Abstract
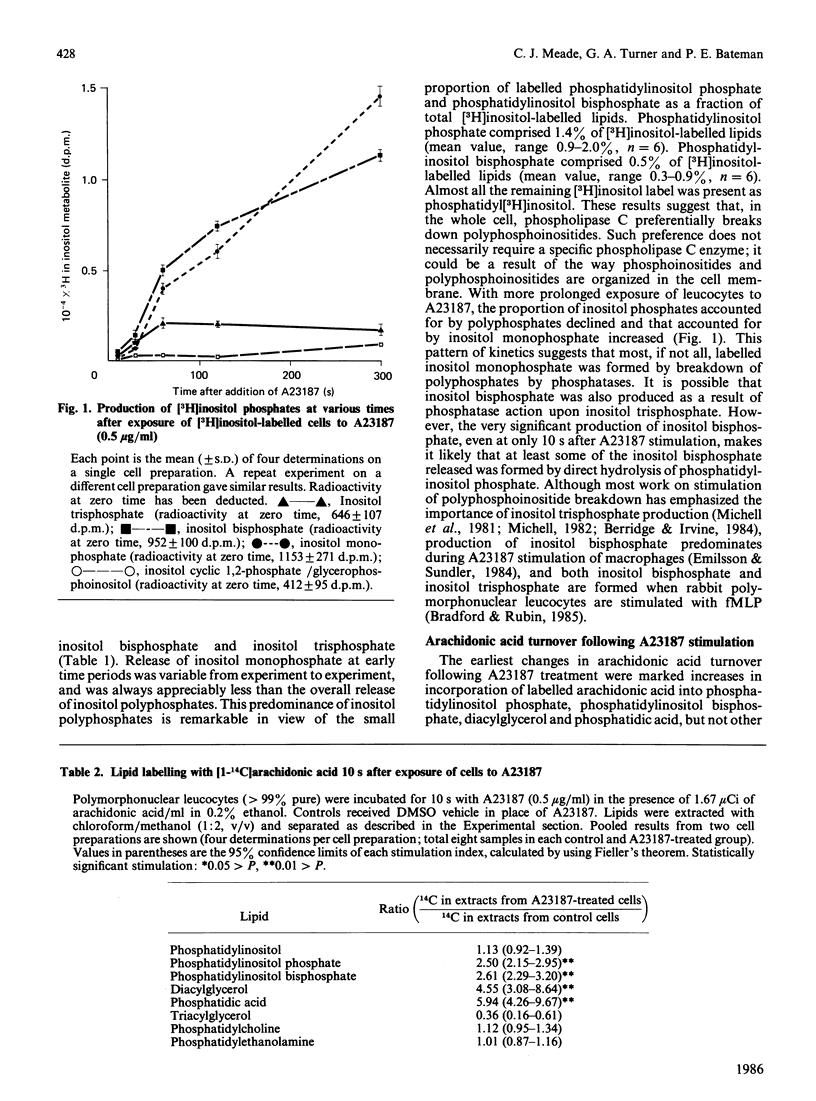
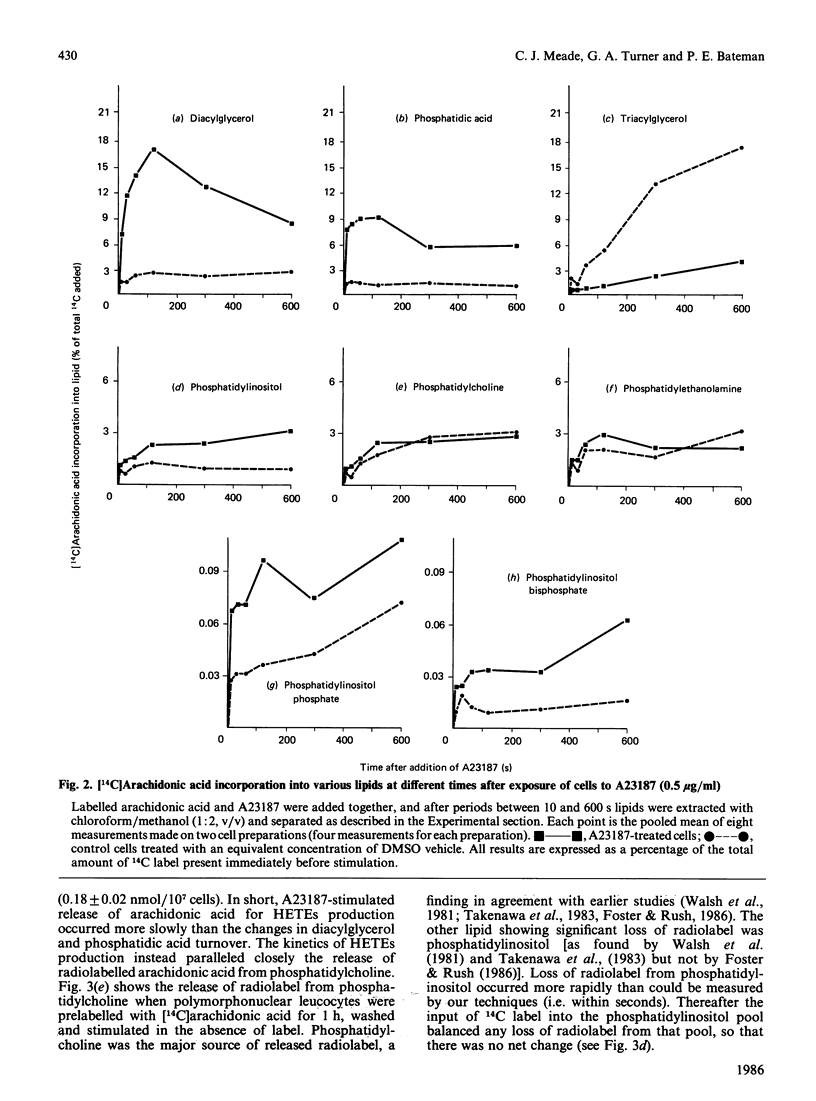
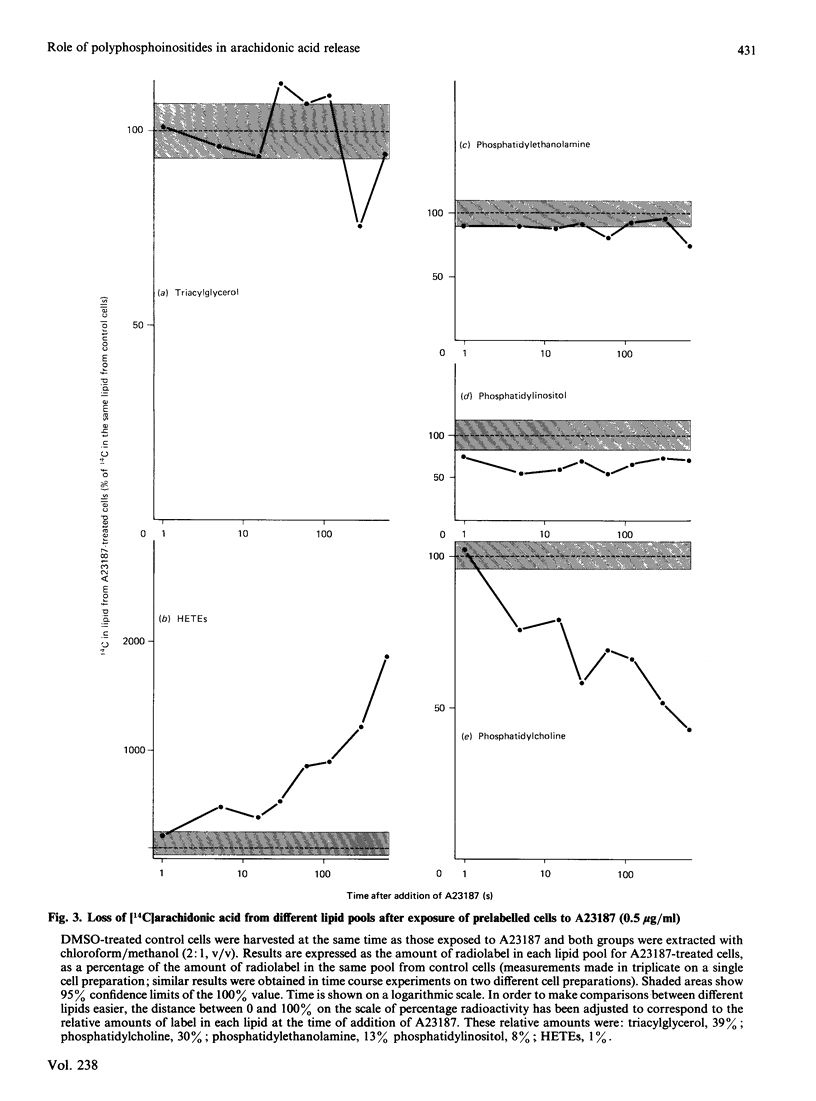
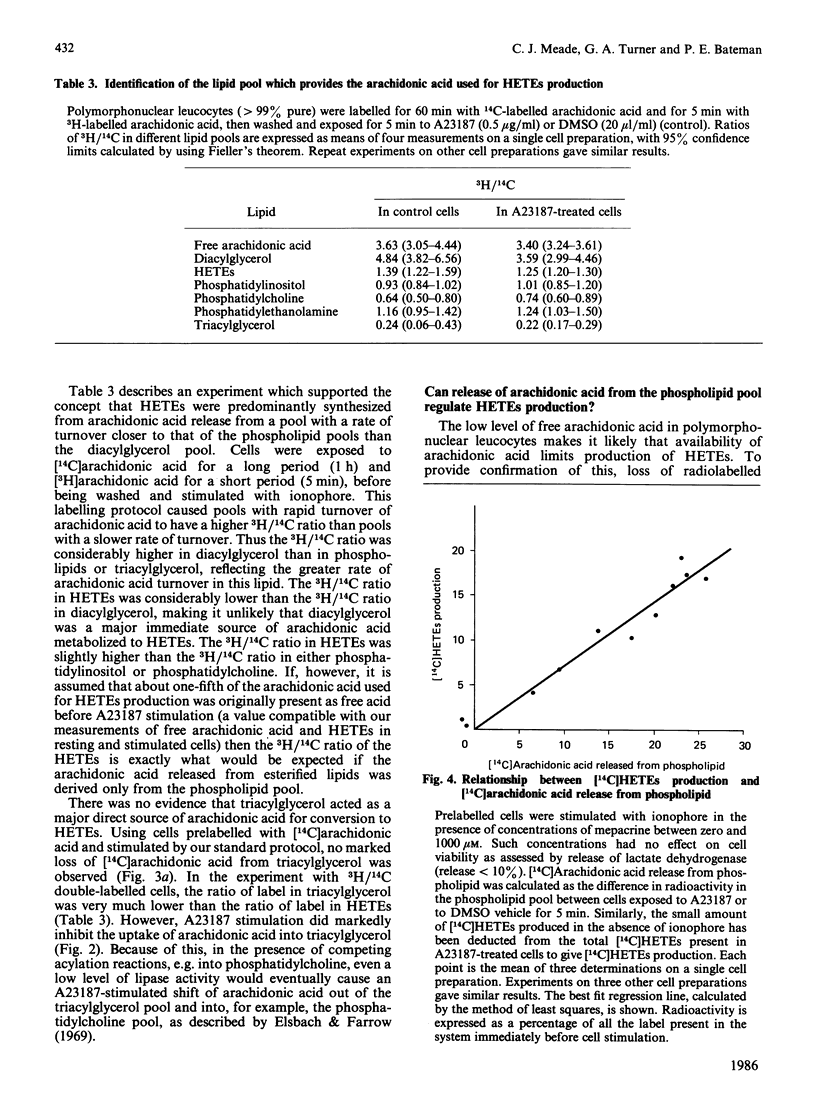
Stimulation of rabbit polymorphonuclear leucocytes with A23187 causes phospholipase C mediated breakdown of polyphosphoinositides, as evidenced by accumulation of [3H]inositol-labelled inositol bisphosphate and inositol trisphosphate. At the same time the polyphosphoinositides and the products of their breakdown, diacylglycerol and phosphatidic acid, label rapidly with radioactive arachidonic acid. Enhancement of polyphosphoinositide labelling is not as great as enhancement of diacylglycerol or phosphatidic acid labelling, suggesting additional early activation of a second independent synthetic pathway to the last named lipids. Experiments using double (3H/14C) labelling, to distinguish pools with different rates of turnover, suggest the major pool of arachidonic acid used for synthesis of lipoxygenase metabolites turns over more slowly than arachidonic acid in diacylglycerol, but at about the same rate as arachidonic acid esterified in phosphatidylcholine or phosphatidylinositol. Further, when cells are prelabelled with [14C]arachidonic acid, then stimulated for 5 min, it is only from phosphatidylcholine, and to a lesser extent phosphatidylinositol, that radiolabel is lost. Release of arachidonic acid is probably via phospholipase A2, since it is blocked by the phospholipase A2 inhibitor manoalide. The absence of accumulated lysophosphatides can be explained by reacylation and, in the case of lysophosphatidylinositol, deacylation. The importance of phospholipase A2 in phosphatidylinositol breakdown contrasts with the major role of phospholipase C in polyphosphoinositide hydrolysis. Measurements of absolute free fatty acid levels, as well as studies showing a correlation between production of radiolabelled hydroxyeicosatetraenoic acids and release of radiolabel from the phospholipid pool, both suggest that hydrolysis of arachidonic acid esterified into phospholipids is the limiting factor regulating formation of lipoxygenase metabolites. By contrast with A23187, fMet-Leu-Phe (a widely used polymorphonuclear leucocyte activator) is a poor stimulant for arachidonic acid release unless a 'second signal' (e.g. cytochalasin B, or a product of A23187-stimulated cells) is also present. In the presence of cytochalasin B, fMet-Leu-Phe, like A23187, stimulates release of radiolabelled arachidonic acid principally from phosphatidylcholine.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Scott W. A., Cohn Z. A. Evidence for sequential signals in the induction of the arachidonic acid cascade in macrophages. J Exp Med. 1986 Jan 1;163(1):139–154. doi: 10.1084/jem.163.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. R., Thompson W. Selective acylation of 1-acylglycerophosphorylinositol by rat brain microsomes. Comparison with 1-acylglycerophosphorylcholine. J Biol Chem. 1973 Oct 25;248(20):7060–7065. [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Use of cytochalasin B to distinguish between early and late events in neutrophil activation. Biochim Biophys Acta. 1980 Oct 2;601(3):584–591. doi: 10.1016/0005-2736(80)90560-x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Meade C. J., Turner G. A., Klaus G. G. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985 Jul;41(3):999–1006. doi: 10.1016/s0092-8674(85)80080-5. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Evidence for multiple metabolic pools of phosphatidylinositol in stimulated platelets. J Biol Chem. 1982 Oct 25;257(20):11856–11859. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Formation of lysophosphatidylinositol in platelets stimulated with thrombin or ionophore A23187. J Biol Chem. 1982 May 10;257(9):5196–5200. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Rapid decrease of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. J Biol Chem. 1982 Nov 10;257(21):12705–12708. [PubMed] [Google Scholar]
- Bjerve K. S., Daae L. N., Bremer J. The selective loss of lysophospholipids in some commonly used lipid-extraction procedures. Anal Biochem. 1974 Mar;58(1):238–245. doi: 10.1016/0003-2697(74)90463-1. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. Formation of a novel dihydroxyeicosatetraenoic acid. J Biol Chem. 1979 Apr 25;254(8):2643–2646. [PubMed] [Google Scholar]
- Bormann B. J., Huang C. K., Mackin W. M., Becker E. L. Receptor-mediated activation of a phospholipase A2 in rabbit neutrophil plasma membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):767–770. doi: 10.1073/pnas.81.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Characterization of formylmethionyl-leucyl-phenylalanine stimulation of inositol trisphosphate accumulation in rabbit neutrophils. Mol Pharmacol. 1985 Jan;27(1):74–78. [PubMed] [Google Scholar]
- Clancy R. M., Dahinden C. A., Hugli T. E. Arachidonate metabolism by human polymorphonuclear leukocytes stimulated by N-formyl-Met-Leu-Phe or complement component C5a is independent of phospholipase activation. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7200–7204. doi: 10.1073/pnas.80.23.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. The dependence on Ca2+ of phosphatidylinositol breakdown and enzyme secretion in rabbit neutrophils stimulated by formylmethionyl-leucylphenylalanine or ionomycin. Biochem J. 1981 Dec 15;200(3):501–508. doi: 10.1042/bj2000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S. Ca2+-dependent conversion of phosphatidylinositol to phosphatidate in neutrophils stimulated with fMet-Leu-Phe or ionophore A23187. Biochim Biophys Acta. 1984 Aug 15;795(1):37–46. doi: 10.1016/0005-2760(84)90102-4. [DOI] [PubMed] [Google Scholar]
- Cohen P., Derksen A. Comparison of phospholipid and fatty acid composition of human erythrocytes and platelets. Br J Haematol. 1969 Oct;17(4):359–371. doi: 10.1111/j.1365-2141.1969.tb01382.x. [DOI] [PubMed] [Google Scholar]
- Cosentino M. J., Ellis L. C. A rapid simple radiometric assay for phospholipase A2 activity. Prostaglandins. 1981 Aug;22(2):309–322. doi: 10.1016/0090-6980(81)90045-9. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Clarke N. D-myoinositol 1:2-cyclic phosphate 2-phosphohydrolase. Biochem J. 1972 Mar;127(1):113–118. doi: 10.1042/bj1270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. B., Galliard T., Hawthorne J. N. Phosphoinositides. 5. The inositol lipids of ox brain. Biochem J. 1963 Jul;88(1):125–131. doi: 10.1042/bj0880125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Farrow S. Cellular triglyceride as a source of fatty acid for lecithin synthesis during phagocytosis. Biochim Biophys Acta. 1969 Mar 4;176(2):438–441. doi: 10.1016/0005-2760(69)90208-2. [DOI] [PubMed] [Google Scholar]
- Emilsson A., Sundler R. Differential activation of phosphatidylinositol deacylation and a pathway via diphosphoinositide in macrophages responding to zymosan and ionophore A23187. J Biol Chem. 1984 Mar 10;259(5):3111–3116. [PubMed] [Google Scholar]
- GRADO C., BALLOU C. E. Myo-inositol phosphates obtained by alkaline hydrolysis of beef brain phosphoinositide. J Biol Chem. 1961 Jan;236:54–60. [PubMed] [Google Scholar]
- Glaser K. B., Jacobs R. S. Molecular pharmacology of manoalide. Inactivation of bee venom phospholipase A2. Biochem Pharmacol. 1986 Feb 1;35(3):449–453. doi: 10.1016/0006-2952(86)90218-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sastre F., Folch-Pi J. Thin-layer chromatography of the phosphoinositides. J Lipid Res. 1968 Jul;9(4):532–533. [PubMed] [Google Scholar]
- Harvey J., Osborne D. J. A rapid method for detecting inhibitors of both cyclo-oxygenase lipoxygenase metabolites of arachidonic acid. J Pharmacol Methods. 1983 Apr;9(2):147–155. doi: 10.1016/0160-5402(83)90006-2. [DOI] [PubMed] [Google Scholar]
- Hax W. M., van Kessel W. S. High-performance liquid chromatographic separation and photometric detection of phospholipids. J Chromatogr. 1977 Nov 11;142:735–741. doi: 10.1016/s0021-9673(01)92081-3. [DOI] [PubMed] [Google Scholar]
- Hirata F. The regulation of lipomodulin, a phospholipase inhibitory protein, in rabbit neutrophils by phosphorylation. J Biol Chem. 1981 Aug 10;256(15):7730–7733. [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Meade C. J., Dawson R. M. One-dimensional thin-layer chromatographic separation of the lipids involved in arachidonic acid metabolism. J Pharmacol Methods. 1984 Oct;12(3):171–182. doi: 10.1016/0160-5402(84)90057-3. [DOI] [PubMed] [Google Scholar]
- Koch-Kallnbach M. E., Diringer H. Isolation and separation of inositol 1-phosphate, cyclic inositol 1,2-phosphate, and glycerylphosphoinositol from tissue culture cells labeled with [3H]inositol. Hoppe Seylers Z Physiol Chem. 1977 Mar;358(3):367–375. doi: 10.1515/bchm2.1977.358.1.367. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M., Cuatrecasas P. Rapid acylation and deacylation of arachidonic acid into phosphatidic acid of horse neutrophils. J Biol Chem. 1980 Nov 25;255(22):10966–10970. [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M. The importance of the production of phosphatidic acid for the release of arachidonic acid in stimulated platelets. Agents Actions. 1981 Dec;11(6-7):536–537. doi: 10.1007/BF01978728. [DOI] [PubMed] [Google Scholar]
- Lombardo D., Dennis E. A. Cobra venom phospholipase A2 inhibition by manoalide. A novel type of phospholipase inhibitor. J Biol Chem. 1985 Jun 25;260(12):7234–7240. [PubMed] [Google Scholar]
- Michell R. H. Inositol lipid metabolism in dividing and differentiating cells. Cell Calcium. 1982 Oct;3(4-5):429–440. doi: 10.1016/0143-4160(82)90028-8. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Monaco M. E. The phosphatidylinositol cycle in WRK-1 cells. Evidence for a separate, hormone-sensitive phosphatidylinositol pool. J Biol Chem. 1982 Mar 10;257(5):2137–2139. [PubMed] [Google Scholar]
- Murase S., Okuyama H. A membrane-bound phospholipase C with an apparent specificity for lysophosphatidylinositol in porcine platelets. J Biol Chem. 1985 Jan 10;260(1):262–265. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Osborne D. J., Peters B. J., Meade C. J. The separation of leukotrienes and hydroxyeicosatetraenoic acid metabolites of arachidonic acid by high performance liquid chromatography (HPLC). Prostaglandins. 1983 Nov;26(5):817–832. doi: 10.1016/0090-6980(83)90065-5. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Deykin D. The mobilization of arachidonic acid in platelets exposed to thrombin or ionophore A23187. Effects of adenosine triphosphate deprivation. J Clin Invest. 1977 Aug;60(2):495–498. doi: 10.1172/JCI108801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse S. E. Activation of human platelet phospholipase C by ionophore A23187 is totally dependent upon cyclo-oxygenase products and ADP. Biochem J. 1984 Aug 15;222(1):103–110. doi: 10.1042/bj2220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse S. E., Allen C. L. Synergistic activation by collagen and 15-hydroxy-9 alpha,11 alpha-peroxidoprosta-5,13-dienoic acid (PGH2) of phosphatidylinositol metabolism and arachidonic acid release in human platelets. J Clin Invest. 1982 Dec;70(6):1216–1224. doi: 10.1172/JCI110720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. P., Sink L. E., Freer R. J. Activation of (arachidonyl) phosphatidylinositol turnover in rabbit neutrophils by the calcium ionophore A23187. Biochem J. 1981 Feb 15;194(2):497–505. doi: 10.1042/bj1940497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978 Nov;19(8):1063–1067. [PubMed] [Google Scholar]
- Smith J. B., Dangelmaier C., Mauco G. Measurement of arachidonic acid liberation in thrombin-stimulated human platelets. Use of agents that inhibit both the cyclooxygenase and lipoxygenase enzymes. Biochim Biophys Acta. 1985 Jul 9;835(2):344–351. doi: 10.1016/0005-2760(85)90290-5. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Homma Y., Nagai Y. Role of Ca2+ in phosphatidylinositol response and arachidonic acid release in formylated tripeptide- or Ca2+ ionophore A23187-stimulated guinea pig neutrophils. J Immunol. 1983 Jun;130(6):2849–2855. [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Walsh C. E., Waite B. M., Thomas M. J., DeChatelet L. R. Release and metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1981 Jul 25;256(14):7228–7234. [PubMed] [Google Scholar]


