Abstract
The development of tissue-selective virus-based vectors requires a better understanding of the role of receptors in gene transfer in vivo, both to rid the vectors of their native tropism and to introduce new specificity. CAR and αv integrins have been identified as the primary cell surface components that interact with adenovirus type 5 (Ad5)-based vectors during in vitro transduction. We have constructed a set of four vectors, which individually retain the wild-type cell interactions, lack CAR binding, lack αv integrin binding, or lack both CAR and αv integrin binding. These vectors have been used to examine the roles of CAR and αv integrin in determining the tropism of Ad vectors in a mouse model following intrajugular or intramuscular injection. CAR was found to play a significant role in liver transduction. The absence of CAR binding alone, however, had little effect on the low level of expression from Ad in other tissues. Binding of αv integrins appeared to have more influence than did binding of CAR in promoting the expression in these tissues and was also found to be important in liver transduction by Ad vectors. An effect of the penton base modification was a reduction in the number of vector genomes that could be detected in several tissues. In the liver, where CAR binding is important, combining defects in CAR and αv integrin binding was essential to effectively reduce the high level of expression from Ad vectors. While there may be differences in Ad vector tropism among species, our results indicate that both CAR and αv integrins can impact vector distribution in vivo. Disruption of both CAR and αv integrin interactions may be critical for effectively reducing native tropism and enhancing the efficacy of specific targeting ligands in redirecting Ad vectors to target tissues.
Adenoviruses (Ad) have been widely used for gene therapy applications because they can accommodate relatively large transgenes, be manufactured to high titer and purity, and transduce a relatively wide range of cells independent of their replicative state. Applications for Ad vectors have been limited by the native tropism of the virus, which restricts their ability to transduce some tissues of interest. In addition, the native tropism-dependent transduction of nontarget tissues may limit applications because of undesired side effects. Therefore, development of Ad vectors that could target specific tissues following systemic or minimally invasive administration would greatly enhance their therapeutic potential and expand their application.
Two interactions between Ad coat proteins and cell surface receptors have been identified as determinants of transduction by Ad vectors. The knob domain of the fiber protein binds to the CAR protein, and an RGD motif within a loop of the penton base protein binds to αv integrins. As a target for high-affinity binding by the fiber knob, the CAR protein plays a prominent role in transduction of cells in vitro (10, 11). This interaction is conserved among the majority of Ad serotypes (19). The murine homologue of CAR is highly conserved in sequence relative to the human protein and functions as a receptor for human Ad (4, 22). CAR is expressed in a broad range of human tissues, including heart, prostate, pancreas, brain, kidney, liver, and lung, while in mice CAR is expressed in similar tissues but its expression in the liver is more prominent (4, 7, 22). In mice the liver is the major tissue transduced following intravenous administration of Ad vectors and is also the site where fiber knobs accumulate when the soluble protein is injected into the bloodstream (33). On the other hand, absence of CAR expression on the surface of human airway epithelia may limit gene delivery to this tissue (24, 32). Nevertheless, the role of CAR in determining which tissues are transduced is not clear (7).
The interaction between penton base and αv integrins was identified for its role in promoting the internalization of virus after it attaches to the cell surface (27). Integrins direct Ad to clathrin-mediated endocytosis and may have additional functions in promoting escape from the endosome (14). The integrin interaction does not appear essential for Ad transduction in vitro (3). Nevertheless, some of the current limitations of Ad vectors in vivo have been attributed to absence or inappropriate distribution of αv integrin expression in the target tissue (8, 16).
We constructed a panel of four Ad vectors which bind CAR and integrins, bind CAR only, bind integrins only, or bind neither CAR nor integrins. These tropism-modified vectors are still capable of being produced to high titer in a specialized production cell line. The panel of vectors was used to examine the role of these interactions in determining the native tropism of Ad in an animal model. Our results indicate that the vector doubly ablated for both CAR and αv integrin binding represents the best candidate for a base vector that could be redirected by incorporation of specific targeting ligands.
MATERIALS AND METHODS
Cells.
A549, Ramos, and CHO cells were obtained from the American Type Culture Collection (Manassas, Va.) and cultured under standard conditions. 293-HA cells have been described previously (6). AE25 is an E1-complementing cell line derived from A549 cells. AE25-HA cells were obtained by Geneticin (Life Technologies, Gaithersburg, Md.) selection of AE25 cells following transduction with a retrovirus encoding a membrane-anchored single-chain antibody reactive with an influenza virus hemagglutinin epitope (HA) (6). The retrovirus stock was produced by transfection of RetroPackPT67 cells (Clontech, Palo Alto, Calif.) with a pLNCX-based plasmid (Clontech) expressing the anti-HA sFv.
Ad vector construction AdL is an E1- and E3-deleted Ad5-based vector that carries the luciferase transgene in the E1 region under the control of the cytomegalovirus promoter. AdL.F* has a modified AB loop in fiber (R412S, A415G, E416G, and K417G) that abolishes CAR binding (20). In addition, AdL.F* has an insertion in the HI loop, between residues 543 and 544, of the sequence SRGFKSYPYDVPDYAG, where the HA epitope is underlined. Oligonucleotide-mediated mutagenesis of the Ad5 fiber was performed on a pNS-based vector (28) utilizing the QuikChange kit (Stratagene, La Jolla, Calif.) as specified by the manufacturer. The primers R415-417(S) and R415-417(A), with sequences TCTCCTAACTGTAGCCTAAATGGAGGGGGTGATGCTAAACTC and GAGTTTAGCATCACCCCCTCCATTTAGGCTACAGTTAGGAGA, respectively, were used first to modify the AB loop. The resultant plasmid was mutagenized a second time with the primers HA-TAGS (GGTACACAGGAAACAGGGTCTAGAGGAT T TAAATCTGGATCCTAC C C CTACGACGTGCCCGACTACGCCGGCGACACAACTCCAAGTGCA) and HA-TAGa (TGCAC T TGGAG T TG T G T CGCCGGCG T AGTC GGGCACG T CGTAGGGG TAGGATCCAGATTTAAATCCTCTAGACCCTGTTTCCTGTGTACC). The modified fiber was then isolated as a NheI-MunI fragment and cloned into pAS(Sc)E3(10X), which carries Ad5 sequences extending from the AgeI site at map unit 73 to the right end of the genome minus the XbaI fragment in E3. A DrdI fragment from this plasmid was used to construct the complete vector genome of AdL.F* by recombination in Escherichia coli.
Replacement of the penton base RGD by an SpeI linker has been described previously (26). To produce AdL.PB*, the HA epitope was inserted into this SpeI site by ligation with the annealed primers SpeHAs (CTAGTTATCCATATGATGTTCCAGATTATGCTT) and SpeHAa (CTAGAAGCATAATCTGGAACATCATATGGATAA). As a result, the penton base protein in this construct has TSYPYDVPDYASS in place of the wild-type HAIRGDTF sequence. The modified penton base was recombined into an Ad vector plasmid in E. coli. To generate AdL.PB*F*, the modified fiber described above was recombined into AdL.PB*.
Vector genomes were excised from plasmids by PacI digestion, and cut DNA was transfected into 293 cells (AdL and AdL.PB*) or 293-HA cells (AdL.F* and AdL.PB*F*) using calcium phosphate. Cell lysates were passaged to produce vector stocks, and vectors were purified by three successive bandings on cesium and then stored at −80°C.
In vitro transduction assays.
Transduction assays were performed on 105 cells. The cells were preincubated with purified fiber protein (5 μg/ml), purified penton base (100 μg/ml), a combination of the two, or medium alone for 60 min (18). The higher concentration of penton-base protein accommodates the 30 fold-lower affinity of this interaction relative to fiber (27). Vector was then added at 50 particles per cell for AE25, AE25-HA, and Ramos cells or 1,000 particles per cell for CHO cells. After a 60-min incubation with vector, the cells were washed twice and incubated for 16 to 20 h before being analyzed. The cells were lysed in 100 μl of 1× reporter lysis buffer and assayed for luciferase (Promega, Madison, Wis.).
Vector expression in vivo.
For analysis of vector distribution following systemic delivery, 8- to 10-week-old female BALB/c mice (Charles River Laboratories, Wilmington, Mass.) were injected intrajugularly with 1011 particle units (PU) of vector in a volume of 100 μl. At 24 h postinjection the animals were sacrificed and tissues were collected and frozen immediately in liquid nitrogen. Direct injections were administered into the gastrocnemius muscle on each side of the mouse using 1010 PU of vector in 50 μl per injection. Tissues were collected 24 h later, as for the systemic administrations. Lysates were prepared from ground tissues by extraction in 1× cell culture lysis reagent (Promega). The protein content of lysates was determined by the Bradford assay (Bio-Rad, Hercules, Calif.) using bovine serum albumin as a standard.
Southern blots.
Viral DNA was isolated along with cellular genomic DNA by using DNeasy tissue kits (Qiagen, Valencia, Calif.). Tissue fragments were incubated overnight in proteinase K and then treated with RNase A. DNA was purified on minicolumns and quantitated by spectroscopy. Following digestion with KpnI, 5 μg of genomic DNA was loaded on a 0.8% agarose gel and transferred to a Zeta-Probe membrane (Bio-Rad) following electrophoresis. The probe was labeled by random priming from a template containing the Ad5 pol region using [32P]dCTP and the Rediprime II DNA-labeling system (Amersham Pharmacia Biotech, Piscataway, N.J.). The probe was hybridized overnight in 7% sodium dodecyl sulfate (SDS)–0.5M Na2HPO4 (pH 7.2) at 65°C. The membrane was washed at 65°C in 40 mM Na2HPO4–5% SDS followed by 40 mM Na2HPO4–1% SDS. Bound probe was detected by autoradiography and was quantitated using an InstantImager (Packard Instrument Co., Meriden, Conn.).
PCR detection of vector sequences in tissue.
Tissue DNA, isolated as described for Southern blots, was also analyzed by TaqMan PCR to detect vector DNA. Primers for amplification were located in the pIX region with the sequences CGCGGGATTGTGACTGACT (sense) and GCCAAAAGAGCCGTCAACTT (antisense). The fluorogenic detection probe had the sequence AGCAGTGCAGCTTCCCGTTCATCC, with 6-carboxyfluorescein at the 5′ end and 6-carboxy-N,N,N′,N′-tetramethylrhodamine at the 3′ end. Samples were amplified in 50 μl for 40 cycles in an ABI Prism 7700 sequence detector with continuous fluorescence monitoring. The data were processed using the instrument's SDS 1.6 software package.
RESULTS
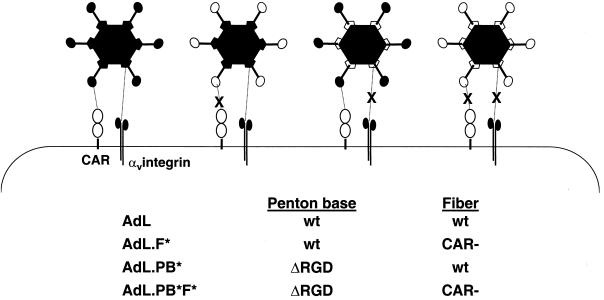
Ad vectors modified to abolish native receptor interactions are depicted in Fig. 1. All express luciferase from a cytomegalovirus promoter in the E1 region. The fiber protein of AdL.F* carries amino acid substitutions within the AB loop which disable CAR binding, whereas AdL.PB* lacks the RGD motif within the penton base which binds αv integrins. An HA epitope was incorporated within the HI loop of the fiber protein of AdL.F* and in place of the penton base RGD in AdL.PB*. The fiber and penton base modifications, including the HA epitopes, were combined in AdL.PB*F*. Vector genomes were constructed by recombination in E. coli, and plasmids carrying the complete genomes of these vectors were transfected into 293 cells (AdL and AdL.PB*) or 293-HA cells which express a membrane-anchored single-chain antibody capable of binding the HA epitope (AdL.F* and AdL.PB*F*). Expansion of the vectors in production runs using 2 × 108 to 1 × 109 cells infected at a multiplicity of infection of 20 PU showed comparable yields for the ablated and unmodified vectors (Table 1). The activities of AdL.F* and AdL.PB*F* were measured in focus-forming unit (FFU) assays on 293-HA cells. While AdL.F* exhibited PU/FFU ratios similar to those for AdL, AdL.PB*F* gave PU/FFU ratios about fourfold higher than those for AdL. This is consistent with the finding that luciferase expression from AdL.PB*F* is about 20% of the level detected from AdL following addition of equal particle numbers to 293-HA cells (data not shown).
FIG. 1.
Schematic diagram of Ad vectors with altered cell surface interactions. AdL.F* has a modification in the AB loop of fiber (see Materials and Methods) that abolishes CAR binding and has an HA epitope inserted in the fiber HI loop. In AdL.PB*, the penton base RGD motif has been replaced by an HA epitope. AdL.PB*F* combines the modifications found in AdL.PB* and AdL.F*.
TABLE 1.
Summary of vector yield and activity from multiple production runs.
| Vector | No. of lots | Yielda
|
|
|---|---|---|---|
| Pu/cell | Pu/FFu | ||
| AdL | 10 | 2.57 × 104 ± 1.13 × 104 | 17.1 ± 2.5 |
| AdL.F* | 5 | 1.51 × 104 ± 2.6 × 103 | 19.0 ± 1.4 |
| AdL.PB* | 5 | 7.25 × 104 ± 3.1 × 104 | 24.9 ± 7.1 |
| AdL.PB*F* | 8 | 1.63 × 104 ± 5.8 × 103 | 68.6 ± 13.7 |
Purified vector was analyzed by spectroscopy to determine the concentration in Pu, and the total yield was normalized to the starting number of cells used in the production run. FFU were determined in immunofluorescent-focus assays on 293 cells (AdL and AdL.PB*) or 293-HA cells (AdL.F* and AdL.PB*F*). The results are expressed as mean ± standard error.
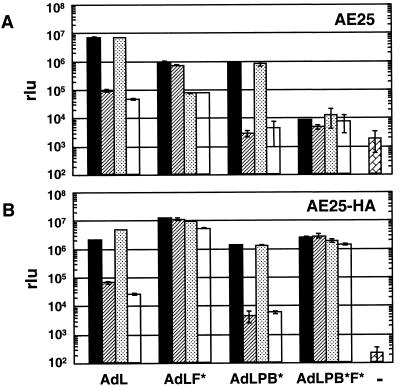
The efficiency of gene transfer was analyzed by assaying luciferase expression in cells following incubation with Ad vectors. The effects of the vector modifications on transduction of AE25 cells are shown in Fig 2A. Whereas the addition of soluble fiber reduced AdL transduction by >70-fold, it had no effect on transduction by AdL.F*. This result is consistent with a model of transduction where the only role of fiber is to bind CAR. Interestingly, transduction by AdL.F* was reduced 12-fold in the presence of penton base protein, which had no effect on AdL transduction. Penton base affected AdL transduction only on combination with fiber, when a small additional reduction was seen. For AdL.PB*, only fiber inhibited transduction. The absence of additional penton base interactions for this vector is indicated by the lack of effect of penton base even when combined with fiber. Based on these results, a vector able to bind neither CAR nor αv integrins was constructed. This vector, designated AdL.PB*F* and also referred to as doubly ablated, transduced AE25 cells to a level nearly 3 orders of magnitude below what was seen with AdL. This residual transduction was less than 10-fold above the background measured in the absence of vector. Transduction by this vector was unaffected in the presence of either fiber or penton base or a combination of the two. Thus, it appeared that penton base inhibited transduction only when fiber was blocked from binding CAR and that this was mediated by the RGD motif. The significantly reduced transduction for the doubly ablated vector suggested that removal of the RGD motif could be critical for disabling the native transduction activity of Ad vectors in vivo.
FIG. 2.
Specificity of transduction of AE25 (A) and AE25-HA (B) cells by the panel of vectors. Cells were incubated with vector (50 Pu/cell) or medium alone (−) for 60 min, washed to remove vector, and incubated for 18 h before being harvested. Competitors (fiber at 5 μg/ml, penton base at 100 μg/ml, or a combination of the two) were incubated with the cells for 60 min before addition of the vector. Cell lysates were assayed for luciferase expression. rlu, relative light units. Symbols: █ no competitor; , fiber; ░⃞, penton base; □, fiber plus penton base.
On cells expressing a membrane-anchored single-chain antibody that binds to the HA peptide epitope incorporated in the coat proteins of the modified vectors, no significant differences were seen in transduction among the four vectors in the absence of competitors (Fig. 2B). Thus, the ability to bind cells via the anti-HA antibody overcomes the transduction deficiencies resulting from loss of CAR and αv integrins. Luciferase levels detected following infection with AdL in the presence or absence of competitors indicated no differences between AE25-HA and AE25 cells. On AE25-HA cells, unlike AE25 cells, AdL.F* exhibited no sensitivity to competition by penton base, presumably because the anti-HA–HA epitope interaction promotes binding, which masks this just as fiber-CAR binding does for AdL. AdL.PB*, on the other hand, remained very sensitive to competition by fiber protein on the AE25-HA cells. This suggested that the HA epitope inserted in place of the RGD motif in penton base was not effectively bound by the anti-HA protein. Transduction by AdL.PB*F* was dramatically increased on AE25-HA cells compared to that on AE25 cells. The results obtained with AE25-HA cells indicated that the CAR-ablated vector AdL.F* and the doubly ablated vector AdL.PB*F* efficiently transduced cells capable of binding the inserted HA epitope. Production of these vectors is dependent on this surrogate interaction, and this observation supports the idea that by incorporating appropriate ligands the modified vectors remain highly active in transduction.
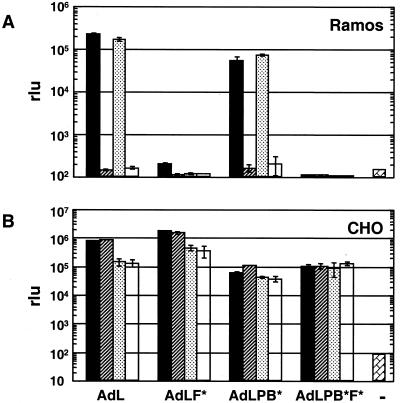
The modified vectors were further evaluated on Ramos cells, which do not express αv integrins but do express CAR, and on CHO cells, which lack CAR but express αv integrins. In the presence of fiber protein, transduction of Ramos cells by AdL, at multiplicity of infection of 50 Pu/cell, dropped 3 orders of magnitude, virtually to background (Fig. 3A). Ramos cells were refractory to AdL.F*, so the additional modification of penton base gave no further reduction in transduction. CHO cells are poorly transduced by AdL, so all vectors were added at 1,000 Pu/cell. In contrast to the Ramos cells, CHO cells exhibited no effect of ablating CAR binding (AdL.F*) or competing with fiber (Fig. 3B). Transduction by AdL and by AdL.F*, however, were both inhibited about fivefold in the presence of penton base. The transduction phenotypes of the modified vectors on both Ramos and CHO cells further demonstrated the specificity of the modifications to fiber and penton base, which inhibit the interactions with CAR and αv integrins, respectively.
FIG. 3.
Transduction of Ramos (A) and CHO (B) cells by a panel of vectors. Cells (105) were incubated with vector at 50 Pu/cell (Ramos) or 1,000 Pu/cell (CHO) or with medium alone (−). Competitors and vectors were incubated with cells as described in the legend to Fig. 2, and cells were assayed for luciferase expression at 18 h. rlu, relative light units. Symbols are as in Fig. 2.
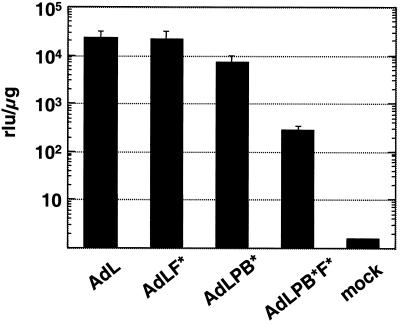
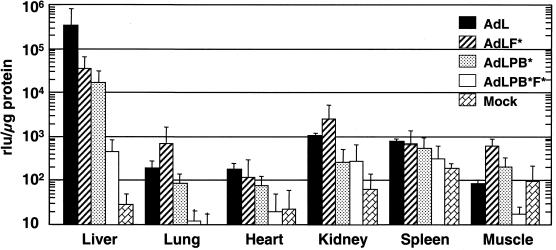
The activities of the panel of vectors in vivo were examined by direct intramuscular administration. Following injection into the gastrocnemius muscle, AdL.F* resulted in similar transduction to that due to AdL (Fig. 4), indicating that CAR does not play a prominent role in transduction of skeletal muscle by Ad vectors. Transduction of muscle was decreased 3-fold for the penton-modified vector AdL.PB*, but AdL.PB*F* caused a dramatic reduction of nearly 100-fold. Thus, while knocking out CAR binding alone had no effect on expression following intramuscular injection, the combination of this modification with the penton base alteration resulted in a 25-fold drop relative to AdL.PB*.
FIG. 4.
Effects of fiber and penton base mutations in vivo on transduction of skeletal muscle. Luciferase activities were assayed in the gastrocnemius muscle 24 h after direct intramuscular injection of 1010 Pu of vector or buffer alone (mock) and were normalized to the protein content of the samples (relative light units [rlu] per microgram). Each column shows the average from four samples, and the error bars represent the standard deviations.
Intravenous administration of Ad5-based vectors in mice results in preferential expression in the liver, which is also a prominent organ for CAR expression. Following intrajugular administration, the CAR-ablated vector AdL.F* caused reduced transduction of the liver compared to AdL (Fig. 5), but luciferase expression remained nearly 3 orders of magnitude above that seen in livers from mock-injected animals. Deletion of the RGD motif from penton base (AdL.PB*) was as effective in reducing liver transduction as was disabling CAR binding. The vector disabled for both binding CAR and binding αv integrins (AdL.PB*F*) exhibited a drop in liver transduction of more than 700-fold compared to AdL. The reduced transduction by AdL.PB*F* was significantly lower than that by either AdL.F* (P < 0.03) or AdL.PB* (P < 0.03). The last two vectors exhibited decreases, respectively, of 10- and 20-fold versus AdL. Thus, the two modifications had a synergistic effect in reducing transduction of the liver. These results, together with the results from intramuscular injection, indicate that removing both the CAR and the αv integrin binding of Ad vectors is critical for reducing the native tropism of Ad vectors in vivo.
FIG. 5.
Transduction of specific tissues following intravenous administrations with the panel of vectors. Animals received 1011 Pu of vector intrajugularly. Liver, lung, heart, kidney, spleen, and muscle (gastrocnemius) were collected at 24 h postinjection and analyzed as described in the legend to Fig. 4. Four samples were used for each vector except for AdL, for which three were used. rlu, relative light units.
Additional tissues from these mice were analyzed for luciferase expression to examine the roles of CAR and αv integrin interactions. The level of transduction detected with AdL in all these tissues was ≤1% of that found in the liver. In the lung, heart, kidney, spleen, and muscle, abolishing CAR binding resulted in no reduction in transduction (Fig. 5). In fact, lung, kidney, and muscle transduction appeared elevated. In contrast to ablating CAR binding, ablating αv integrin binding alone reduced transduction in the lung, heart, and kidney (P < 0.05, P < 0.01, and P < 0.01, respectively), although transduction of muscle was not significantly different from that by AdL. The doubly ablated vector exhibited reduced transduction relative to AdL in the spleen (P = 0.02) as well as the lung, heart, kidney, and muscle (P < 0.01 for all). With the exception of the spleen, ablating integrin binding was more effective than abolishing CAR binding for limiting transduction. In lung, heart, and muscle, AdL.PB*F* resulted in significantly less transduction than did AdL.PB* (P < 0.02, P < 0.05, and P < 0.04, respectively), and in all five tissues the transduction measured for AdL.PB*F* was not significantly above background. We detected no difference among the vectors in the low level of transduction of the superficial inguinal node or the diaphragm (data not shown). In all tissues where transduction by AdL was detected, AdL.PB*F* gave reduced luciferase expression.
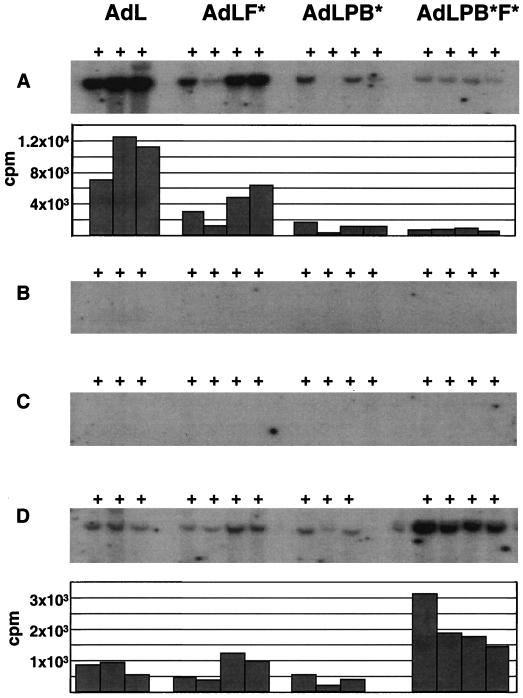
Southern blot analyses were performed on DNA isolated from individual tissues to examine vector distribution independent of expression. Clear differences were seen among the panel of vectors in terms of genomes detected in the liver (Fig. 6A). Quantitation of bound probe showed a two- to threefold decrease for AdL.F* versus AdL, regardless of whether the weakest AdL.F* sample was included in calculating the means. A 9-fold decrease relative to AdL was seen for AdL.PB*, while the vector signal was reduced by 13-fold for AdL.PB*F*. Thus, in terms of vector particles localizing to the liver, αv integrin binding had a greater impact than CAR binding. The synergistic effect on transduction of combining the CAR and integrin binding modifications was not observed at the level of localization of particles to the liver.
FIG. 6.
Detection of vector DNA by Southern blot analysis, DNA isolated from liver (A), lung (B), heart (C), and spleen (D) was digested with KpnI and hybridized to a probe from the pol region of Ad5. The blots were exposed to X-ray film for 24 h (A) or 48 h (B to D). Quantitation of the bound probe using an InstantImager instrument is shown in the lower panels of A and D. The counts per minute reported were corrected by subtraction of background determined in adjacent regions of the blot. Samples from mock-treated animals were not above background.
AdL DNA was localized primarily in the liver at 24 h postadministration. In the lung and heart, the AdL DNA level was at or below the level of detection (Fig. 6B and C). As reported above, expression in these tissues was less than 1% of that detected in the liver. A similar reduction in vector genomes would give a signal barely above background in the Southern blot analysis. Vector DNA was more readily detected in the spleen (Fig. 6D). In addition, a different pattern was seen for the panel of vectors in this tissue. There was no difference between the amounts of AdL.F* and AdL, while the amount of AdL.PB* was reduced about twofold. By contrast, the amount of AdL.PB*F* was increased nearly threefold versus AdL. Quantitation of bound probe indicated that the amount of AdL detected per 5 μg of splenic DNA was 8% of that detected with an equal quantity of liver DNA. Since the normal mouse liver is about nine times the size of the normal spleen in terms of weight, the fraction of the AdL dose present in the spleen was quite small compared to that in the liver. While the AdL.PB*F* level was elevated in the spleen relative to that of AdL and was also nearly threefold higher than that of AdLPB*F* in the liver on a per-microgram-of-DNA basis, the size difference between the two organs indicates that the majority of this vector also localized to the liver.
We used real-time PCR analysis to further quantify differences in genome copies between the vectors within specific tissues. The results are presented as percentages of the numbers of copies detected for AdL in the respective tissue, since differences in PCR efficiency among organs limit a direct comparison of the number of vector copies detected in different tissues (Table 2). The number of relative copies detected for each vector in the liver by PCR agreed well with the Southern blot results. Abolition of CAR binding resulted in two- to threefold drop in the number of vector genomes detected. For AdL.PB*, the reduction was 10-fold relative to AdL and significantly reduced relative to AdL.F* (P < 0.03). The level of the doubly ablated vector was reduced 15-fold relative to that of AdL and was not significantly lower than that of AdL.PB*. PCR detection of the different vectors in the spleen also matched the results of Southern blotting, with AdLPB*F* giving an elevation in the number of vector copies. We were able to detect vector DNA in the lung, heart, kidney, and diaphragm and found that loss of CAR binding did not result in a statistically significant reduction in the level of vector genomes in these tissues (Table 2). In contrast, AdL.PB* levels were significantly reduced relative to AdL levels in the lung (P < 0.01) and heart (P < 0.01), as were the levels of AdL.PB*F* (P < 0.01 for both). The levels of AdL.PB*F* were also significantly reduced relative to those of AdL in the diaphragm (P < 0.02) and relative to those of AdL.F* in the kidney (P < 0.04). Thus, the penton base modification had a broader effect than the fiber modification in reducing the number of vector copies in these tissues.
TABLE 2.
Relative copies for the panel of vectors within specific tissues as detected by Taqman PCRa
| Tissue | Relative no. of copies (% of AdL
value)b
|
|||
|---|---|---|---|---|
| AdL | AdL.F* | AdL.PB* | AdL.PB*F* | |
| Liver | 100 ± 20 | 39 ± 11 | 10 ± 3.4 | 7.4 ± 1.7 |
| Spleen | 100 ± 44 | 69 ± 13 | 41 ± 16 | 171 ± 36 |
| Lung | 100 ± 24 | 103 ± 53 | 7.4 ± 4.8 | 14 ± 4.5 |
| Heart | 100 ± 20 | 87 ± 30 | 9.3 ± 5.6 | 17 ± 6.6 |
| Kidney | 100 ± 77 | 22 ± 8 | 7.4 ± 5.2 | 4.8 ± 0.5 |
| Diaphragm | 100 ± 41 | 82 ± 76 | 29 ± 18 | 8.8 ± 2.2 |
Tissue DNA was analyzed using primers and probes that recognize the pIX region of Ad5. Results were obtained as the number of of copies per nanogram of DNA.
To control for tissue differences, the results are reported as percentages of the number of copies of AdL detected in the respective tissue following subtraction of background. The values listed are expressed as mean ± standard error.
DISCUSSION
Using a panel of Ad vectors disabled for CAR binding, αv integrin binding, or both, we have found that ablating interactions with both CAR and αv integrin is essential to significantly reduce the native tropism of an Ad vector. While fiber protein efficiently inhibited infection by the wild-type Ad vector in vitro, fiber modifications that abolish CAR binding resulted in a vector that was resistant to this inhibition. Therefore, there appeared to be no additional role of fiber in vector attachment. The vector that lacked CAR binding, however, exhibited sensitivity to inhibition by purified penton base that was not seen for the wild-type vector. This suggested that an interaction involving penton base could mediate transduction in the absence of fiber-CAR binding and prompted us to construct a vector ablated for both CAR and αv integrin binding. The significant reduction in transduction for the doubly ablated vector, AdL.PB*F*, relative to the CAR-ablated vector, AdL.F*, indicated that an interaction between penton base and αv integrins was involved in the residual transduction by AdL.F*. The inability of penton base protein to inhibit AdL.PB*F* transduction confirmed that the RGD loop of penton base was involved in the penton base-mediated transduction by AdL.F* and that additional interactions of penton base with cellular receptors were not involved in vector uptake.
The importance of abolishing both CAR and αv integrin binding for ablating native vector tropism was borne out by in vivo analysis. Following intravenous injection into mice, transgene expression from Ad was preferentially localized to the liver. Vectors unable to bind CAR caused a 10- to 20-fold reduction in transgene expression in the liver. This is consistent with what has been reported for Ad vectors complexed with neutralizing anti-fiber antibodies attached to targeting ligands (9, 17). Deleting only the RGD motif that mediates the αv integrin interaction, however, had as great an effect in reducing liver transduction as did loss of CAR binding. However, the most dramatic result was the drop in liver transduction measured with the doubly ablated vector. The fiber and penton base modifications had a synergistic effect, resulting in a > 700-fold decrease of luciferase expression in the liver. The residual transduction resulted in expression that was within 1 log unit of that seen in mock-treated animals.
The penton base-αv integrin interaction has been proposed to be important for efficient internalization of Ad from the cell surface via receptor-mediated endocytosis, and this interaction may also be important for cell signaling and for release of vector cores from the endosome (reviewed in reference 14). Based on these observations, one might expect that the impact of the penton base RGD mutation on in vivo transduction is inefficient internalization. This would suggest that at least part of the reduced expression from the doubly ablated vector resulted from loss of transduction activity of the particles rather than from targeting. If this were the case, employing the doubly ablated vector as a base for targeted vectors might require, for example, selecting target receptors that could mediate endocytosis of the vector. While differences in receptor internalization may influence the relative effectiveness of retargeting the doubly ablated vector to specific novel receptors, our results suggest that the consequences of the RGD deletion in reducing transduction could take effect prior to internalization. First, no difference in transduction activity among the unmodified, the CAR-ablated, the integrin-ablated, and the doubly ablated vectors was detected on AE25-HA cells. It appears that under these conditions, attachment via the anti-HA receptor or CAR is sufficient for efficient internalization. Second, the RGD deletion caused a marked reduction in the level of vector genomes detected in a number of tissues. Unless the Southern and PCR analyses preferentially detected internalized genomes, these results point to an influence of the RGD deletion on vector distribution rather than just to vector internalization.
Expression data from experiments with additional tissues besides the liver underscored the importance of deleting the penton base RGD motif for altering Ad tropism. No significant decrease in transduction was seen in the lung, heart, kidney, spleen, or skeletal muscle for the CAR-ablated vector, AdL.F*. Following intravenous injection, AdL.F* gave elevated expression in skeletal muscle and some evidence for elevated expression in the lung and kidney. Expression from AdL.PB*, on the other hand, was significantly reduced relative to that from AdL in the lung, heart, and kidney. In the spleen, a combination of the penton base and fiber modifications was necessary to reduce expression.
Both AdL.F* and AdL.PB* showed a decrease in the number of vector genomes in the liver by Southern blot analysis, but the penton base modification had a greater effect. With the possible exception of the kidney, Taqman PCR detected no significant decrease in the numbers of AdL.F* genomes in tissues other than the liver. In contrast, the numbers of AdL.PB* genomes were reduced in the lung, heart, kidney, spleen and diaphragm. The reduction of the numbers of AdL.PB* genomes was similar to that of the numbers of AdL.PB*F* genomes, except in the spleen, where the AdL.PB*F* level was elevated relative to that of AdL. These results indicate not only that interactions other than those with the fiber receptor can influence vector distribution but also that in most tissues the penton base interaction has more influence than binding of CAR by fiber.
In the liver, both AdL.PB* and AdL.PB*F* showed a reduction in vector copies of about 10-fold, but whereas AdL.PB* expression was decreased about 20-fold relative to that of AdL, the reduction of AdL.PB*F* expression was 700-fold. The magnitude of the decrease in expression of AdL.PB*F* greatly exceeds the reduction in the number of detectable genomes. In AdL.F*, we detected a 2- to 3-fold drop in the number of vector copies together with a 10- to 20-fold drop in expression. The greater effect on expression of the CAR-ablating modification in the context of the RGD deletion suggests that the penton base can mediate transduction in the liver, just as we observed in vitro. It is likely that there are multiple mechanisms causing localization of Ad vectors to the liver, and future studies are needed to analyze the specific types of cells in the liver that take up vector.
The data reported here were collected from tissues harvested at 24 h after vector administration. DNA analysis has indicated that as much as 90% of an intravenous dose of Ad vector disappears within the first 24 h via clearance and degradation in the liver (30). When circulation through the liver was bypassed, systemic administration of Ad to mice resulted in persistence of the vector in the circulation and enhanced transduction of the lung, kidney, and intestine (31). Kupffer cells appear to play a role in clearance by the liver, but the precise mechanism has not been elucidated (1, 12, 21, 29). The increase in the vector DNA level detected in the spleen for AdL.PB*F* could reflect altered vector circulation in the absence of CAR and integrin binding. The magnitude of the increase seen for AdL.PB*F* DNA in the spleen, however, was insufficient to account for the decrease of AdL.PB*F* DNA in other tissues and does not indicate any increase in the total amount of vector DNA detected at 24 h. Based on these results, it appears that absence of CAR and αv integrin binding does not enable an Ad vector to escape rapid clearance and degradation following intravenous administration.
The enhanced transduction in lung, kidney, and skeletal muscle that we detected for AdL.F* and the increased levels of AdL.PB*F* in the spleen provide evidence that altering the interactions of Ad vectors with CAR and αv integrin can alter their distribution in vivo. Given the similar levels of expression from AdL and AdL.F* following direct intramuscular injection, the greater expression from AdL.F* following systemic administration appears to reflect a difference in distribution of the two vectors. At the same time, interactions beyond those with CAR and αv integrins affect the fate of Ad vectors following systemic delivery (7, 31). While access to specific target tissues can be limited by physical barriers (5, 13, 25), the rapid clearance of Ad vectors in the liver impacts any application where a vector might be administered systemically. The vectors we evaluated have ablated receptor specificity. While limiting the interaction of Ad vectors with native receptors is critical for development of vectors that have greater specificity for target tissues, the efficacy of novel receptor specificities incorporated in the doubly ablated vector remains to be tested.
Since the liver is the major site of transgene expression following intravenous administration of Ad vectors in mice, the dramatic reduction in transduction of this organ by the doubly ablated vector represents a significant finding for developing targeted Ad vectors. The reduced levels of this vector in most tissues examined should enhance the targeting specificity of forms of the vector carrying novel targeting sequences. Targeting of phage particles to specific tissues has been demonstrated in vivo through the incorporation of short linear peptide motifs (2, 15). Targeted Ad vectors could be developed by incorporating these ligands (23), or Ad vectors might be used directly to identify ligands. Vectors with ablated tropism, through loss of CAR and αv integrin binding, provide an improved platform for evaluating the targeting potential of peptide ligands incorporated in Ad capsids. Further modifications of the vector to avoid rapid clearance from the circulation might result in even greater targeting efficiency, as well as in advantages from enabling therapeutic efficacy at lower vector doses.
REFERENCES
- 1.Alemany R, Suzuki K, Curiel D T. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 2.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 3.Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson J M, Krithivas A, Celi L, Droguett G, Horwitz M S, Wickham T, Crowell R L, Finberg R W. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho W K, Ebihara S, Nalbantoglu J, Gilbert R, Massie B, Holland P, Karpati G, Petrof B J. Modulation of Starling forces and muscle fiber maturity permits adenovirus-mediated gene transfer to adult dystrophic (mdx) mice by the intravascular route. Hum Gene Ther. 2000;11:701–714. doi: 10.1089/10430340050015608. [DOI] [PubMed] [Google Scholar]
- 6.Einfeld D A, Brough D E, Roelvink P W, Kovesdi I, Wickham T J. Construction of a pseudoreceptor that mediates transduction by adenoviruses expressing a ligand in fiber or penton base. J Virol. 1999;73:9130–9136. doi: 10.1128/jvi.73.11.9130-9136.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, Schoemaker R, Veghel R, Houtsmuller A, Schultheiss H P, Lamers J, Poller W. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- 8.Goldman M, Su Q, Wilson J M. Gradient of RGD-dependent entry of adenoviral vector in nasal and intrapulmonary epithelia: implications for gene therapy of cystic fibrosis. Gene Ther. 1996;3:811–818. [PubMed] [Google Scholar]
- 9.Gu D L, Gonzalez A M, Printz M A, Doukas J, Ying W, D'Andrea M, Hoganson D K, Curiel D T, Douglas J T, Sosnowski B A, Baird A, Aukerman S L, Pierce G F. Fibroblast growth factor 2 retargeted adenovirus has redirected cellular tropism: evidence for reduced toxicity and enhanced antitumor activity in mice. Cancer Res. 1999;59:2608–2614. [PubMed] [Google Scholar]
- 10.Hemmi S, Geertsen R, Mezzacasa A, Peter I, Dummer R. The presence of human coxsackievirus and adenovirus receptor is associated with efficient adenovirus-mediated transgene expression in human melanoma cell cultures. Hum Gene Ther. 1998;9:2363–2373. doi: 10.1089/hum.1998.9.16-2363. [DOI] [PubMed] [Google Scholar]
- 11.Leon R P, Hedlund T, Meech S J, Li S, Schaack J, Hunger S P, Duke R C, DeGregori J. Adenoviral-mediated gene transfer in lymphocytes. Proc Natl Acad Sci USA. 1998;95:13159–13164. doi: 10.1073/pnas.95.22.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieber A, He C Y, Meuse L, Schowalter D, Kirillova I, Winther B, Kay M A. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logeart D, Hatem S N, Rucker-Martin C, Chossat N, Nevo N, Haddada H, Heimburger M, Perricaudet M, Mercadier J J. Highly efficient adenovirus-mediated gene transfer to cardiac myocytes after single-pass coronary delivery. Hum Gene Ther. 2000;11:1015–1022. doi: 10.1089/10430340050015329. [DOI] [PubMed] [Google Scholar]
- 14.Nemerow G R, Stewart P L. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev. 1999;63:725–734. doi: 10.1128/mmbr.63.3.725-734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasqualini R. Vascular targeting with phage peptide libraries. Q J Nucl Med. 1999;43:159–162. [PubMed] [Google Scholar]
- 16.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds P N, Zinn K R, Gavrilyuk V D, Balyasnikova I V, Rogers B E, Buchsbaum D J, Wang M H, Miletich D J, Grizzle W E, Douglas J T, Danilov S M, Curiel D T. A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol Ther. 2000;2:562–578. doi: 10.1006/mthe.2000.0205. [DOI] [PubMed] [Google Scholar]
- 18.Roelvink P W, Kovesdi I, Wickham T J. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roelvink P W, Lizonova A, Lee J G, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roelvink P W, Mi Lee G, Einfeld D A, Kovesdi I, Wickham T J. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- 21.Tao N, Gao G P, Parr M, Johnston J, Baradet T, Wilson J M, Barsoum J, Fawell S E. Sequestration of adenoviral vector by kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- 22.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trepel M, Grifman M, Weitzman M D, Pasqualini R. Molecular adaptors for vascular-targeted adenoviral gene delivery. Hum Gene Ther. 2000;11:1971–1981. doi: 10.1089/10430340050143408. [DOI] [PubMed] [Google Scholar]
- 24.Walters R W, Grunst T, Bergelson J M, Finberg R W, Welsh M J, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 25.Wang G, Zabner J, Deering C, Launspach J, Shao J, Bodner M, Jolly D J, Davidson B L, McCray P. Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo. Am J Respir Cell Mol Biol. 2000;22:129–138. doi: 10.1165/ajrcmb.22.2.3938. [DOI] [PubMed] [Google Scholar]
- 26.Wickham T J, Carrion M E, Kovesdi I. Targeting of adenovirus penton base to new receptors through replacement of its RGD motif with other receptor-specific peptide motifs. Gene Ther. 1995;2:750–756. [PubMed] [Google Scholar]
- 27.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 28.Wickham T J, Tzeng E, Shears L L, 2nd, Roelvink P W, Li Y, Lee G M, Brough D E, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolff G, Worgall S, van Rooijen N, Song W R, Harvey B G, Crystal R G. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worgall S, Wolff G, Falck-Pedersen E, Crystal R G. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 31.Ye X, Jerebtsova M, Ray P E. Liver bypass significantly increases the transduction efficiency of recombinant adenoviral vectors in the lung, intestine, and kidney. Hum Gene Ther. 2000;11:621–627. doi: 10.1089/10430340050015806. [DOI] [PubMed] [Google Scholar]
- 32.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Investig. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zinn K R, Douglas J T, Smyth C A, Liu H G, Wu Q, Krasnykh V N, Mountz J D, Curiel D T, Mountz J M. Imaging and tissue biodistribution of 99mTc-labeled adenovirus knob (serotype 5) Gene Ther. 1998;5:798–808. doi: 10.1038/sj.gt.3300659. [DOI] [PubMed] [Google Scholar]