Abstract
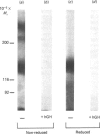
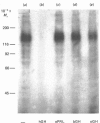
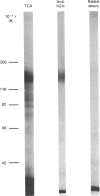
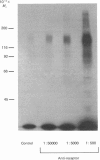
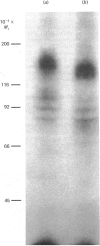
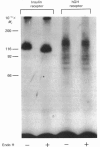
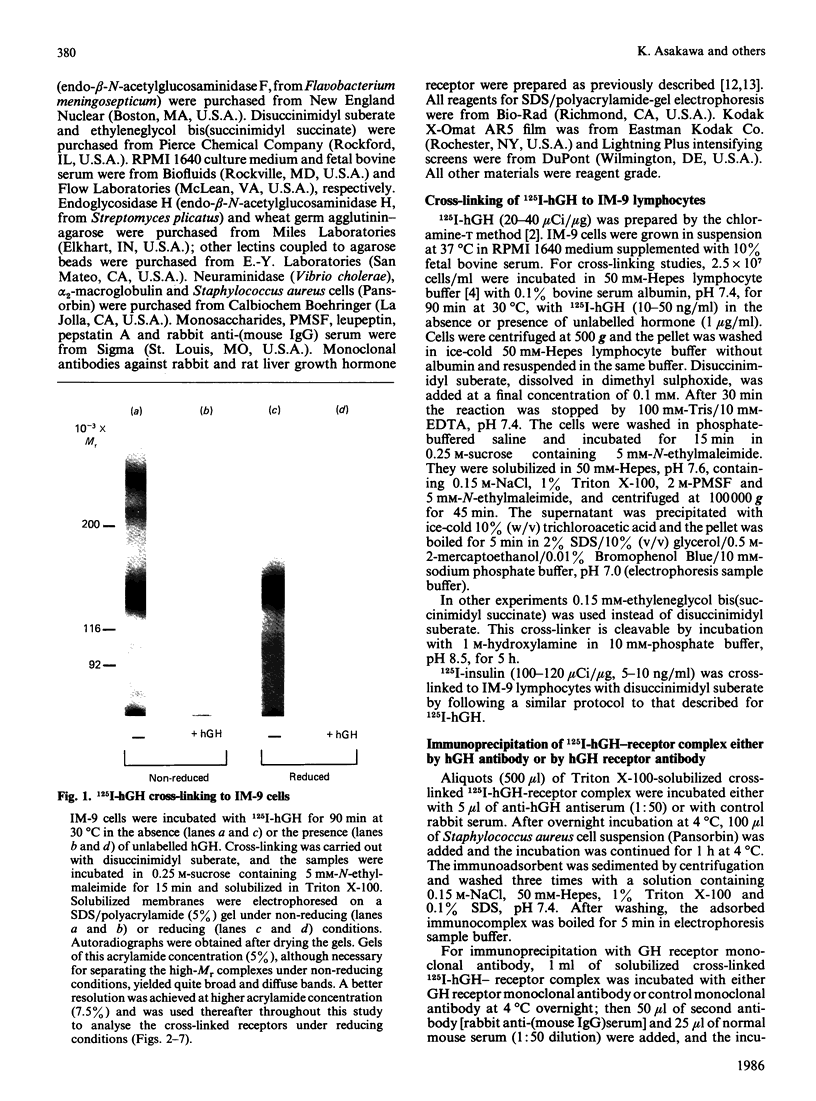
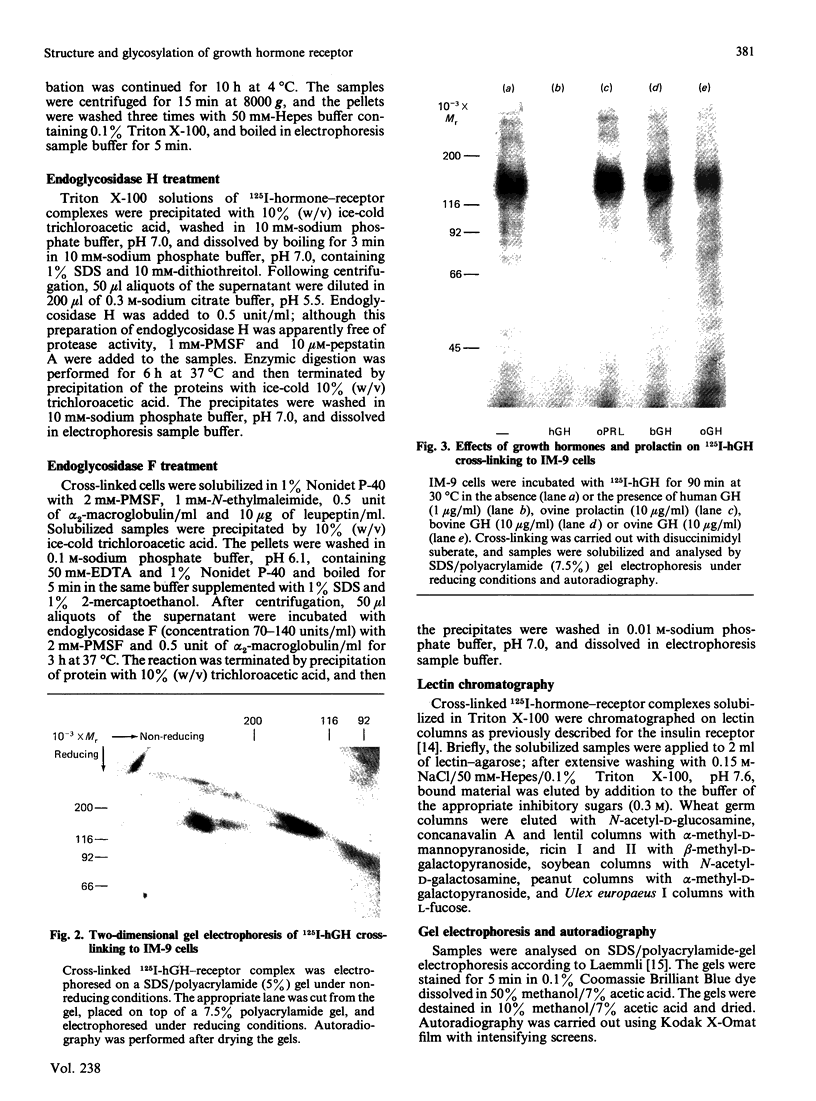
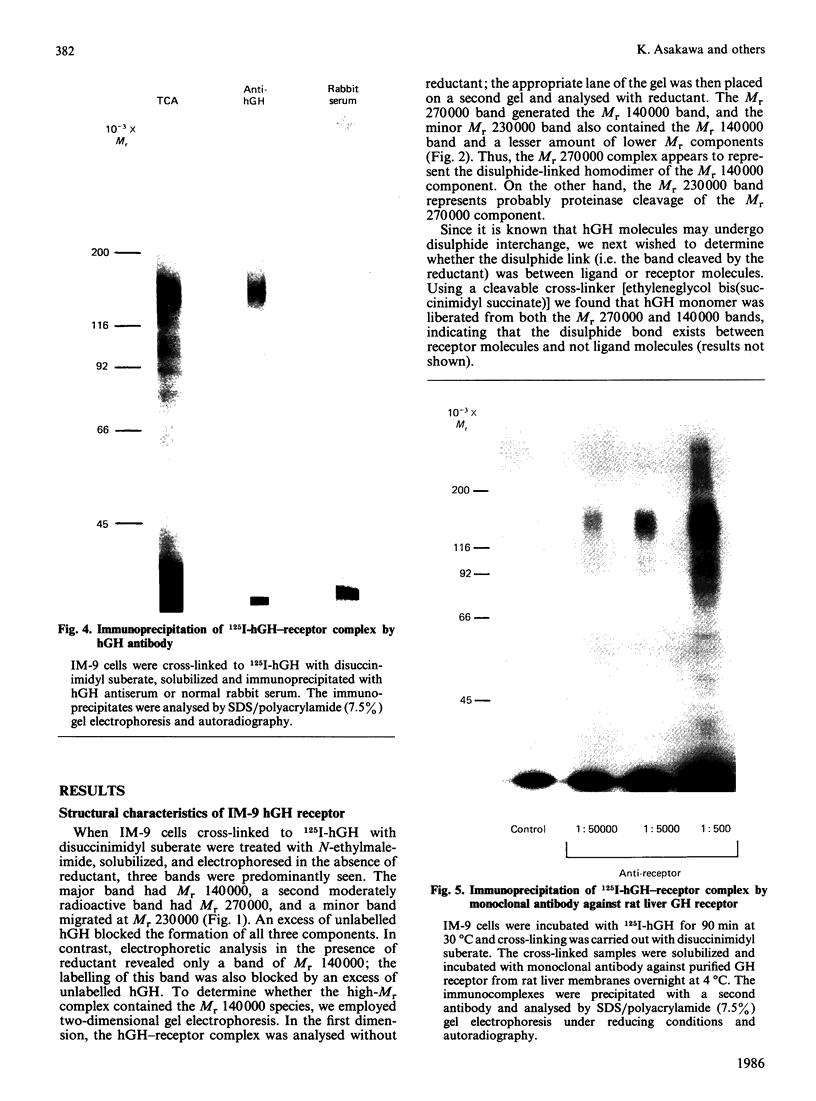
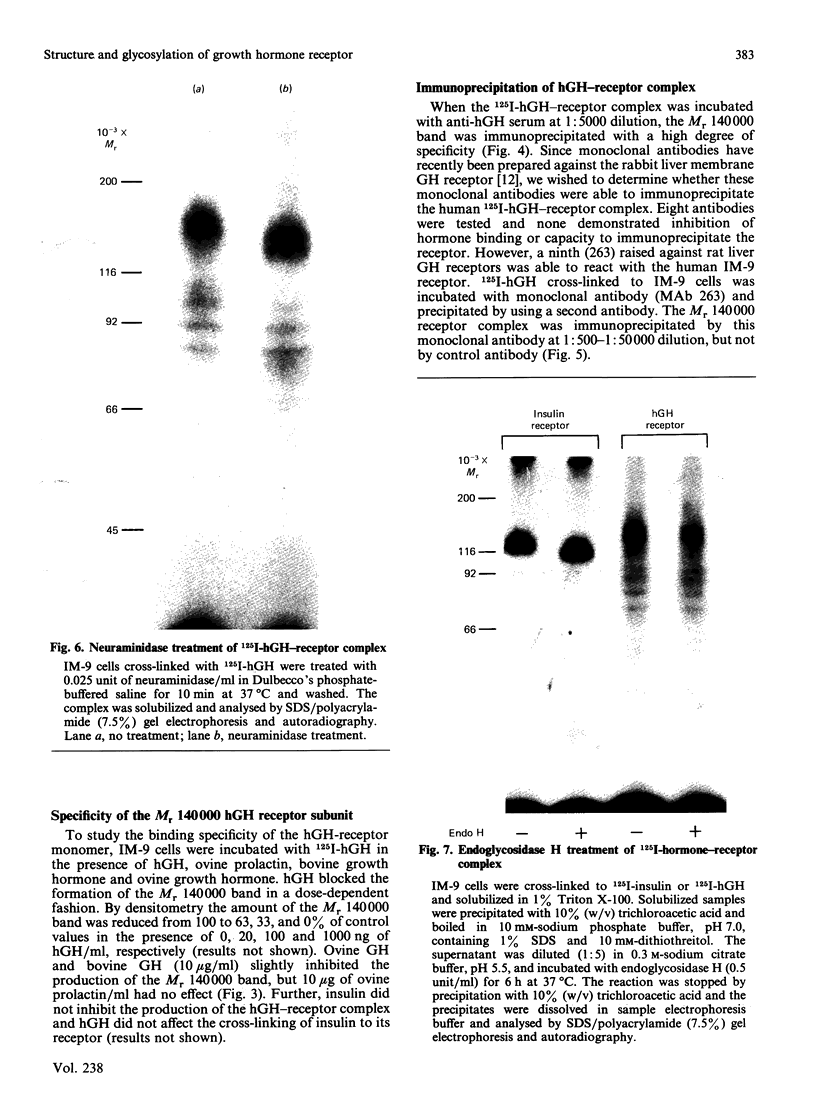
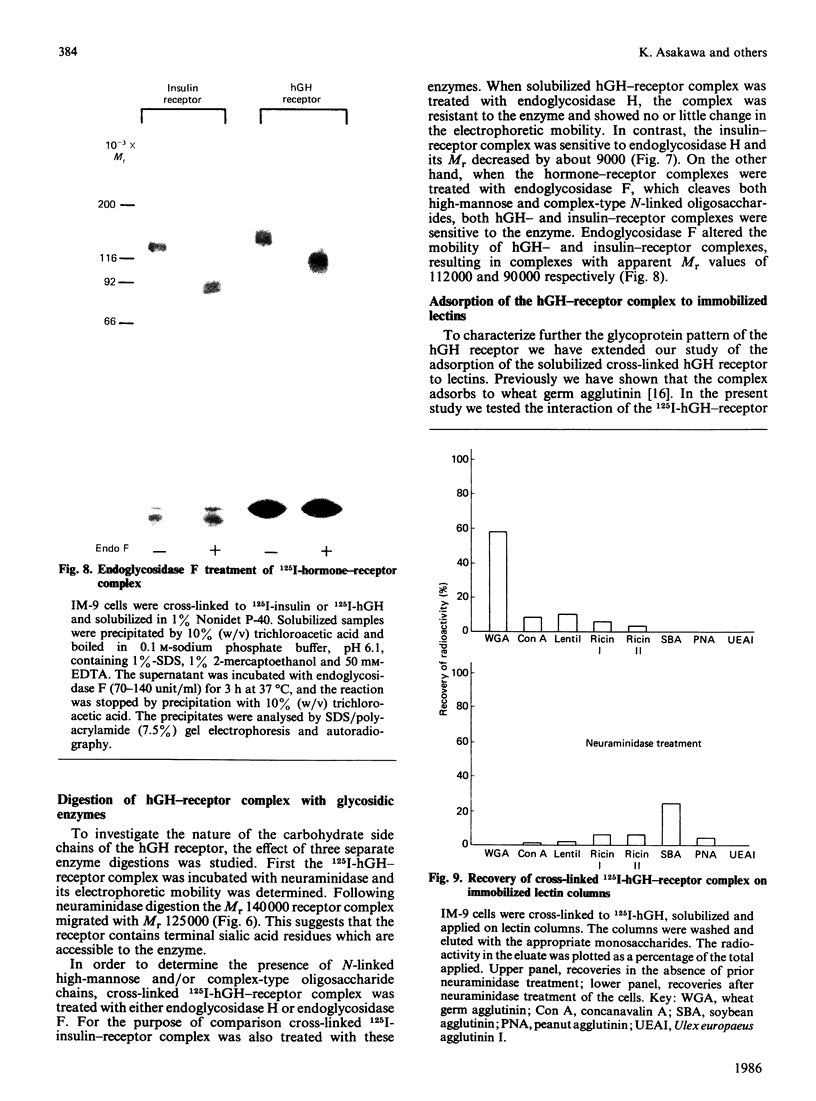
The structural characteristics and glycoprotein nature of the human growth hormone (hGH) receptor in cultured lymphocytes (IM-9 cell line) were studied with the use of a bifunctional reagent (disuccinimidyl suberate) to couple 125I-hGH covalently to intact cells. After cross-linking, the hormone-receptor complexes were analysed by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. A single band of Mr 140,000 was identified under reducing conditions. The labelling of this band was blocked by unlabelled hGH but not by insulin, ovine prolactin, bovine or ovine growth hormones. The Mr 140,000 band was immunoprecipitated by either anti-hGH antibody or by a monoclonal antibody against rat liver growth hormone receptor. In the absence of reductant two major bands of Mr 270,000 and 140,000 were found. On two-dimensional gel electrophoresis, with the first dimension in the absence of reductant and the second in its presence, the Mr 270,000 complex generated the Mr 140,000 band. The nature of the oligosaccharide chains of the receptor was studied by treatment with different glycosidases. The electrophoretic mobility of the Mr 140,000 receptor complex was markedly increased after digestion with endoglycosidase F but showed no or little change after digestion with endoglycosidase H. The Mr 140,000 band was also sensitive to neuraminidase treatment. In addition the 125I-hGH-receptor complex was adsorbed by immobilized wheat germ agglutinin and to a smaller extent by immobilized concanavalin A, lentil lectin, ricin I and ricin II. In conclusion, taking into account that hGH is a Mr 22,000 polypeptide, the binding subunit of the GH receptor in human IM-9 lymphocytes has an Mr of approx. 120,000. The native receptor may exist as a homodimer of the binding subunit formed by disulphide bonds. Furthermore, the GH receptor subunit contains asparagine N-linked type of oligosaccharide chains. Most, if not all, of these chains are of the complex type and appear to be sialylated whereas no high-mannose type chains are detectable in the mature form of the receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakawa K., Grunberger G., McElduff A., Gorden P. Polypeptide hormone receptor phosphorylation: is there a role in receptor-mediated endocytosis of human growth hormone? Endocrinology. 1985 Aug;117(2):631–637. doi: 10.1210/endo-117-2-631. [DOI] [PubMed] [Google Scholar]
- Barazzone P., Lesniak M. A., Gorden P., Van Obberghen E., Carpentier J. L., Orci L. Binding, internalization, and lysosomal association of 125I-human growth hormone in cultured human lymphocytes: a quantitative morphological and biochemical study. J Cell Biol. 1980 Nov;87(2 Pt 1):360–369. doi: 10.1083/jcb.87.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard R., Bundesen P. G., Rylatt D. B., Waters M. J. Monoclonal antibodies to the rabbit liver growth hormone receptor: production and characterization. Endocrinology. 1984 Nov;115(5):1805–1813. doi: 10.1210/endo-115-5-1805. [DOI] [PubMed] [Google Scholar]
- Blossey H. C. Studies on the molecular architecture and the composition of the GH receptor from rabbit liver. Horm Metab Res. 1979 Nov;11(11):616–621. doi: 10.1055/s-0028-1092788. [DOI] [PubMed] [Google Scholar]
- Carter-Su C., Schwartz J., Kikuchi G. Identification of a high affinity growth hormone receptor in rat adipocyte membranes. J Biol Chem. 1984 Jan 25;259(2):1099–1104. [PubMed] [Google Scholar]
- Donner D. B. Covalent coupling of human growth hormone to its receptor on rat hepatocytes. J Biol Chem. 1983 Feb 25;258(4):2736–2743. [PubMed] [Google Scholar]
- Eastman R. C., Lesniak M. A., Roth J., De Meyts P., Gorden P. Regulation of receptor by homologous hormone enhances sensitivity and broadens scope of radioreceptor assay for human growth hormone. J Clin Endocrinol Metab. 1979 Aug;49(2):262–268. doi: 10.1210/jcem-49-2-262. [DOI] [PubMed] [Google Scholar]
- Gorin E., Goodman H. M. Covalent binding of growth hormone to surface receptors on rat adipocytes. Endocrinology. 1984 Apr;114(4):1279–1286. doi: 10.1210/endo-114-4-1279. [DOI] [PubMed] [Google Scholar]
- Hedo J. A., Harrison L. C., Roth J. Binding of insulin receptors to lectins: evidence for common carbohydrate determinants on several membrane receptors. Biochemistry. 1981 Jun 9;20(12):3385–3393. doi: 10.1021/bi00515a013. [DOI] [PubMed] [Google Scholar]
- Hedo J. A., Kahn C. R., Hayashi M., Yamada K. M., Kasuga M. Biosynthesis and glycosylation of the insulin receptor. Evidence for a single polypeptide precursor of the two major subunits. J Biol Chem. 1983 Aug 25;258(16):10020–10026. [PubMed] [Google Scholar]
- Hedo J. A., Kasuga M., Van Obberghen E., Roth J., Kahn C. R. Direct demonstration of glycosylation of insulin receptor subunits by biosynthetic and external labeling: evidence for heterogeneity. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4791–4795. doi: 10.1073/pnas.78.8.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizuka N., Gorden P., Lesniak M. A., Van Obberghen E., Carpentier J. L., Orci L. Polypeptide hormone degradation and receptor regulation are coupled to ligand internalization. A direct biochemical and morphologic demonstration. J Biol Chem. 1981 May 10;256(9):4591–4597. [PubMed] [Google Scholar]
- Hughes J. P., Simpson J. S., Friesen H. G. Analysis of growth hormone and lactogenic binding sites cross-linked to iodinated human growth hormone. Endocrinology. 1983 Jun;112(6):1980–1985. doi: 10.1210/endo-112-6-1980. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Hedo J. A., Yamada K. M., Kahn C. R. The structure of insulin receptor and its subunits. Evidence for multiple nonreduced forms and a 210,000 possible proreceptor. J Biol Chem. 1982 Sep 10;257(17):10392–10399. [PubMed] [Google Scholar]
- Keefer L. M., De Meyts P. Glycosylation of cell surface receptors: tunicamycin treatment decreases insulin and growth hormone binding to different levels in cultured lymphocytes. Biochem Biophys Res Commun. 1981 Jul 16;101(1):22–29. doi: 10.1016/s0006-291x(81)80005-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lesniak M. A., Gorden P., Roth J., Gavin J. R., 3rd Binding of 125I-human growth hormone to specific receptors in human cultured lymphocytes. Characterization of the interaction and a sensitive radioreceptor assay. J Biol Chem. 1974 Mar 25;249(6):1661–1667. [PubMed] [Google Scholar]
- Lesniak M. A., Gorden P., Roth J. Reactivity of non-primate growth hormones and prolactins with human growth hormone receptors on cultured human lymphocytes. J Clin Endocrinol Metab. 1977 May;44(5):838–849. doi: 10.1210/jcem-44-5-838. [DOI] [PubMed] [Google Scholar]
- Lesniak M. A., Roth J., Gorden P., Gavin J. R., 3rd Human growth hormone radioreceptor assay using cultured human lymphocytes. Nat New Biol. 1973 Jan 3;241(105):20–22. doi: 10.1038/newbio241020a0. [DOI] [PubMed] [Google Scholar]
- Lesniak M. A., Roth J. Regulation of receptor concentration by homologous hormone. Effect of human growth hormone on its receptor in IM-9 lymphocytes. J Biol Chem. 1976 Jun 25;251(12):3720–3729. [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- Tsushima T., Murakami H., Wakai K., Isozaki O., Sato Y., Shizume K. Analysis of hepatic growth hormone binding sites of pregnant rabbit crosslinked to 125I-labelled human growth hormone. FEBS Lett. 1982 Oct 4;147(1):49–53. doi: 10.1016/0014-5793(82)81009-0. [DOI] [PubMed] [Google Scholar]
- Tsushima T., Sasaki N., Imai Y., Matsuzaki F., Friesen H. G. Characteristics of solubilized human-somatotropin-binding protein from the liver of pregnant rabbits. Biochem J. 1980 May 1;187(2):479–492. doi: 10.1042/bj1870479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. J., Friesen H. G. Purification and partial characterization of a nonprimate growth hormone receptor. J Biol Chem. 1979 Jul 25;254(14):6815–6825. [PubMed] [Google Scholar]