Abstract
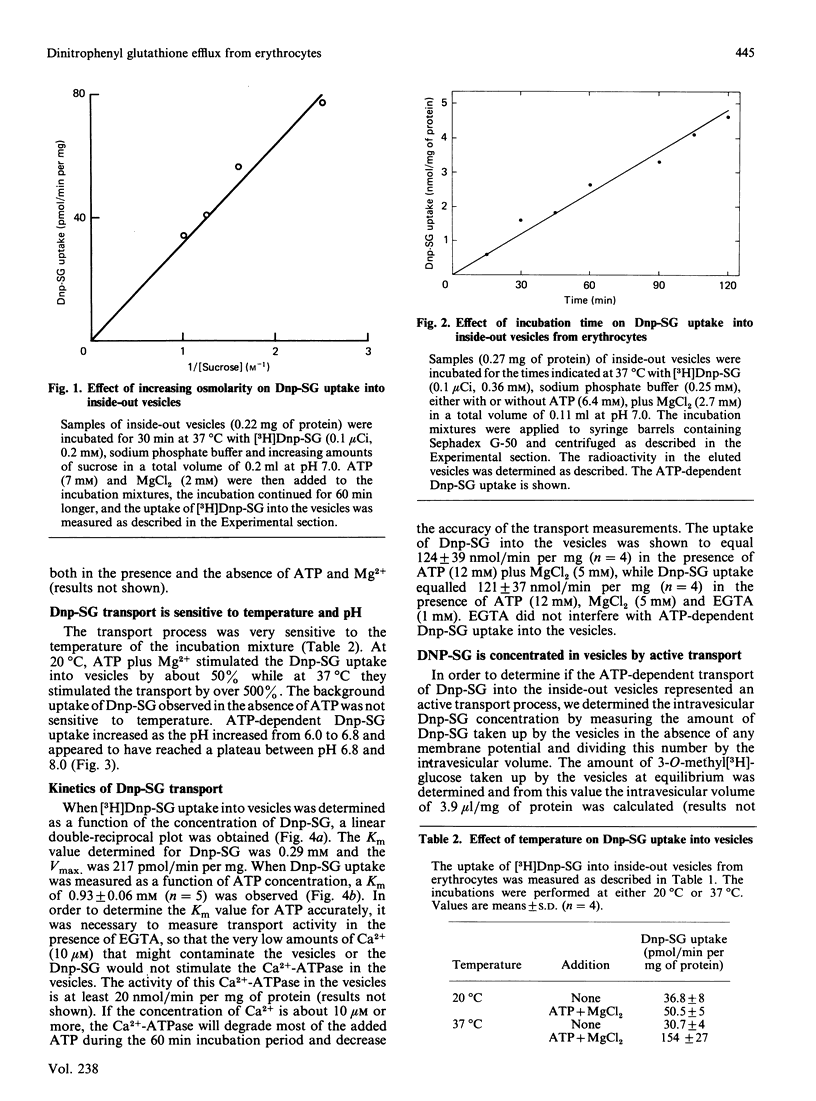
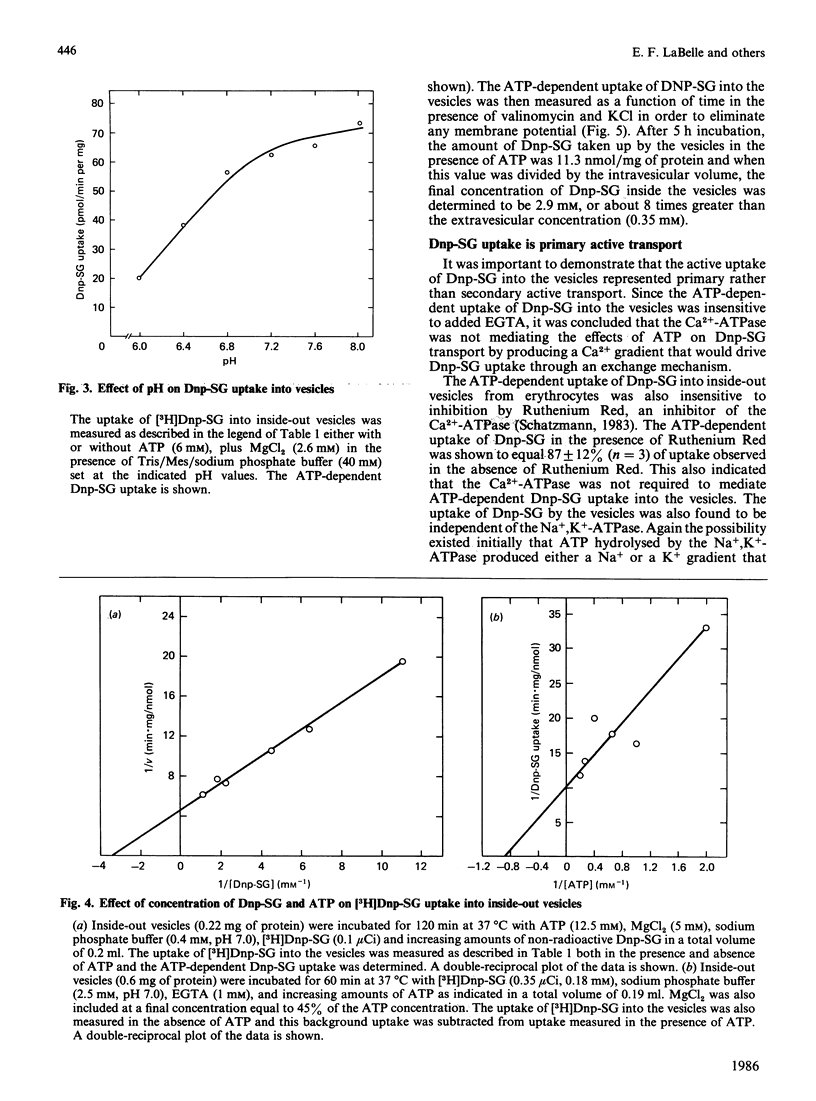
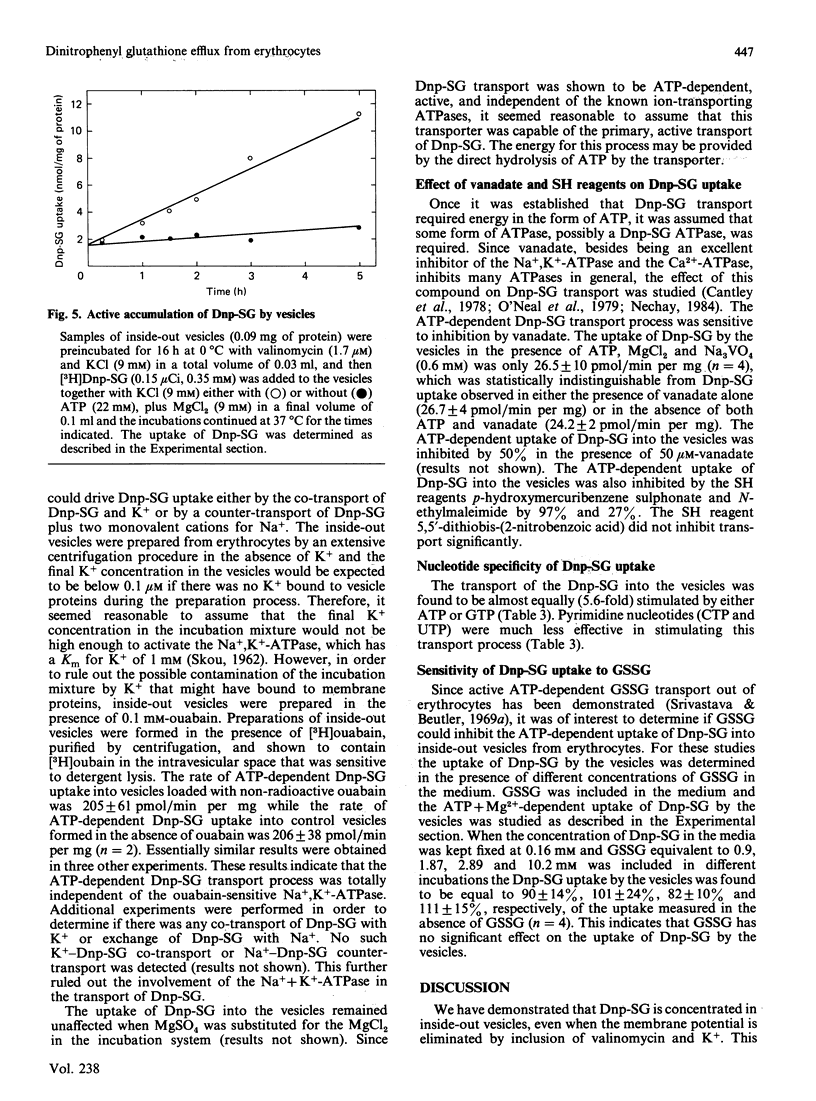
Dinitrophenyl S-glutathione is accumulated by inside-out vesicles made from human erythrocytes in a process totally dependent on ATP and Mg2+. The vesicles were shown to accumulate dinitrophenyl S-glutathione against a concentration gradient. The vesicles were able to concentrate this glutathione derivative even in the absence of membrane potential. This indicated that the ATP-dependent uptake of dinitrophenyl S-glutathione by inside-out vesicles represented an active transport process. Neither extravesicular EGTA nor intravesicular ouabain inhibited the transport process, indicating that neither the Ca2+-ATPase nor the Na+, K+-ATPase were involved. These results indicated that dinitrophenyl S-glutathione uptake by inside-out vesicles probably represented primary active transport. The uptake of dinitrophenyl S-glutathione was a linear function of time (up to 5 h) and vesicle protein. The rate of uptake was optimal between pH 7.0 and 8.0 and at 37 degrees C. The Km values determined for dinitrophenyl S-glutathione and ATP were 0.29 mM and 1 mM, respectively. The transport process was completely inhibited by vanadate and by p-hydroxymercuribenzene sulphonate and inhibited to a lesser extent by N-ethylmaleimide. GTP could efficiently substitute for ATP as an energy source for the transport process, but CTP and UTP were comparatively much less effective.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Bilzer M., Sies H. Competition between transport of glutathione disulfide (GSSG) and glutathione S-conjugates from perfused rat liver into bile. FEBS Lett. 1982 Apr 5;140(1):73–76. doi: 10.1016/0014-5793(82)80523-1. [DOI] [PubMed] [Google Scholar]
- Akerboom T., Inoue M., Sies H., Kinne R., Arias I. M. Biliary transport of glutathione disulfide studied with isolated rat-liver canalicular-membrane vesicles. Eur J Biochem. 1984 May 15;141(1):211–215. doi: 10.1111/j.1432-1033.1984.tb08177.x. [DOI] [PubMed] [Google Scholar]
- Awasthi Y. C., Garg H. S., Dao D. D., Partridge C. A., Srivastava S. K. Enzymatic conjugation of erythrocyte glutathione with 1-chloro-2,4-dinitrobenzene: the fate of glutathione conjugate in erythrocytes and the effect of glutathione depletion on hemoglobin. Blood. 1981 Oct;58(4):733–738. [PubMed] [Google Scholar]
- Awasthi Y. C., Misra G., Rassin D. K., Srivastava S. K. Detoxification of xenobiotics by glutathione S-transferases in erythrocytes: the transport of the conjugate of glutathione and 1-chloro-2,4-dinitrobenzene. Br J Haematol. 1983 Nov;55(3):419–425. doi: 10.1111/j.1365-2141.1983.tb02156.x. [DOI] [PubMed] [Google Scholar]
- Awasthi Y. C., Singh S. V. Purification and characterization of a new form of glutathione S-transferase from human erythrocytes. Biochem Biophys Res Commun. 1984 Dec 28;125(3):1053–1060. doi: 10.1016/0006-291x(84)91390-1. [DOI] [PubMed] [Google Scholar]
- Board P. G. Transport of glutathione S-conjugate from human erythrocytes. FEBS Lett. 1981 Feb 23;124(2):163–165. doi: 10.1016/0014-5793(81)80127-5. [DOI] [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyland E., Chasseaud L. F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:173–219. doi: 10.1002/9780470122778.ch5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GLUTATHIONE PEROXIDASE: THE PRIMARY AGENT FOR THE ELIMINATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES. Biochemistry. 1963 Nov-Dec;2:1420–1428. doi: 10.1021/bi00906a038. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Resh M. D., Guidotti G. Vanadate inhibits the red cell (Na+, K+) ATPase from the cytoplasmic side. Nature. 1978 Apr 6;272(5653):552–554. doi: 10.1038/272552a0. [DOI] [PubMed] [Google Scholar]
- Inoue M., Akerboom T. P., Sies H., Kinne R., Thao T., Arias I. M. Biliary transport of glutathione S-conjugate by rat liver canalicular membrane vesicles. J Biol Chem. 1984 Apr 25;259(8):4998–5002. [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. Glutathione transport across hepatocyte plasma membranes. Analysis using isolated rat-liver sinusoidal-membrane vesicles. Eur J Biochem. 1984 Feb 1;138(3):491–495. doi: 10.1111/j.1432-1033.1984.tb07943.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Esterbauer H., Sies H. Role of cardiac glutathione transferase and of the glutathione S-conjugate export system in biotransformation of 4-hydroxynonenal in the heart. J Biol Chem. 1986 Feb 5;261(4):1576–1581. [PubMed] [Google Scholar]
- Ishikawa T., Sies H. Cardiac transport of glutathione disulfide and S-conjugate. Studies with isolated perfused rat heart during hydroperoxide metabolism. J Biol Chem. 1984 Mar 25;259(6):3838–3843. [PubMed] [Google Scholar]
- Kondo T., Dale G. L., Beutler E. Glutathione transport by inside-out vesicles from human erythrocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6359–6362. doi: 10.1073/pnas.77.11.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Dale G. L., Beutler E. Studies on glutathione transport utilizing inside-out vesicles prepared from human erythrocytes. Biochim Biophys Acta. 1981 Jul 6;645(1):132–136. doi: 10.1016/0005-2736(81)90520-4. [DOI] [PubMed] [Google Scholar]
- Kondo T., Murao M., Taniguchi N. Glutathione S-conjugate transport using inside-out vesicles from human erythrocytes. Eur J Biochem. 1982 Jul;125(3):551–554. doi: 10.1111/j.1432-1033.1982.tb06717.x. [DOI] [PubMed] [Google Scholar]
- LaBelle E. F., Valentine M. E. Inhibition by amiloride of 22Na+ transport into toad bladder microsomes. Biochim Biophys Acta. 1980 Sep 2;601(1):195–205. doi: 10.1016/0005-2736(80)90524-6. [DOI] [PubMed] [Google Scholar]
- Marcus C. J., Habig W. H., Jakoby W. B. Glutathione transferase from human erythrocytes. Nonidentity with the enzymes from liver. Arch Biochem Biophys. 1978 Jun;188(2):287–293. doi: 10.1016/s0003-9861(78)80011-3. [DOI] [PubMed] [Google Scholar]
- Nechay B. R. Mechanisms of action of vanadium. Annu Rev Pharmacol Toxicol. 1984;24:501–524. doi: 10.1146/annurev.pa.24.040184.002441. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Baldi C., Svensson S. A., Larsson R., Bellomo G., Orrenius S. Glutathione S-conjugates stimulate ATP hydrolysis in the plasma membrane fraction of rat hepatocytes. FEBS Lett. 1985 Jul 22;187(1):121–125. doi: 10.1016/0014-5793(85)81226-6. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Moore M., Bellomo G., Mirabelli F., Orrenius S. Demonstration and partial characterization of glutathione disulfide-stimulated ATPase activity in the plasma membrane fraction from rat hepatocytes. J Biol Chem. 1985 Feb 25;260(4):1999–2002. [PubMed] [Google Scholar]
- O'Neal S. G., Rhoads D. B., Racker E. Vanadate inhibition of sarcoplasmic reticulum Ca2+-ATPase and other ATPases. Biochem Biophys Res Commun. 1979 Aug 13;89(3):845–850. doi: 10.1016/0006-291x(79)91855-2. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Prchal J., Srivastava S. K., Beutler E. Active transport of GSSG from reconstituted erythrocyte ghosts. Blood. 1975 Jul;46(1):111–117. [PubMed] [Google Scholar]
- SKOU J. C. Preparation from mammallian brain and kidney of the enzyme system involved in active transport of Na ions and K ions. Biochim Biophys Acta. 1962 Apr 9;58:314–325. doi: 10.1016/0006-3002(62)91015-6. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. The red cell calcium pump. Annu Rev Physiol. 1983;45:303–312. doi: 10.1146/annurev.ph.45.030183.001511. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Ansari N. H., Awasthi Y. C. Lens glutathione depletion of 1-chloro-2,4-dinitrobenzene and oxidative stress. Curr Eye Res. 1984 Jan;3(1):117–119. doi: 10.3109/02713688408997192. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Accurate measurement of oxidized glutathione content of human, rabbit, and rat red blood cells and tissues. Anal Biochem. 1968 Oct 24;25(1):70–76. doi: 10.1016/0003-2697(68)90082-1. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Cataract produced by tyrosinase and tyrosine systems in rabbitens in vitro. Biochem J. 1969 May;112(4):421–425. doi: 10.1042/bj1120421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Permeability of normal and cataractous rabbit lenses to glutathione. Proc Soc Exp Biol Med. 1968 Feb;127(2):512–514. doi: 10.3181/00379727-127-32727. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. The transport of oxidized glutathione from human erythrocytes. J Biol Chem. 1969 Jan 10;244(1):9–16. [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]


