent on NADPH addition and was prevented by SKF-525A, an inhibitor of cytochrome P-450-dependent enzymes. Arachidonate metabolites of cortical microsomes were identified by g.c.-m.s. as 20- and 19-hydroxyeicosatetraenoic acid, 11,12-epoxyeicosatrienoic acid and 11,12-dihydroxyeicosatrienoic acid. The profile of arachidonic acid metabolites was the same for the medullary microsomes. Induction of cytochrome P-450 by 3-methylcholanthrene and beta-naphthoflavone increased cytochrome P-450 content and aryl-hydrocarbon hydroxylase activity by 2-fold in the cortex and medulla, and this correlated with a 2-fold increase in arachidonic acid metabolites via the cytochrome P-450 pathway. These changes can also be demonstrated in cells isolated from the medullary segment of the thick ascending limb of the loop of Henle, which previously have been shown to metabolize arachidonic acid specifically via the cytochrome P-450-dependent pathway. The specific activity for the formation of arachidonic acid metabolites by this pathway is higher in the kidney than in the liver, the highest activity being in the outer medulla, namely 7.9 microgram as against 2.5 micrograms of arachidonic acid transformed/30 min per nmol of cytochrome P-450 for microsomes obtained from outer medulla and liver respectively. These findings are consistent with high levels of cytochrome P-450 isoenzyme(s), specific for arachidonic acid metabolism, primarily localized in the outer medulla.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders M. W. Metabolism of drugs by the kidney. Kidney Int. 1980 Nov;18(5):636–647. doi: 10.1038/ki.1980.181. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E. Cytochrome P-450 heme and the regulation of hepatic heme oxygenase activity. Arch Biochem Biophys. 1976 Sep;176(1):91–102. doi: 10.1016/0003-9861(76)90144-2. [DOI] [PubMed] [Google Scholar]
- Capdevila J., Chacos N., Falck J. R., Manna S., Negro-Vilar A., Ojeda S. R. Novel hypothalamic arachidonate products stimulate somatostatin release from the median eminence. Endocrinology. 1983 Jul;113(1):421–423. doi: 10.1210/endo-113-1-421. [DOI] [PubMed] [Google Scholar]
- Capdevila J., Chacos N., Werringloer J., Prough R. A., Estabrook R. W. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5362–5366. doi: 10.1073/pnas.78.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J., Marnett L. J., Chacos N., Prough R. A., Estabrook R. W. Cytochrome P-450-dependent oxygenation of arachidonic acid to hydroxyicosatetraenoic acids. Proc Natl Acad Sci U S A. 1982 Feb;79(3):767–770. doi: 10.1073/pnas.79.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J., Parkhill L., Chacos N., Okita R., Masters B. S., Estabrook R. W. The oxidative metabolism of arachidonic acid by purified cytochromes P-450. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1357–1363. doi: 10.1016/0006-291x(81)91597-7. [DOI] [PubMed] [Google Scholar]
- Dees J. H., Coe L. D., Yasukochi Y., Masters B. S. Immunofluorescence of NADPH-cytochrome c (P-450) reductase in rat and minipig tissues injected with phenobarbital. Science. 1980 Jun 27;208(4451):1473–1475. doi: 10.1126/science.6770464. [DOI] [PubMed] [Google Scholar]
- Dees J. H., Masters B. S., Muller-Eberhard U., Johnson E. F. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin and phenobarbital on the occurrence and distribution of four cytochrome P-450 isozymes in rabbit kidney, lung, and liver. Cancer Res. 1982 Apr;42(4):1423–1432. [PubMed] [Google Scholar]
- Endou H. Cytochrome P-450 monooxygenase system in the rabbit kidney: its intranephron localization and its induction. Jpn J Pharmacol. 1983 Apr;33(2):423–433. doi: 10.1254/jjp.33.423. [DOI] [PubMed] [Google Scholar]
- Ferreri N. R., Schwartzman M., Ibraham N. G., Chander P. N., McGiff J. C. Arachidonic acid metabolism in a cell suspension isolated from rabbit renal outer medulla. J Pharmacol Exp Ther. 1984 Nov;231(2):441–448. [PubMed] [Google Scholar]
- Ibraham N. G., Friedland M. L., Levere R. D. Heme metabolism in erythroid and hepatic cells. Prog Hematol. 1983;13:75–130. [PubMed] [Google Scholar]
- Ibrahim N. G., Hoffstein S. T., Freedman M. L. Induction of liver cell haem oxygenase in iron-overloaded rats. Biochem J. 1979 May 15;180(2):257–263. doi: 10.1042/bj1800257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim N. G., Nelson J. C., Levere R. D. Control of delta-aminolaevulinate synthase and haem oxygenase in chronic-iron-overloaded rats. Biochem J. 1981 Oct 15;200(1):35–42. doi: 10.1042/bj2000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lu A. Y., West S. B. Multiplicity of mammalian microsomal cytochromes P-45. Pharmacol Rev. 1979 Dec;31(4):277–295. [PubMed] [Google Scholar]
- Maines M. D., Ibrahim N. G., Kappas A. Solubilization and partial purification of heme oxygenase from rat liver. J Biol Chem. 1977 Aug 25;252(16):5900–5903. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt stimulation of heme degradation in the liver. Dissociation of microsomal oxidation of heme from cytochrome P-450. J Biol Chem. 1975 Jun 10;250(11):4171–4177. [PubMed] [Google Scholar]
- Morrison A. R., Pascoe N. Metabolism of arachidonate through NADPH-dependent oxygenase of renal cortex. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7375–7378. doi: 10.1073/pnas.78.12.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzyme. J Biol Chem. 1968 Dec 10;243(23):6242–6249. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Oliw E. H., Guengerich F. P., Oates J. A. Oxygenation of arachidonic acid by hepatic monooxygenases. Isolation and metabolism of four epoxide intermediates. J Biol Chem. 1982 Apr 10;257(7):3771–3781. [PubMed] [Google Scholar]
- Oliw E. H., Oates J. A. Rabbit renal cortical microsomes metabolize arachidonic acid to trihydroxyeicosatrienoic acids. Prostaglandins. 1981 Dec;22(6):863–871. doi: 10.1016/0090-6980(81)90017-4. [DOI] [PubMed] [Google Scholar]
- Parnham M. J., Bragt P. C., Bast A., Zijlstra F. J. Comparison of the effects of inhibitors of cytochrome P-450-mediated reactions on human platelet aggregation and arachidonic acid metabolism. Biochim Biophys Acta. 1981 Oct 12;677(2):165–173. doi: 10.1016/0304-4165(81)90081-7. [DOI] [PubMed] [Google Scholar]
- Schwartzman M., Ferreri N. R., Carroll M. A., Songu-Mize E., McGiff J. C. Renal cytochrome P450-related arachidonate metabolite inhibits (Na+ + K+)ATPase. Nature. 1985 Apr 18;314(6012):620–622. doi: 10.1038/314620a0. [DOI] [PubMed] [Google Scholar]
- Snyder G. D., Capdevila J., Chacos N., Manna S., Falck J. R. Action of luteinizing hormone-releasing hormone: involvement of novel arachidonic acid metabolites. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3504–3507. doi: 10.1073/pnas.80.11.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Zenser T. V., Mattammal M. B., Davis B. B. Differential distribution of the mixed-function oxidase activities in rabbit kidney. J Pharmacol Exp Ther. 1978 Dec;207(3):719–725. [PubMed] [Google Scholar]


