Abstract
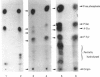
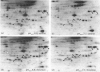
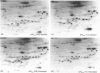
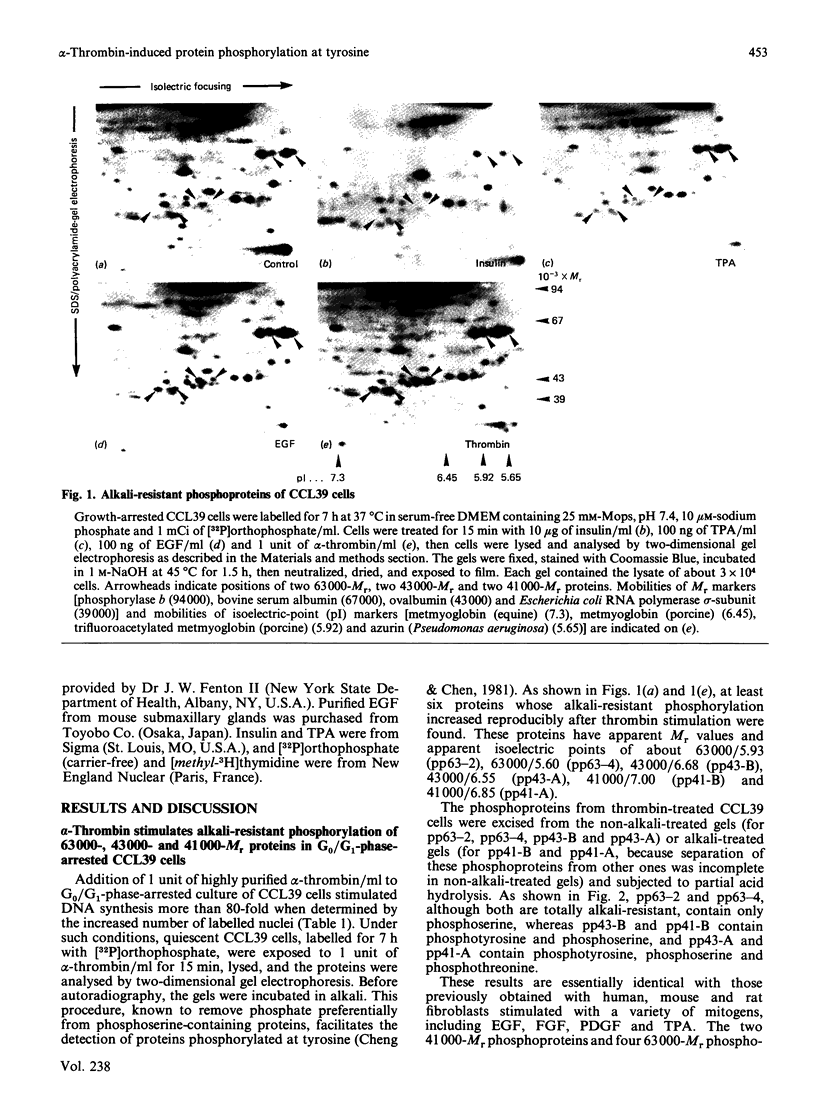
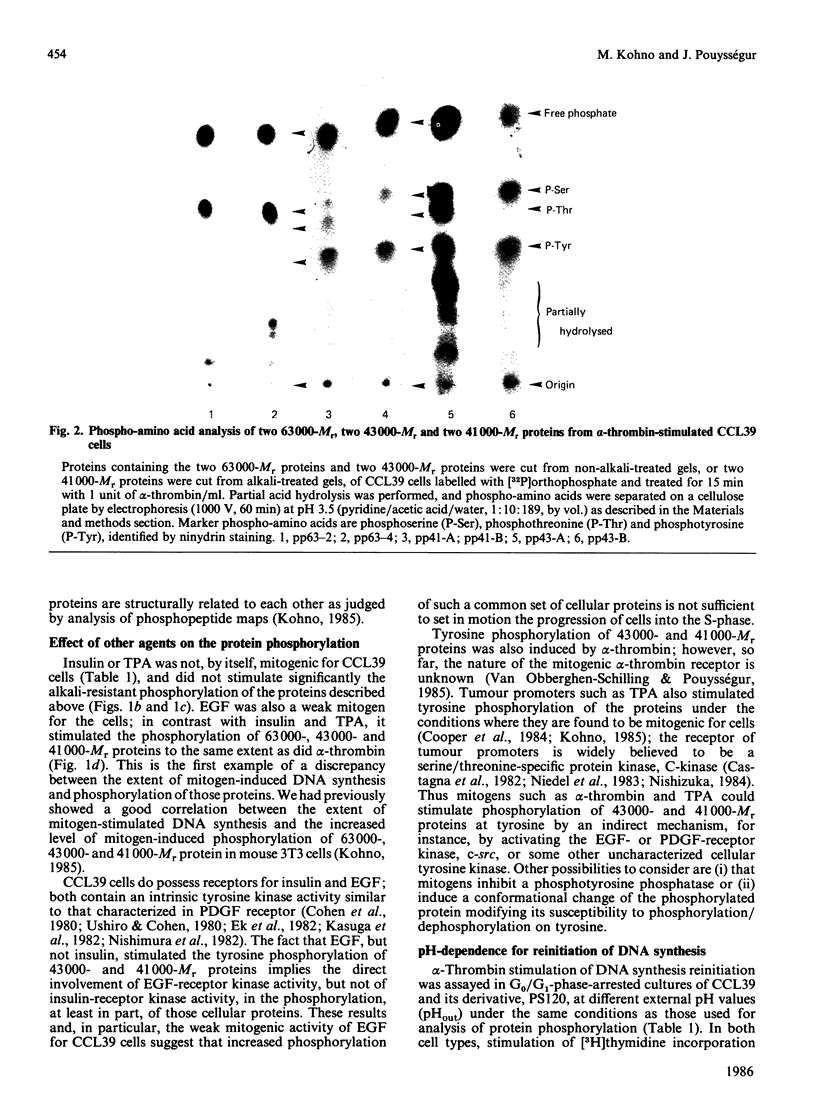
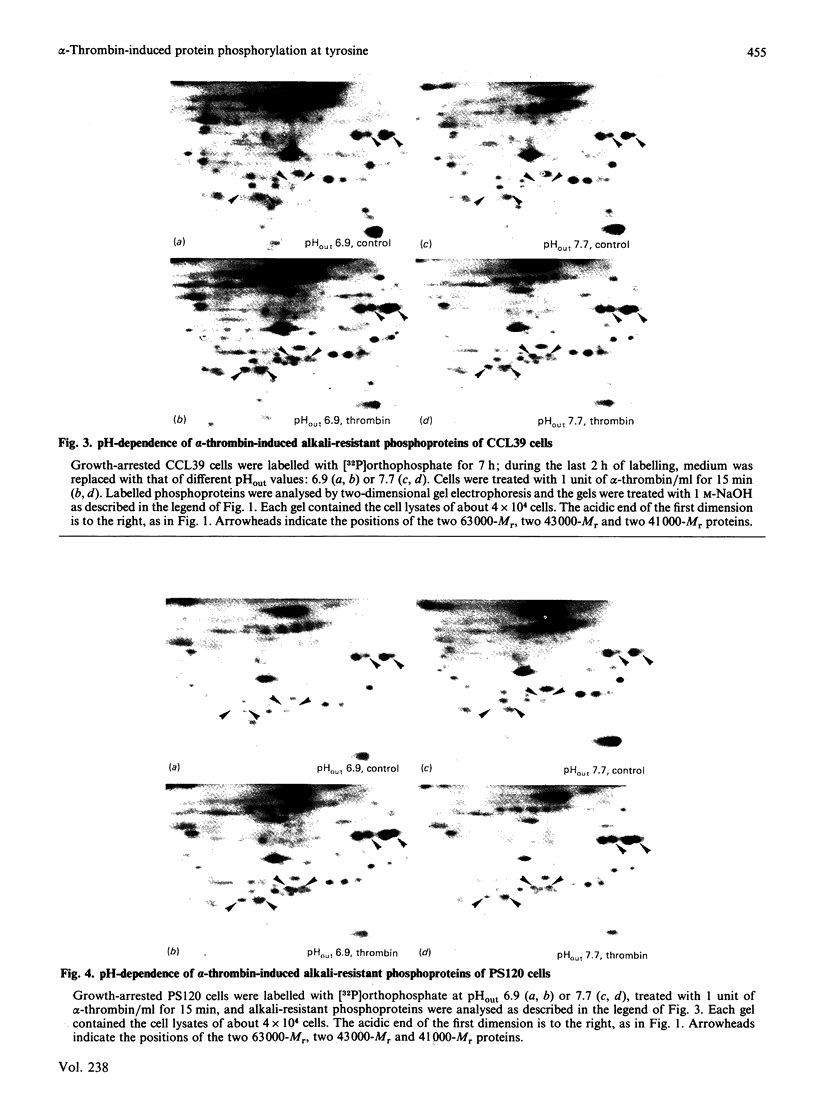
Incubation of quiescent Chinese-hamster fibroblasts (CCL39) with alpha-thrombin, a potent mitogen for the cells, was found to stimulate the rapid phosphorylation of two 43,000-Mr and two 41,000-Mr proteins at tyrosine, threonine and/or serine, and two 63,000-Mr proteins at serine. Insulin, 12-O-tetradecanoylphorbol 13-acetate (TPA) and epidermal growth factor (EGF) are weak mitogens for cells; insulin and TPA did not stimulate the phosphorylation of those proteins significantly, whereas EGF stimulated their phosphorylation to the same extent as did alpha-thrombin. We analysed alpha-thrombin-induced protein phosphorylation at different external pH values in CCL39 and in the mutant derivative PS120, which lacks Na+/H+-antiport activity. We showed that cytoplasmic alkalinization, a common and early response to mitogens, is not required to trigger phosphorylation of 63,000-, 43,000- and 41,000-Mr proteins, either at tyrosine or serine and threonine residues. This finding contrasts with the phosphorylation of ribosomal protein S6, which takes place only at permissive pH for reinitiation of DNA synthesis. These results, demonstrating that phosphorylation of 63,000-, 43,000- and 41,000-Mr proteins and cytoplasmic alkalinization are not coupled, reinforce the idea that the site of action of intracellular pH controlling the commitment of G0/G1-phase-arrested cells to DNA synthesis might be restricted to mitogen-stimulated S6 phosphorylation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Jakobs K. H. Ni-mediated inhibition of human platelet adenylate cyclase by thrombin. Eur J Biochem. 1984 Dec 3;145(2):333–338. doi: 10.1111/j.1432-1033.1984.tb08558.x. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chambard J. C., Franchi A., Le Cam A., Pouysségur J. Growth factor-stimulated protein phosphorylation in G0/G1-arrested fibroblasts. Two distinct classes of growth factors with potentiating effects. J Biol Chem. 1983 Feb 10;258(3):1706–1713. [PubMed] [Google Scholar]
- Cheng Y. S., Chen L. B. Detection of phosphotyrosine-containing 34,000-dalton protein in the framework of cells transformed with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2388–2392. doi: 10.1073/pnas.78.4.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Diverse mitogenic agents induce the phosphorylation of two related 42,000-dalton proteins on tyrosine in quiescent chick cells. Mol Cell Biol. 1984 Jan;4(1):30–37. doi: 10.1128/mcb.4.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Gilmore T., Martin G. S. Phorbol ester and diacylglycerol induce protein phosphorylation at tyrosine. Nature. 1983 Dec 1;306(5942):487–490. doi: 10.1038/306487a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Kohno M. Diverse mitogenic agents induce rapid phosphorylation of a common set of cellular proteins at tyrosine in quiescent mammalian cells. J Biol Chem. 1985 Feb 10;260(3):1771–1779. [PubMed] [Google Scholar]
- Kohno M., Kuwata S., Namba Y., Hanaoka M. Interleukin 2 induces rapid phosphorylation of cellular proteins in murine T-lymphocytes. FEBS Lett. 1986 Mar 17;198(1):33–37. doi: 10.1016/0014-5793(86)81179-6. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Franchi A., Cragoe E., Jr, Pouysségur J. Blockade of the Na+/H+ antiport abolishes growth factor-induced DNA synthesis in fibroblasts. Structure-activity relationships in the amiloride series. J Biol Chem. 1984 Apr 10;259(7):4313–4319. [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- Nakamura K. D., Martinez R., Weber M. J. Tyrosine phosphorylation of specific proteins after mitogen stimulation of chicken embryo fibroblasts. Mol Cell Biol. 1983 Mar;3(3):380–390. doi: 10.1128/mcb.3.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., Huang J. S., Deuel T. F. Platelet-derived growth factor stimulates tyrosine-specific protein kinase activity in Swiss mouse 3T3 cell membranes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4303–4307. doi: 10.1073/pnas.79.14.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Chambard J. C., Franchi A., Paris S., Van Obberghen-Schilling E. Growth factor activation of an amiloride-sensitive Na+/H+ exchange system in quiescent fibroblasts: coupling to ribosomal protein S6 phosphorylation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3935–3939. doi: 10.1073/pnas.79.13.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., L'Allemain G., Paris S. Cytoplasmic pH, a key determinant of growth factor-induced DNA synthesis in quiescent fibroblasts. FEBS Lett. 1985 Oct 7;190(1):115–119. doi: 10.1016/0014-5793(85)80439-7. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., Silvestre P. Relationship between increased aerobic glycolysis and DNA synthesis initiation studied using glycolytic mutant fibroblasts. Nature. 1980 Oct 2;287(5781):445–447. doi: 10.1038/287445a0. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Sardet C., Franchi A., L'Allemain G., Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Pouysségur J. Affinity labeling of high-affinity alpha-thrombin binding sites on the surface of hamster fibroblasts. Biochim Biophys Acta. 1985 Dec 12;847(3):335–343. doi: 10.1016/0167-4889(85)90039-4. [DOI] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Pérez-Rodríguez R., Franchi A., Chambard J. C., Pouysségur J. Analysis of growth factor "relaxation" in Chinese hamster lung fibroblasts required for tumoral expression. J Cell Physiol. 1983 May;115(2):123–130. doi: 10.1002/jcp.1041150204. [DOI] [PubMed] [Google Scholar]