Abstract
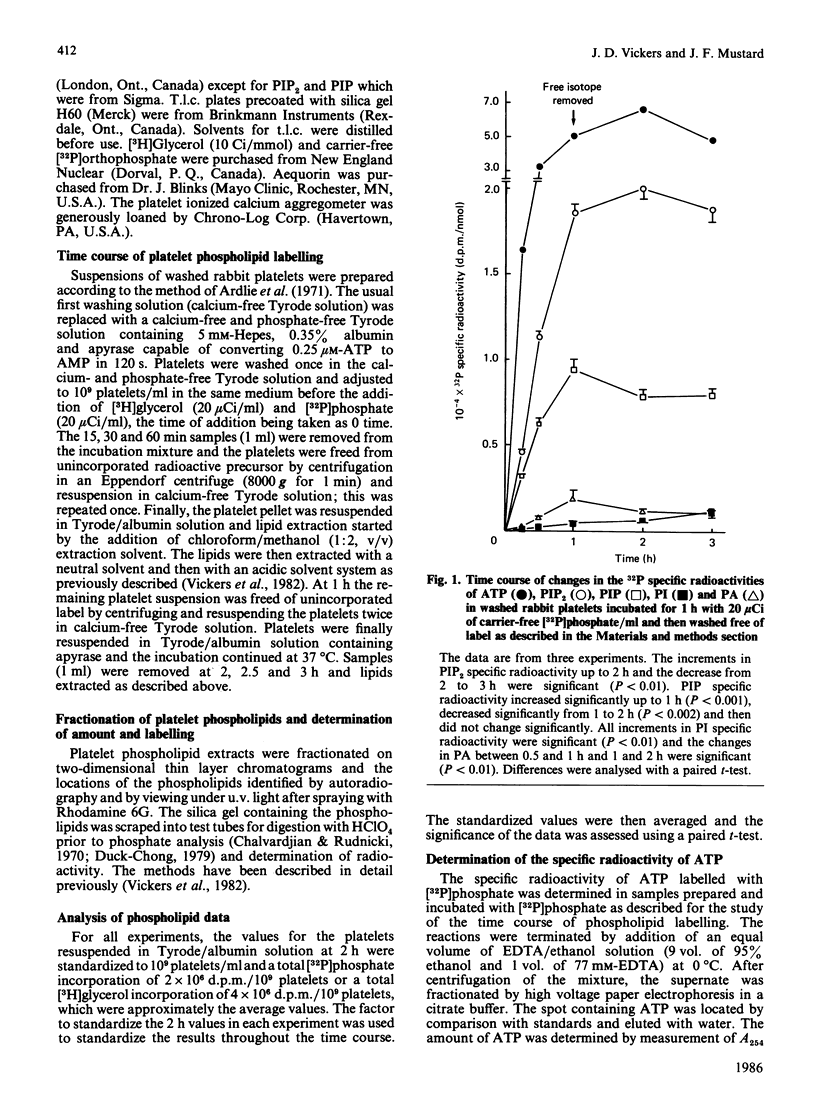
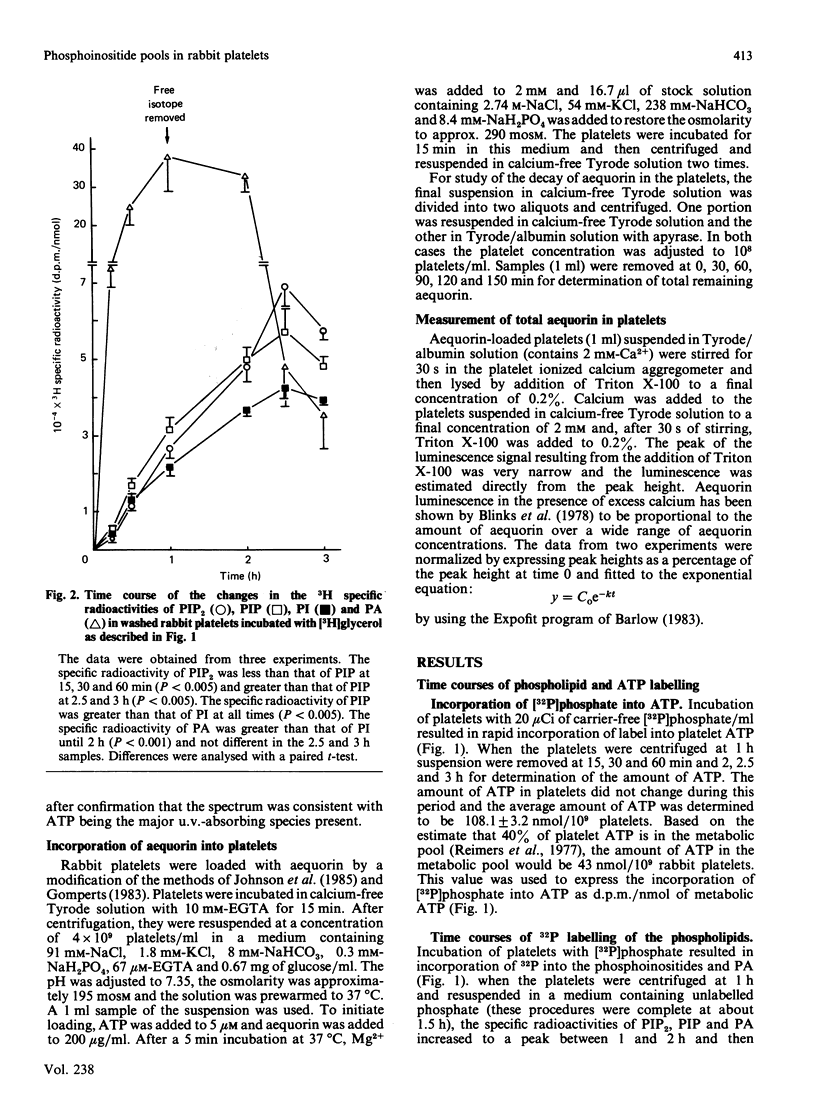
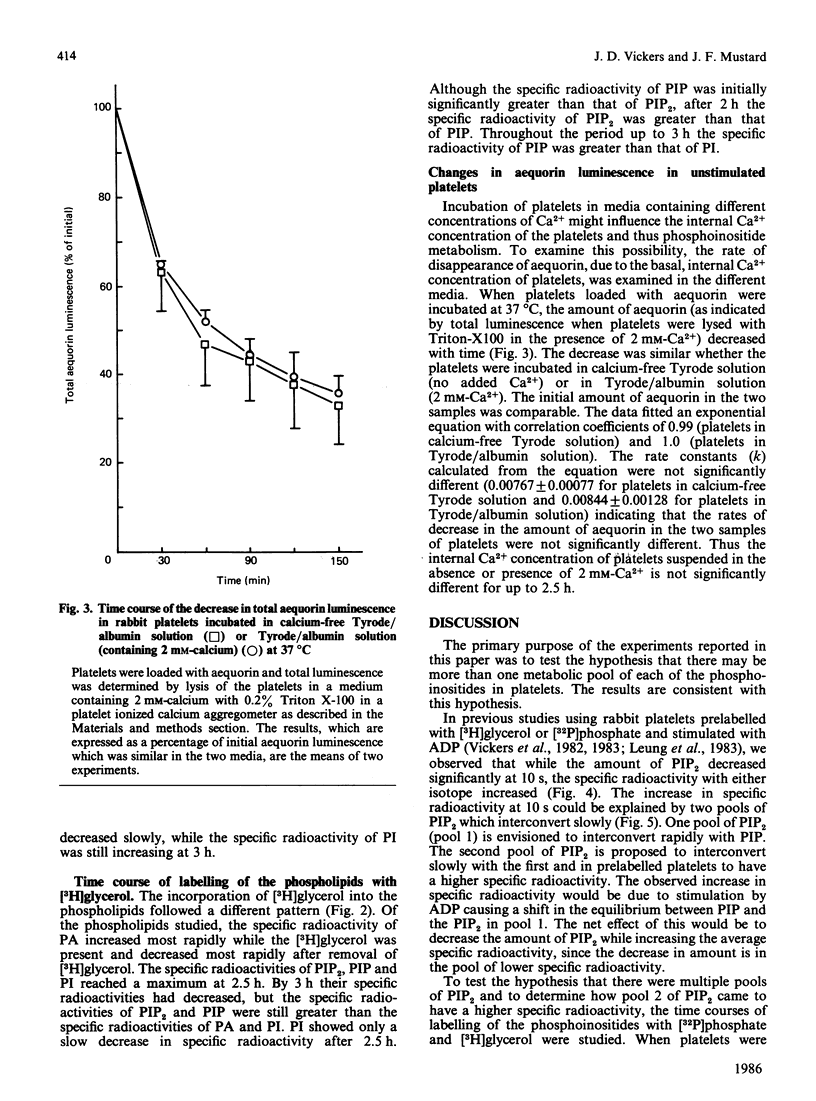
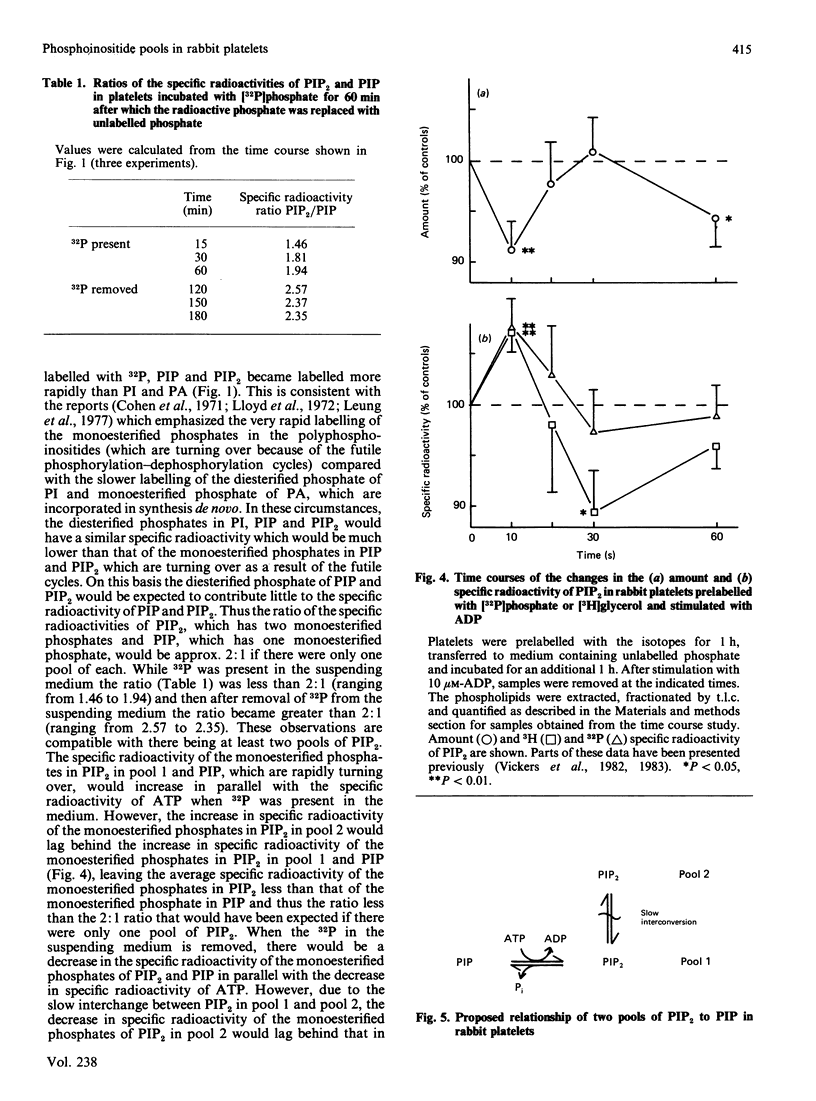
The labelling of the phosphoinositides and phosphatidic acid in washed rabbit platelets incubated with [32P]phosphate or [3H]glycerol was studied in the presence of isotope and after unincorporated isotope had been removed. With both isotopes the increase in the specific radioactivity of phosphatidylinositol 4,5-bisphosphate (PIP2) lagged behind that of phosphatidylinositol 4-phosphate (PIP) but the specific radioactivity remained higher after unincorporated isotope had been removed. This result was consistent with the presence of a second pool of PIP2, which interconverted slowly with the pool of PIP2 which was in direct equilibrium with PIP, proposed to explain the increase in specific radioactivity of PIP2 which accompanies the decrease in amount of PIP2 at 10 s in ADP-stimulated platelets. In platelets labelled with [3H]glycerol, the specific radioactivity of PIP2 became higher than that of PIP and the specific radioactivity of PIP became higher than that of phosphatidylinositol (PI). These results were interpreted to indicate that there were two pools of PIP; of these the pool with the higher specific radioactivity was the precursor of PIP2. Similarly, two pools of PI were proposed. The presence of pools of the phosphoinositides with different specific radioactivities necessitates the measurement of chemical amount of these compounds when studying the effect of stimulation of the platelets, since changes in labelling may not accurately reflect changes in the amount of the phosphoinositides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardlie N. G., Perry D. W., Packham M. A., Mustard J. F. Influence of apyrase on stability of suspensions of washed rabbit platelets. Proc Soc Exp Biol Med. 1971 Apr;136(4):1021–1023. doi: 10.3181/00379727-136-35419. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 and phospholipase C activities of platelets. Differential substrate specificity, Ca2+ requirement, pH dependence, and cellular localization. J Biol Chem. 1980 Nov 10;255(21):10227–10231. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Evidence for multiple metabolic pools of phosphatidylinositol in stimulated platelets. J Biol Chem. 1982 Oct 25;257(20):11856–11859. [PubMed] [Google Scholar]
- Chalvardjian A., Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal Biochem. 1970 Jul;36(1):225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- Chap H., Perret B., Mauco G., Simon M. F., Douste-Blazy L. Organization and role of platelet membrane phospholipids as studied with purified phospholipases. Agents Actions. 1979 Oct;9(4):400–406. doi: 10.1007/BF01970668. [DOI] [PubMed] [Google Scholar]
- Cohen P., Broekman M. J., Verkley A., Lisman J. W., Derksen A. Quantification of human platelet inositides and the influence of ionic environment on their incorporation of orthophosphate-32P. J Clin Invest. 1971 Apr;50(4):762–772. doi: 10.1172/JCI106547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Dangelmaier C. A., Holmsen H. K. Thrombin-induced platelet responses differ in requirement for receptor occupancy. Evidence for tight coupling of occupancy and compartmentalized phosphatidic acid formation. J Biol Chem. 1981 Sep 25;256(18):9393–9396. [PubMed] [Google Scholar]
- Johnson P. C., Ware J. A., Cliveden P. B., Smith M., Dvorak A. M., Salzman E. W. Measurement of ionized calcium in blood platelets with the photoprotein aequorin. Comparison with Quin 2. J Biol Chem. 1985 Feb 25;260(4):2069–2076. [PubMed] [Google Scholar]
- Laffont F., Chap H., Soula G., Douste-Blazy L. Phospholipid exchange proteins from platelet cytosol possibly involved in phospholipid effect. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1366–1371. doi: 10.1016/s0006-291x(81)80162-3. [DOI] [PubMed] [Google Scholar]
- Lagarde M., Menashi S., Crawford N. Localisation of phospholipase A2 and diglyceride lipase activities in human platelet intracellular membranes. FEBS Lett. 1981 Feb 9;124(1):23–26. doi: 10.1016/0014-5793(81)80045-2. [DOI] [PubMed] [Google Scholar]
- Leung N. L., Kinlough-Rathbone R. L., Mustard J. F. Incorporation of 32PO4 into phospholipids of blood platelets. Br J Haematol. 1977 Jul;36(3):417–425. doi: 10.1111/j.1365-2141.1977.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Leung N. L., Vickers J. D., Kinlough-Rathbone R. L., Reimers H. J., Mustard J. F. ADP-induced changes in [32P]phosphate labeling of phosphatidylinositol-4,5-bisphosphate in washed rabbit platelets made refractory by prior ADP stimulation. Biochem Biophys Res Commun. 1983 Jun 15;113(2):483–490. doi: 10.1016/0006-291x(83)91751-5. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Nishizawa E. E., Haldar J., Mustard J. F. Changes in 32 p-labelling of platelet phospholipids in response to ADP. Br J Haematol. 1972 Nov;23(5):571–585. doi: 10.1111/j.1365-2141.1972.tb07092.x. [DOI] [PubMed] [Google Scholar]
- Low M. G., Finean J. B. Specific release of plasma membrane enzymes by a phosphatidylinositol-specific phospholipase C. Biochim Biophys Acta. 1978 Apr 20;508(3):565–570. doi: 10.1016/0005-2736(78)90100-1. [DOI] [PubMed] [Google Scholar]
- Perret B., Chap H. J., Douste-Blazy L. Asymmetric distribution of arachidonic acid in the plasma membrane of human platelets. A determination using purified phospholipases and a rapid method for membrane isolation. Biochim Biophys Acta. 1979 Oct 5;556(3):434–446. doi: 10.1016/0005-2736(79)90131-7. [DOI] [PubMed] [Google Scholar]
- Reimers H. J., Packham M. A., Cazenave J. P., Mustard J. F. Effect of reserpine on adenosive uptake and metabolism, and subcellular transport of platelet adenosine triphosphate in washed rabbit platelets. Biochem Pharmacol. 1977 Sep 15;26(18):1657–1665. doi: 10.1016/0006-2952(77)90142-3. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick P. K., Kurica K. B., Chacko G. K. Location of phosphatidylethanolamine and phosphatidylserine in the human platelet plasma membrane. J Clin Invest. 1976 May;57(5):1221–1226. doi: 10.1172/JCI108390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S. D., Coleman R., Finean J. B., Michell R. H. Selective release of plasma-membrane enzymes from rat hepatocytes by a phosphatidylinositol-specific phospholipase C. Biochem J. 1980 Apr 1;187(1):277–280. doi: 10.1042/bj1870277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S. D. Minireview. Phosphatidylinositol specific phospholipases C. Life Sci. 1982 Apr 19;30(16):1323–1335. doi: 10.1016/0024-3205(82)90016-9. [DOI] [PubMed] [Google Scholar]
- Vickers J. D., Kinlough-Rathbone R. L., Mustard J. F. Changes in phosphatidylinositol-4,5-bisphosphate 10 seconds after stimulation of washed rabbit platelets with ADP. Blood. 1982 Dec;60(6):1247–1250. [PubMed] [Google Scholar]


